Summary We assessed the impacts of hay-scented fern (Dennstaedtia punctilobula (Michx.) Moore) and subsoil lim-ing (CaO amendments) on root and shoot growth of green-house-grown, first-year, northern red oak (Quercus rubra L.) seedlings. Red oak seedlings and ferns were grown in recon-structed soil profiles of four common Pennsylvanian forest soils. When grown in the presence of hay-scented ferns, with or without subsoil liming, red oak seedlings had significantly reduced height growth, and foliar, stem and total root biomass. Fern foliar biomass was significantly reduced when ferns were grown with red oaks, but there was no significant difference in total belowground biomass of ferns. Belowground fern biomass was concentrated in the upper soil profile, whereas red oak roots showed a variety of distributions. In the presence of ferns, fine root branching in red oak was reduced in the organic horizons of three of the four soils tested. In both the presence and absence of ferns, root branching in red oak was also significantly and negatively correlated with the concentration of 0.01 M SrCl2-extractable aluminum in the mineral horizons (r2 = 0.77). Subsoil liming generally improved root branching in red oaks. The presence of ferns significantly reduced ecto-mycorrhizal infection frequency in red oak. We conclude that hay-scented fern inhibited root branching and suppressed above- and belowground biomass accumulation of first-year northern red oak seedlings.
Keywords: root competition, soil acidity, subsoil liming.
Introduction
Hay-scented fern (Dennstaedtia punctilobula (Michx.) Moore) is a rhizomatous, invasive, gap-colonizing species that grows across wide gradients of edaphic and light conditions (Conard 1908, Cody et al. 1977, Burnside 1988, Brach et al. 1993), and exhibits great phenotypic plasticity above- and belowground (Horsley 1984, Hammen 1993). Dense ground covers of hay-scented fern have been implicated in reducing hardwood seedling growth and causing regeneration delays and failures in Allegheny and northern hardwood forests (Horsley and Marquis 1983, Maquire and Forman 1983, Drew
1988, 1990, Horsley 1993a) and oak forests (Bowersox and McCormick 1987, Horsley 1988, Kolb et al. 1990). In recent decades, hay-scented fern has become a large-scale forest management problem in Pennsylvania; it has proliferated in forest understories, disturbed areas, shelterwood cuts, and clearcuts (Horsley 1981, Marquis et al. 1990, Groninger and McCormick 1991, McWilliams et al. 1994).
Numerous mechanisms have been proposed to explain how hay-scented fern interferes with the growth of hardwood seed-lings. These include: reductions in photon flux density and changes in light quality (R/FR ratio) under fern canopies (Horsley 1986, Burnside 1988, Kolb et al. 1990, Horsley 1993a), competition for nutrients and water by fern root sys-tems (Drew 1988), allelopathy (Horsley 1977, 1993a) and physical occupation of organic soil horizons by fern root sys-tems (Hammen 1993). Most research has focused on hay-scented fern interference with growth of black cherry (Prunus serotina Ehrh.) seedlings (Horsley 1993a, 1993b). There is only limited information about mechanisms underlying fern interference with other hardwood seedlings, including north-ern red oak (Quercus rubra L.) (Bowersox and McCormick 1987).
In recent decades, northern red oak has regenerated poorly in parts of its range (Crow 1988). Although regeneration diffi-culties result from numerous interacting factors (Lorimer 1989, Kelly et al. 1990, Walters and Auchmoody 1993), inter-ference by hay-scented fern has been cited as an important factor limiting seedling growth and regeneration success in some areas (Bowersox and McCormick 1987, Kolb et al. 1989, Kolb and Steiner 1990, McWilliams et al. 1994).
In the current greenhouse study, reconstructed soil profiles were used to approximate the soil types that red oak seedlings typically face under field conditions. Red oak growth re-sponses to subsoil liming and established fern covers were examined. The greenhouse study was designed to eliminate the effect of shading by the ferns and, under these conditions, test the following hypotheses: (1) ferns reduce aboveground growth of red oak; (2) ferns reduce the belowground growth of red oak; (3) ferns alter the shoot/root ratio of red oak; (4) sub-soil liming improves root growth and overall growth of red oak
Hay-scented fern (
Dennstaedtia punctilobula
(Michx.) Moore)
interference with growth of northern red oak (
Quercus rubra
L.)
seedlings
JONATHAN LYON
1,2and WILLIAM E. SHARPE
11 School of Forest Resources, Environmental Resources Research Institute, The Pennsylvania State University, University Park, PA 16802, USA 2 Present address: Department of Natural Science, Edgewood College, 855 Woodrow Street, Madison, WI 53711, USA
Received October 25, 1995
seedlings when grown alone or in the presence of ferns; and (5) ferns reduce ectomycorrhizal infection frequency in red oak.
Materials and methods
In June 1992, soil was collected from four field sites, Cook-port, Clymer, Wharton, and Hazleton/Dekalb, in north-central Pennsylvania. These sites support forests with at least half the overstory composed of northern red oak (for detailed site information, see Lyon and Sharpe 1995). All soil horizons to a depth of 30 cm, but excluding the organic horizons, were collected from excavated pits. Bulk densities of each mineral horizon at the four field sites were determined. Each horizon of the excavated soils was sieved through a 4-mm mesh. Mineral soil horizons were then reconstructed in plastic, cylin-drical growth cores (mini-terracosms; 15 cm diameter × 30 cm depth). The growth cores had a total volume of 5025 cm3, a volume/depth ratio of approximately 168, an adequate surface area for O2 exchange, and ample water storage capacity (Han-son et al. 1987). Identical soil weights of individual horizons (within each soil type) were placed in the growth cores and the horizons were compacted to specific volumes to match meas-ured field bulk density values (Reicosky et al. 1972). Calcium amendments (subsoil liming) were achieved by inter-mixing CaO at a rate of 500 kg Ca ha−1 in the deepest mineral horizon of each soil type before horizon reconstruction. The rate of Ca application was designed to reduce Al concentrations without radically altering the balance of cations in the soil.
Organic horizons were collected in August 1992. Based on soil analyses at each site, fern and non-fern samples were chosen from areas of similar soil chemical status. Intact or-ganic horizon cores (15.5 cm diameter × 3 cm depth) were used to reduce soil disturbance and nitrification potential. Fern fronds were cut back to the soil surface before cores were removed from the ground. Each fern core was inspected to ensure that all cores had similar rhizome densities and were devoid of large fern roots or rocks. All recognized non-fern roots and large pieces of leaf litter were removed. Organic horizons were immediately taken to the laboratory and weighed. Cores used in the study were of the same weight
± 25 g. Small samples were collected from each core for chemical analysis, and the cores were then fitted on top of the appropriate mineral soil profiles. The completed mini-terra-cosms were wrapped with clear plastic and aluminum foil to eliminate light penetration and reduce heat absorbency. The bottom of each mini-terracosm was covered with a 400 µ m-mesh nylon screen equipped with a plastic cap connected with a drainage tube to a collection bottle. The net volume of water moving through the cores and the solution chemistry of the soil effluent were determined. The mini-terracosms were kept in the greenhouse and watered twice weekly with deionized water until January 1993.
Soil analysis
Soil samples from each horizon (160 mini-terracosms × 3 horizons = 480 samples) were air-dried and sieved (2-mm
mesh) before analysis. Soil tests were conducted when the soils were collected and at the end of the study. Exchangeable cations were determined for a subsample (n = 2) from each horizon (North Dakota State University 1988). Soil pH was measured in 0.01 M CaCl2 (1/1, w/v). Extractable Ca and Al were determined for all 480 samples in extracts of 0.01 M SrCl2 (Joslin and Wolfe 1989). All SrCl2 soil extracts were analyzed by atomic absorption spectrometry (Thermo-Jarrel-Ash (IL) Video 22 spectrometer, Milton, MA).
Red oak acorns and seedling growth
In October 1992, red oak acorns were collected from a single parent tree growing on University Park Campus, Pennsylvania State University, and immediately processed as described by Olson (1974). After cold stratification, acorns of similar weight (5.5 ± 0.1 g) were germinated on moist potting soil until radicle emergence (1--2 cm long). Acorns were planted flush with the surface of the organic horizons. Two weeks before acorn planting, fern foliage was cut back to the soil surface to ensure that adequate light was available for the red oak seed-lings. This procedure simulated the phenology of fern growth under field conditions, where early seedling growth generally occurs before the emergence of fern fronds (Cody et al. 1977). The impact of cutting on subsequent frond growth appeared to be minimal; fronds grew back quickly and reached heights similar to pre-cut levels (pre-cut grand mean height = 47.6 cm; post-cut grand mean height = 49.7 cm). To prevent shading of red oak seedlings during the study, fronds were restrained by plastic rods and string. Red oak seedling cotyledons were severed from all seedlings at Day 60 of the study to eliminate their continued influence. The study was terminated 130 days after acorn planting.
Greenhouse conditions and experimental design
Growth cores were placed on greenhouse benches equipped with a 20% shade cloth. The greenhouse was illuminated artificially to provide a 16-h photoperiod and temperature was regulated by an automatic ventilation system. Maximum and minimum temperatures ranged from 35 to 22 °C and from 30 to 14 °C, respectively. Relative humidity ranged from 30 to 90%. The experimental design was a completely randomized block with replication. The experimental units were individual mini-terracosms. There were four soil profiles, five treatments and eight replications of each treatment (= 160 mini-terra-cosms). The five treatments were: (1) red oak grown alone; (2) red oak grown with Ca-amended subsoil; (3) fern grown alone; (4) fern grown with red oak; and (5) fern grown with red oak with Ca-amended subsoil.
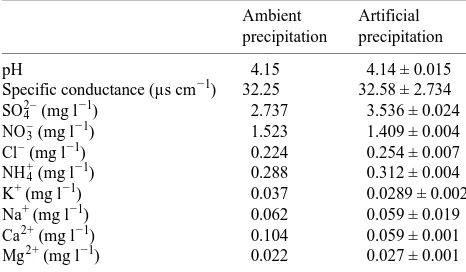
properties of the simulated precipitation mix with 1990 ambi-ent precipitation chemistry means are summarized in Table 1. Sulfate concentrations in the artificial precipitation were higher than ambient values to match the conductivity and pH values.
Red oak and fern shoot and root growth analyses
Periodic measures of red oak height growth (every 7 to 10 days) were made. After 130 days, red oak seedlings and ferns were harvested, red oak foliar and stem biomass were determined, and mini-terracosms were separated by horizon. Red oak and fern roots and rhizomes were separated by hori-zon, taking care to collect all fine roots. Plant tissues were then washed in deionized water and frozen until processed. After thawing, rhizomes and roots were carefully washed in dilute Alconox® solution (Alconox, Inc., New York) followed by three rinses in deionized water. Red oak root systems were then separated into taproot and lateral roots, and fern tissues were separated into rhizomes and fine roots. All plant tissues were dried to constant weight at 80 °C to determine dry weight biomass.
Measurements of red oak roots followed the terminology of Lyford (1980). The primary laterals were counted in two cate-gories (> 3 cm length and < 3 cm length). The total length of the primaries > 3 cm long was also measured (Tennant 1975). The five longest primary laterals within each horizon were measured for diameter, total number of secondary laterals > 2 cm long, and total length of secondary laterals. Total sec-ondary lateral root length was calculated by multiplying the combined length of the five longest secondary laterals by the proportion of the remaining length of primary laterals. All inter-horizon roots (roots not attached to the taproot) were measured separately, but were added to primary length totals for horizons in which they occurred. All root measurements were analyzed by horizon but were summed across horizons to calculate total root system measures. Mycorrhizal infection frequency was determined as described by Mitchell et al. (1984). Hay-scented fern rhizome measurements included to-tal rhizome length, rhizome diameters of five randomly chosen
rhizome segments, and the number, length and diameter of fine roots found on each the five rhizome segments.
Statistical analysis
Statistical analysis was performed using Minitab 8.2 software (Minitab, Inc. Data Tech Industries, Valley Forge, PA). Signifi-cance testing of continuous distribution variables between treatments was conducted by ANOVA. Mean separations were performed with Fisher’s protected LSD test. Comparisons be-tween pre- and post-study soil parameters were made with Dunnett’s test. All significant differences reported are at α = 0.05, unless otherwise noted. Variables were transformed when necessary to meet the assumption of normality. Some measure-ments of red oak and fern belowground tissues were recalcu-lated on a volumetric basis and others were normalized to compare proportional allocation of roots by horizon. Linear and polynomial regressions were also run using normalized root morphological variables and root branching index values. Differences in regression slope coefficients were determined as described by Sokal and Rohlf (1981).
Results
During the 130-day study, there were no significant changes in soil pH in the limed treatments of any of the four soils tested (Table 2). Calcium concentrations in the limed treatments exhibited significant changes in some soils; however, the Ca concentrations of the limed horizons were still an order of magnitude higher than those of the non-limed horizons at the end of the study. Furthermore, Al concentrations at the end of the study were lower in the limed soils than in the non-limed soils. We conclude, therefore, that the effects of the Ca amend-ments were maintained throughout the study.
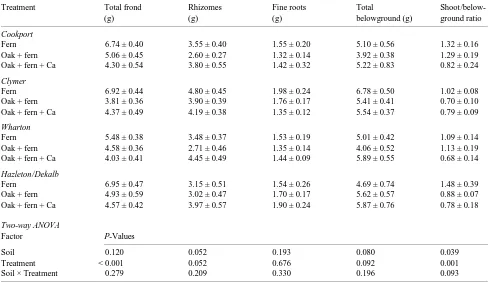
Fern biomass and biomass allocation
The only statistically significant soil type and treatment main effect on fern biomass was on the fern shoot/belowground biomass ratio (Table 3). However, the rhizome and total below-ground biomass P-values were near significant. There were no significant soil × treatment interactions on fern biomass, al-though treatment main effects were significant for both total frond weight and fern shoot/belowground ratio. Frond weights in the fern alone treatment were significantly higher than frond weights in the red oak + fern treatment on all soils except the Wharton soil, and they were significantly higher than frond weights of ferns in the red oak + fern + Ca treatment across all four soils (Table 3). Neither total belowground nor fine root biomass was significantly different between treatments across soil types (but note P-values). Fern shoot/belowground biomass ratios reflected the reduction in shoot biomass relative to constant values of belowground biomass. Fern shoot/below-ground ratios were significantly lower in ferns in the red oak + fern + Ca treatment than in ferns in either the fern alone or red oak + fern treatments on the Cookport and Wharton soils. In the Clymer and Hazleton/Dekalb soils, significant changes in fern biomass allocation occurred only in response to the com-bined impacts of red oak and Ca.
Table 1. Comparisons of the pH, specific conductance and chemical composition of ambient versus artificial precipitation. All values are means (± SE) and are based on eight sampling dates.
Table 2. Comparison of pH and 0.01M SrCl2-extractable Al and Ca in the lowermost mineral horizon B across soil types and treatments. Treatment
means of the soil parameters determined at the end of the study are compared to pre-experimental means using Dunnett’s test. Numbers in columns followed by an asterisk are significantly different from the pre-experimental control value.
Treatment B Horizon B + Lime Horizon
pH Al Ca pH Al Ca
Cookport
Pre-experimental values 3.87 0.162 0.026 4.38 0.010 0.634
Oak 4.05 0.212* 0.019* 4.36 0.030* 0.470*
Fern 4.13* 0.201* 0.031
Oak + fern 4.06* 0.205* 0.028 4.51 0.043* 0.755*
Clymer
Pre-experimental values 4.17 0.387 0.034 4.86 0.032 0.682
Oak 4.16 0.121* 0.033 4.54 0.017 0.456
Fern 4.11 0.123* 0.012*
Oak + fern 4.14 0.183* 0.020* 4.67 0.011 0.578
Wharton
Pre-experimental values 3.64 0.176 0.027 4.02 0.028 0.735
Oak 3.88 0.301* 0.025 4.05 0.051* 0.306*
Fern 3.83 0.287* 0.022
Oak + fern 3.82 0.267* 0.031 4.13 0.068* 0.593*
Hazleton/Dekalb
Pre-experimental values 3.92 0.307 0.035 4.12 0.111 0.769
Oak 3.88 0.283 0.039 4.08 0.134 0.590*
Fern 3.98 0.283 0.028*
Oak + fern 3.95 0.304 0.026* 4.15 0.122 0.516*
Table 3. Hay-scented fern biomass allocation (mean dry weights ± SE) at the end of the study.
Treatment Total frond Rhizomes Fine roots Total
Shoot/below-(g) (g) (g) belowground (g) ground ratio
Cookport
Fern 6.74 ± 0.40 3.55 ± 0.40 1.55 ± 0.20 5.10 ± 0.56 1.32 ± 0.16
Oak + fern 5.06 ± 0.45 2.60 ± 0.27 1.32 ± 0.14 3.92 ± 0.38 1.29 ± 0.19
Oak + fern + Ca 4.30 ± 0.54 3.80 ± 0.55 1.42 ± 0.32 5.22 ± 0.83 0.82 ± 0.24
Clymer
Fern 6.92 ± 0.44 4.80 ± 0.45 1.98 ± 0.24 6.78 ± 0.50 1.02 ± 0.08
Oak + fern 3.81 ± 0.36 3.90 ± 0.39 1.76 ± 0.17 5.41 ± 0.41 0.70 ± 0.10
Oak + fern + Ca 4.37 ± 0.49 4.19 ± 0.38 1.35 ± 0.12 5.54 ± 0.37 0.79 ± 0.09
Wharton
Fern 5.48 ± 0.38 3.48 ± 0.37 1.53 ± 0.19 5.01 ± 0.42 1.09 ± 0.14
Oak + fern 4.58 ± 0.36 2.71 ± 0.46 1.35 ± 0.14 4.06 ± 0.52 1.13 ± 0.19
Oak + fern + Ca 4.03 ± 0.41 4.45 ± 0.49 1.44 ± 0.09 5.89 ± 0.55 0.68 ± 0.14
Hazleton/Dekalb
Fern 6.95 ± 0.47 3.15 ± 0.51 1.54 ± 0.26 4.69 ± 0.74 1.48 ± 0.39
Oak + fern 4.93 ± 0.59 3.02 ± 0.47 1.70 ± 0.17 5.62 ± 0.57 0.88 ± 0.07
Oak + fern + Ca 4.57 ± 0.42 3.97 ± 0.57 1.90 ± 0.24 5.87 ± 0.76 0.78 ± 0.18
Two-way ANOVA
Factor P-Values
Soil 0.120 0.052 0.193 0.080 0.039
Treatment < 0.001 0.052 0.676 0.092 0.001
Figure 1 shows the vertical distribution of fern belowground biomass by soil horizon volume (data were pooled across soils because there were no significant soil or treatment main effects or soil × treatment interactions). The shallow rooting pattern of ferns in the growth cores (Figure 1) closely resembles the growth form in the field (Hammen 1993). Rhizome biomass accounted for 81--89% of the biomass in the organic horizons and for 60--81% of the biomass in the entire soil profile. Overall, the belowground biomass of ferns was not strongly influenced by the red oak or subsoil Ca treatments.
Fern impacts on red oak growth
There were significant soil type (P = 0.038) and treatment main effects (P < 0.001) on the height growth of red oak seedlings (Figure 2). Mean final heights of red oak seedlings in the red oak, red oak + Ca, red oak + fern and red oak + fern + Ca treatments were 45.83, 51.01, 19.86 and 28.23 cm, respectively. On all four soils, final red oak height growth was significantly reduced in the red oak + fern treatment compared with the red oak and red oak + Ca treatments (Table 4). Comparisons between red oak + fern and red oak + fern + Ca treatments showed that there was significantly greater height growth of red oak in the presence of subsoil Ca on the Wharton and Hazleton/Dekalb soils, indicating that subsoil liming of these soils counteracted the depression of red oak height growth associated with the presence of ferns.
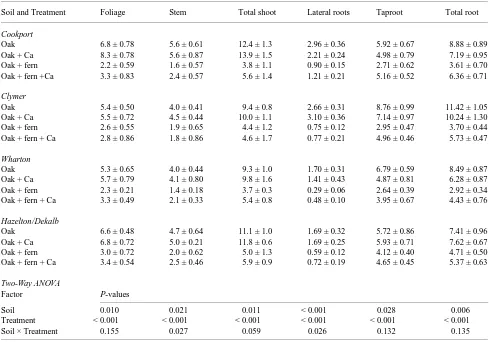
Significant soil type and treatment main effects were found for red oak foliar, stem, total shoot, lateral root, taproot and total root biomass (Table 4). Statistically significant soil × treatment interactions were found only for stem (P = 0.027) and lateral root biomass (P = 0.026). For all measured vari-ables, treatment main effect F-ratios were larger than either soil main effect or soil × treatment interaction F-ratios, indi-cating that ferns exerted the dominant influence on red oak biomass. Overall, seedlings in the red oak and red oak + Ca treatments had significantly greater foliar, stem, total shoot,
taproot and lateral root biomass than seedlings in the red oak + fern and red oak + fern + Ca treatments. This pattern was evident within each soil type (Table 4).
Fern impacts on red oak shoot/root ratios
Shoot/root biomass ratios of red oak seedlings ranged from 0.81 to 1.93, with most at or near 1 (mean = 1.39) (Figure 3). On the Clymer and Wharton soils, there were no significant treatment effects on shoot/root ratios; however, on the Cook-port and Hazleton/Dekalb soils, red oak seedlings allocated a higher proportion of biomass to shoots than roots in the ab-sence of ferns than in the preab-sence of ferns.
Fern impacts on red oak root architecture
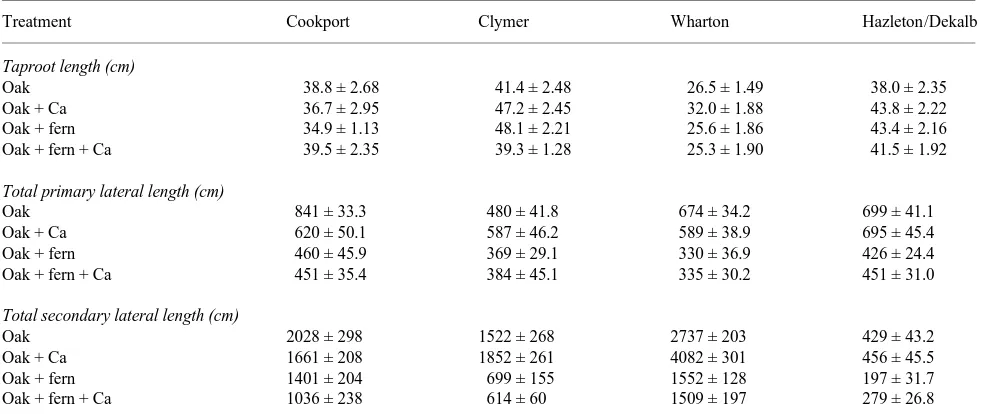
None of the treatments had a significant effect on red oak taproot length in any of the four soils tested (Table 5). No consistent patterns of root biomass reallocation were observed despite the significant reduction in root biomass in the red oak + fern and red oak + fern + Ca treatments. The anticipated shift in red oak root biomass deeper into the soil profile in response to subsoil liming or the presence of hay-scented ferns only occurred in the Cookport and Hazleton/Dekalb soils. Total primary and secondary lateral lengths were significantly re-duced in the presence of ferns, except for secondary laterals in the red oak + fern treatment in Cookport soil (Table 5). Signifi-cant increases in root length in red oak + Ca treatments were noted for primary laterals in Clymer soil and for secondary laterals in Wharton soil. A significant decrease in secondary laterals was noted in the red oak + fern + Ca treatment in Cookport soil.
A graphical summary of root branching patterns of red oak (i.e., total length of secondary laterals/total length of primary laterals) in the organic horizons of the four soils is presented in Figure 4. Significant treatment (P = 0.004) and soil effects (P = 0.037) were noted. Among the four soils examined, overall frequency of root branching was lowest in the
Hazle-Figure 2. Comparison of mean height growth of red oak seedlings on four soils and across four treatments. Soil types are as follows: Cook = Cookport; Clym = Clymer; Whar = Wharton; and Haz/D = Hazle-ton/Dekalb. Significant soil and treatment main effects and a signifi-cant soil × treatment interaction were found.
ton/Dekalb soil. Root branching was significantly reduced in the presence of ferns in all but the Wharton soil. Thus, with the exception of the Wharton soil, inhibition of red oak root branching was a major morphological and architectural effect of fern interference.
Soil aluminum impacts on red oak root growth
Figure 5 illustrates the root branching response of red oak seedlings to soil Al concentrations (0.01 M SrCl2 extractable)
on Day 130. Each point in the regression represents a treatment mean for a mineral soil horizon. Only mineral horizons were used in the regressions because of difficulties in accurately determining Al concentrations in the organic horizons. There was a significant (P < 0.001) negative relationship between red oak root branching and soil Al concentration (r2 = 0.774).
Mycorrhizal infection frequency in red oak seedlings
Across treatments, there was a significant effect of soil (P < 0.001) on the proportion of red oak primary laterals in-fected with ectomycorrhizal fungi, with the Wharton soil showing the lowest overall infection frequency (mean = 3.98%) (Figure 6). There was also a significant (P < 0.001) treatment effect on mycorrhizal infection frequency. Seedlings in the red oak and red oak + Ca treatments had significantly higher infection frequencies than seedlings in the red oak + fern and red oak + fern + Ca treatments. On both the Cookport and Wharton soils, mycorrhizal infection frequencies were significantly reduced in the presence of ferns (red oak + fern and red oak + fern + Ca treatments). No significant differences in infection frequency across treatments were observed in the Clymer soil. On the Hazleton/Dekalb soil, the highest infec-tion frequency was observed when red oak was grown alone.
Table 4. Comparison of northern red oak seedling biomass (dry weight in g) allocation determined at the end of the 130-day growth study. Values are means ± SE.
Soil and Treatment Foliage Stem Total shoot Lateral roots Taproot Total root
Cookport
Oak 6.8 ± 0.78 5.6 ± 0.61 12.4 ± 1.3 2.96 ± 0.36 5.92 ± 0.67 8.88 ± 0.89
Oak + Ca 8.3 ± 0.78 5.6 ± 0.87 13.9 ± 1.5 2.21 ± 0.24 4.98 ± 0.79 7.19 ± 0.95
Oak + fern 2.2 ± 0.59 1.6 ± 0.57 3.8 ± 1.1 0.90 ± 0.15 2.71 ± 0.62 3.61 ± 0.70 Oak + fern +Ca 3.3 ± 0.83 2.4 ± 0.57 5.6 ± 1.4 1.21 ± 0.21 5.16 ± 0.52 6.36 ± 0.71
Clymer
Oak 5.4 ± 0.50 4.0 ± 0.41 9.4 ± 0.8 2.66 ± 0.31 8.76 ± 0.99 11.42 ± 1.05
Oak + Ca 5.5 ± 0.72 4.5 ± 0.44 10.0 ± 1.1 3.10 ± 0.36 7.14 ± 0.97 10.24 ± 1.30
Oak + fern 2.6 ± 0.55 1.9 ± 0.65 4.4 ± 1.2 0.75 ± 0.12 2.95 ± 0.47 3.70 ± 0.44
Oak + fern + Ca 2.8 ± 0.86 1.8 ± 0.86 4.6 ± 1.7 0.77 ± 0.21 4.96 ± 0.46 5.73 ± 0.47
Wharton
Oak 5.3 ± 0.65 4.0 ± 0.44 9.3 ± 1.0 1.70 ± 0.31 6.79 ± 0.59 8.49 ± 0.87
Oak + Ca 5.7 ± 0.79 4.1 ± 0.80 9.8 ± 1.6 1.41 ± 0.43 4.87 ± 0.81 6.28 ± 0.87
Oak + fern 2.3 ± 0.21 1.4 ± 0.18 3.7 ± 0.3 0.29 ± 0.06 2.64 ± 0.39 2.92 ± 0.34
Oak + fern + Ca 3.3 ± 0.49 2.1 ± 0.33 5.4 ± 0.8 0.48 ± 0.10 3.95 ± 0.67 4.43 ± 0.76
Hazelton/Dekalb
Oak 6.6 ± 0.48 4.7 ± 0.64 11.1 ± 1.0 1.69 ± 0.32 5.72 ± 0.86 7.41 ± 0.96
Oak + Ca 6.8 ± 0.72 5.0 ± 0.21 11.8 ± 0.6 1.69 ± 0.25 5.93 ± 0.71 7.62 ± 0.67
Oak + fern 3.0 ± 0.72 2.0 ± 0.62 5.0 ± 1.3 0.59 ± 0.12 4.12 ± 0.40 4.71 ± 0.50
Oak + fern + Ca 3.4 ± 0.54 2.5 ± 0.46 5.9 ± 0.9 0.72 ± 0.19 4.65 ± 0.45 5.37 ± 0.63
Two-Way ANOVA
Factor P-values
Soil 0.010 0.021 0.011 < 0.001 0.028 0.006
Treatment < 0.001 < 0.001 < 0.001 < 0.001 < 0.001 < 0.001
Soil × Treatment 0.155 0.027 0.059 0.026 0.132 0.135
Discussion
To avoid light competition by herbaceous ground covers, inter-mediate shade-tolerant tree seedlings such as northern red oak (Baker 1949) must achieve sufficient height growth to overtop the canopy of ground vegetation. Because of its recurrent growth form (Hanson et al. 1986), red oak has the potential for multiple growth flushes in the same season, although typically only a single flush each year is observed in the field (Crow 1988). In the present study, the first growth flush of red oak seedlings showed no significant differences in height growth or leaf area across soil types or treatments (Lyon 1995), sug-gesting that cotyledon reserves influenced early red oak growth (Carpenter and Guard 1954, Hanson 1986, Crow 1988). However, cotyledons were removed at Day 60, and so did not impact later phases of growth. The relatively robust
height growth observed in seedlings in the fern-free treatments was greater than height growth reported in native soils (McCle-nahen 1987, Joslin and Wolfe 1989, Kolb et al. 1990, Sharpe et al. 1993). Our results may be explained by several factors, including favorable growing conditions (light, humidity, tem-perature, soil water) and the existence of a multi-horizon rooting environment with a nutrient-rich organic horizon. The poor height growth exhibited by red oak seedlings in the presence of ferns, despite the favorable growth conditions, highlights the strong belowground interference by ferns.
Interspecific root competition between two plant species occurs if one species (or both) exhibits a growth reduction
Table 5. Comparison of red oak seedling total taproot length, total primary lateral length, and total secondary lateral length between treatments. Values are means ± SE.
Treatment Cookport Clymer Wharton Hazleton/Dekalb
Taproot length (cm)
Oak 38.8 ± 2.68 41.4 ± 2.48 26.5 ± 1.49 38.0 ± 2.35
Oak + Ca 36.7 ± 2.95 47.2 ± 2.45 32.0 ± 1.88 43.8 ± 2.22
Oak + fern 34.9 ± 1.13 48.1 ± 2.21 25.6 ± 1.86 43.4 ± 2.16
Oak + fern + Ca 39.5 ± 2.35 39.3 ± 1.28 25.3 ± 1.90 41.5 ± 1.92
Total primary lateral length (cm)
Oak 841 ± 33.3 480 ± 41.8 674 ± 34.2 699 ± 41.1
Oak + Ca 620 ± 50.1 587 ± 46.2 589 ± 38.9 695 ± 45.4
Oak + fern 460 ± 45.9 369 ± 29.1 330 ± 36.9 426 ± 24.4
Oak + fern + Ca 451 ± 35.4 384 ± 45.1 335 ± 30.2 451 ± 31.0
Total secondary lateral length (cm)
Oak 2028 ± 298 1522 ± 268 2737 ± 203 429 ± 43.2
Oak + Ca 1661 ± 208 1852 ± 261 4082 ± 301 456 ± 45.5
Oak + fern 1401 ± 204 699 ± 155 1552 ± 128 197 ± 31.7
Oak + fern + Ca 1036 ± 238 614 ± 60 1509 ± 197 279 ± 26.8
Figure 4. Root branching index (total length of secondary laterals/total length of primary laterals) for northern red oak roots growing in the organic horizons of the four soils. Mean branching index values (± SE) are shown for each soil and treatment.
Figure 5. Second-order polynomial regression between 0.01 M SrCl2
when it shares the same soil resources as the other species, and is not limited in growth by aboveground factors. In our study, hay-scented fern interfered with the growth of red oak seed-lings, despite the apparent lack of aboveground interference. Red oak height growth and foliar and stem biomass were all significantly reduced when seedlings were grown with ferns. Similarly, Hippensteel (1992) noted that frond removal did not restore height growth of first-year paper birch (Betula papyrif-era Marsh.) or white ash (Fraxinus americana L.) seedlings to that of non-fern treatments. Beck (1970) reported slow growth rates of red oak seedlings released from overstory and under-story competition in North Carolina. In contrast to our results, Horsley (1993a) reported good height growth of black cherry when fronds of competing ferns were restrained to allow full light to reach the cherry leaf surfaces. The higher growth rate of black cherry compared with red oak (Farmer 1980) may account for the discrepancy between these species.
Many plant growth models predict a greater partitioning of biomass to plant tissues responsible for capturing the most limiting nutrient (Johnson and Thornley 1987). Several red oak seedling studies have documented shifts in biomass partition-ing to root systems in response to grass competition (Watson 1988, Kolb and Steiner 1990) and increasing irradiance (Baz-zaz and Miao 1993). In our study, however, significant in-creases in biomass allocation to roots in response to ferns were observed on only two of the four soils tested (Cookport and Hazleton/Dekalb). Although these soil-specific patterns indi-cate the importance of soil type in predicting the biomass allocation responses of red oak, they provide no information on root architectural changes that might have occurred. Be-cause assessment of root architectural changes can provide a more comprehensive view of root responses to both root com-petition and the soil chemical environment than simple biomass determinations (Fitter et al. 1991), we also determined the effects of ferns on root branching patterns of red oak seedlings.
We observed that red oak root branching (i.e., secondary lateral growth) in the organic horizon was significantly re-duced in the presence of hay-scented fern in all but the Whar-ton soil. This architectural change in red oak roots, which was not reflected in the biomass allocation analysis, represents a specific symptom of root interference between hay-scented
fern and red oak and has important implications for red oak seedling growth because most root hairs in red oak are located on secondary lateral roots (Richardson 1953). It is possible that the reduction in fine root branching was a result of the physical occupation of the organic horizons by fern rhizomes and fine roots (McConnaughay and Bazzaz 1992). If ferns can interfere with fine root development of red oaks in the organic horizons, which typically contain higher concentrations of nutrients and roots than mineral horizons (Vogt et al. 1983, Gale and Grigal 1987, Kelly and Joslin 1989, Ehrenfeld et al. 1992), they can effectively diminish the availability of nutrients to red oaks.
The presence of Al in the subsoil also influenced red oak seedling root growth and architecture. A significant negative relationship between red oak root branching and 0.01 M SrCl2 -extractable Al was observed in both the fern and non-fern treatments. This result is consistent with several previous oak studies reporting that 0.01 M SrCl2-extractable Al was signifi-cantly and negatively correlated with red oak root branching in mineral soil horizons (Kelly et al. 1990). We observed in-creases in red oak root branching in response to subsoil liming, suggesting that soil Al contributed to poor red oak growth. It has been widely recognized that first-year red oak seedlings develop vertical taproots that are able to penetrate to a depth of 45 cm in the first year of growth (Korstian 1927, Rudolf 1929, Toumey 1929, Holch 1931, Biswell 1935, Duncan 1941, Richardson 1953, Carpenter and Guard 1954, Farmer 1975, Lyford 1980). However, if high concentrations of Al in the subsoil reduce fine root branching, then the ability of red oak to exploit subsoil nutrients or water, or both, will be impaired. Hay-scented fern also inhibited ectomycorrhizal infection in red oak roots. Infection rates were relatively low in the current study (4--38%) compared to other studies of red oak (Ruehle 1980, Mitchell et al. 1984). The low infection rates observed on the Wharton soils are consistent with the finding that this soil had the highest nutrient availability of the soils tested. Although we did not measure fern mycorrhizal infection rates, hay-scented fern has also been shown to have mycorrhizal associations (Conard 1908).
We conclude that both hay-scented fern and subsoil Al interfered with aboveground growth or belowground growth or both of first-year northern red oak seedlings. Interference by ferns was more severe than that caused by subsoil Al. The presence of ferns resulted in reduced red oak height, shoot growth, root growth, root branching in the organic horizon and ectomycorrhizal infection rates. The degree of interference by hay-scented fern was often soil specific. Liming treatments were of limited effectiveness in improving red oak biomass in the presence and absence of ferns. However, liming did result in significant increases in red oak height growth in the pres-ence of ferns on two of the four soils. Liming also ameliorated the negative impacts of subsoil Al on red oak root branching. Based on the observed changes in red oak root branching in response to both ferns and subsoil Al, we conclude that changes in root architecture may be a better indicator of below-ground responses to ferns and subsoil Al than simple biomass determinations.
Acknowledgments
Funding and logistical support for this study came from the School of Forest Resources and the Environmental Resource Research Institute at The Pennsylvania State University. We thank the Pennsylvania Bureau of Forestry for access to field sites. Mary Kay Amistadi was indispensable in the soil analyses. We thank Stephen Horsley for his critical review of this manuscript, which greatly improved the quality of this paper. Thanks are also extended to Dave DeWalle, Larry McCormick, Todd Bowersox, and Dale Baker for their help in the study.
References
Baker, F.S. 1949. A revised tolerance table. J. For. 47:179--181. Bazzaz, F.A. and S.L. Miao. 1993. Successional status, seed size, and
responses of tree seedlings to CO2, light, and nutrients. Ecology
74:104--112.
Beck, D.E. 1970. Effect of competition on survival and height growth of red oak seedlings. USDA For. Serv., Res. Pap. SE-56, 6 p. Biswell, H.H. 1935. Effects of environment upon the root habits of
certain deciduous forest trees. Bot. Gaz. 96:676--708.
Bowersox, T.W. and L.H. McCormick. 1987. Herbaceous communi-ties reduce the juvenile growth of northern red oak, white ash, yellow poplar, but not white pine. Proc. 6th Central Hardwood Forest Conf., pp 39--43.
Brach, A.R., S.J. McNaughton and D.J. Raynal. 1993. Photosynthetic adaptability of two fern species of a northern hardwood forest. Am. Fern J. 83:47--53.
Burnside, E.V. 1988. The effects of light intensity on the growth of hay-scented fern. M.Sc. Thesis, The Pennsylvania State Univ., 54 p. Carpenter, I.W. and A.T. Guard. 1954. Anatomy and morphology of the seedling roots of four species of the genus Quercus. J. For. 52:269--274.
Cody, W.S., I.V. Hall and C.W. Cromptom. 1977. The biology of Canadian weeds: 26. Dennstaedtia punctilobula (Michx.) Moore. Can. J. Plant Sci. 57:1159--1168.
Conard, H.S. 1908. The structure and life history of the hay-scented fern. Carnegie Inst. Wash. Publ. 94:1--106.
Crow, T.R. 1988. Reproductive mode and mechanisms for self-re-placement of northern red oak (Quercus rubra)----a review. For. Sci. 34:19--40.
Drew, A.P. 1988. Interference of black cherry by ground flora of the Allegheny uplands. Can J. For. Res. 18:652--656.
Drew, A.P. 1990. Fern and aster effects on black cherry shelterwood regeneration. Can. J. For. Res. 20:1513--1515.
Duncan, W.H. 1941. A study of root development in three soil types in the Duke Forest. Ecol. Monogr. 11:142--164.
Ehrenfeld, J.G., E. Kaldor and R.W. Parmelee. 1992. Vertical distribu-tion of roots along a toposequence in the New Jersey pinelands. Can. J. For. Res. 22:1929--1936.
Farmer, R.E. 1975. Dormancy and root regeneration of northern red oak. Can J. For. Res. 5:176--185.
Farmer, R.E. 1980. Comparative analysis of 1st-year growth in six deciduous tree species. Can. J. For. Res. 10:35--41.
Fitter, A.H., T.R. Strickland, M.L. Harvey and G.W. Wilson. 1991. Architectural analysis of plant root systems. 1. Architectural corre-lates of exploitation efficiency. New Phytol. 118:375--383. Gale, M.R. and D.F. Grigal. 1987. Vertical root distribution of northern
tree species in relation to successional status. Can J. For. Res. 17:829--834.
Groninger, J.W. and L.H. McCormick. 1991. Effects of sulfometuron on hay-scented fern spore emergence. Can. J. For. Res. 21:942--943.
Hammen, S.C.L. 1993. Density-dependent phenotypic variation in the hay-scented fern, Dennstaedtia punctilobula. Bull. Torrey Bot. Club. 120:392--396.
Hanson, P.J. 1986. Studies of Quercus rubra L. seedling dry matter accumulation, morphological development, and carbon dioxide exchange under controlled conditions. Ph.D. Thesis, Univ. Minne-sota, 169 p.
Hanson, P.J., R.E. Dickson, J.G. Isebrands, T.R. Crow and R.K. Dixon. 1986. A morphological index of Quercus seedling onto-geny for use in studies of physiology and growth. Tree Physiol. 2:273--281.
Hanson, P.J., R.K. Dixon and R.E. Dickson. 1987. Effects of container size and shape on the growth of northern red oak seedlings. Hort-Science 22:1293--1295.
Hippensteel, T.E. 1992. Light stress induced by hayscented fern. M.Sc. Thesis, School of Forest Resources, The Pennsylvania State Univ., 87 p.
Holch, A.E. 1931. Development of roots and shoots of certain decidu-ous tree seedlings in different forest sites. Ecology 12:259--298. Horsley, S.B. 1977. Allelopathic inhibition of black cherry by fern,
grass, goldenrod, and aster. Can. J. For. Res. 7:205--216.
Horsley, S.B. 1981. Control of herbaceous weeds in Allegheny hard-wood forests with herbicides. Weed Sci. 29:655--662.
Horsley, S.B. 1984. Hayscented fern rhizome development in uncut and thinned Allegheny hardwood stands. Am. J. Bot. 71:80--81. Horsley, S.B. 1986. Evaluation of hayscented fern influence with
black cherry. Am. J. Bot. 73:668--669.
Horsley, S.B. 1988. How vegetation can influence regeneration. In
Proc. Guidelines for regenerating Appalachian Hardwood Stands. Eds. H.C. Smith, A.W. Perkins and W.E Kidd. SAF Publ., 88-03, West Virginia Univ. Books, pp 38--55.
Horsley S.B. 1993a. Mechanisms of interference between hay-scented fern and black cherry. Can. J. For. Res. 23:2059--2069.
Horsley, S.B. 1993b. Evaluation of allelopathic interference between hay-scented fern and black cherry. J. Chem. Ecol. 19:2737--2755. Horsley, S.B. and D.A. Marquis. 1983. Interference by weeds and deer
with Allegheny hardwood reproduction. Can. J. For. Res. 13:61--69. Johnson, I.R. and J.H.M. Thornley. 1987. A model of shoot:root
partitioning with optimal growth. Ann. Bot. 60:133--142. Joslin, J.D. and M.H. Wolfe. 1989. Aluminum effects on northern red
oak seedling growth in six forest soil horizons. Soil Sci. Soc. Am. J. 53:274--281.
Kelly, J.M. and J.D. Joslin. 1989. Mass and chemical composition of roots in two second-growth oak forests in eastern Tennessee. For. Ecol. Manage. 27:87--92.
Kelly, J.M., M. Schaedle, F.C. Thornton and J.D. Joslin. 1990. Sensi-tivity of tree seedlings to aluminum: II Red oak, sugar maple, and European beech. J. Environ. Qual. 19:172--179.
Kolb, T.E., T.W. Bowersox, L.H. McCormick and K.C. Steiner. 1989. Effects of shade and herbaceous vegetation on first-year germina-tion and growth of direct-seeded northern red oak, white ash, white pine, and yellow poplar. In Proc. 7th Central Hardwood For. Conf. Eds. G. Rink and C.A. Budelsky. USDA For. Serv. Gen. Tech. Rep. NC-132, pp 156--161.
Kolb, T.E. and K.C. Steiner. 1990. Growth and biomass partitioning response of northern red oak genotypes to shading and grass com-petition. For. Sci. 36:293--303.
Kolb, T.E., T.W. Bowersox and L.H. McCormick. 1990. Influences of light intensity on weed-induced stresses of tree seedlings. Can. J. For. Res. 20:503--507.
Lorimer, C.G. 1989. The oak regeneration problem: new evidence on causes and possible solutions. In Proc. 17th Ann. Symp. Hardwood Res. Council, Merrimac, WA, pp 23--40.
Lyford, W.H. 1980. Development of the root system of northern red oak (Quercus rubra L.) Harvard For. Pap. 21, MA, 14 p.
Lynch, J.A., J.W. Grimm and E.S. Corbett. 1991. Atmospheric deposi-tion: spatial and temporal variations in Pennsylvania----1990. ERRI, Penn. State Univ., Publ. No. ER9103, 79 p.
Lyon, J. 1995. The impacts of soils, herbaceous competition and soil liming on the growth and nutrition of northern red oak (Quercus rubra L.) seedlings. Ph.D. Thesis, Intercollege Program in Ecology, The Pennsylvania State Univ., 169 p.
Lyon, J. and W.E. Sharpe. 1995. Impacts of electric deer exclusion fencing and soils on plant species abundance, richness, and diver-sity following clearcutting in Pennsylvania. In Proc. 10th Central Hardwood Forest Conf. Eds. K.W. Gottschalk and S.L.C. Fosbroke. USDA For. Serv. Gen. Tech. Rep. NE-197, pp 47--59.
Maquire, D.A. and R.T.T. Forman. 1983. Herb cover effects on tree seedling patterns in a mature hemlock-hardwood forest. Ecology 64:393--401.
Marquis, D.A., R.L. Ernst and S.L. Stout. 1990. Prescribing silvicul-tural treatments in hardwood stands of the Alleghenies. USDA For. Serv. Gen Tech. Rep. NE-96, 32 p.
McClenahen, J.R. 1987. Effects of simulated throughfall pH and sulfate concentration on a deciduous forest soil. Water Air Soil Pollut. 35:319--333.
McConnaughay, K.D.M. and F.A. Bazzaz. 1992. The occupation and fragmentation of space: consequences of neighboring roots. Funct. Ecol. 6:704--710.
McWilliams, W.H., S.L. Stout, T.W. Bowersox and L.H. McCormick. 1994. Advance tree-seedling regeneration and herbaceous cover in Pennsylvania’s forests. Penn. For. 94:14--17.
Mitchell, R.J., G.S. Cox, R.K. Dixon, H.E. Garrett and I.L. Sander. 1984. Inoculation of three Quercus species with eleven isolates of ectomycorrhizal fungi. II. Foliar nutrient content and isolate effec-tiveness. For. Sci. 30:563--572.
Olson, D.F., Jr. 1974. Quercus L. Oak. In Seeds of Woody Plants in the United States. USDA Agric. Handbook 450:692--703.
Reicosky, D.C., R.J. Millington and D.B. Peters. 1972. Patterns of water uptake and root distribution of soybeans (Glycine max) in the presence of a water table. Agron. J. 64:292--297.
Richardson, S.D. 1953. A note on some differences in root hair formation between seedlings of sycamore and American oak. New Phytol. 52:80--82.
Rudolf, P.O. 1929. A study of the seedling root systems of certain forest trees. M.F. Thesis, Cornell Univ., 72 p.
Ruehle, J.L. 1980. Ectomycorrhizal colonization of container-grown northern red oak as affected by fertility USDA For. Serv. Res. Note SE-297, 7 p.
Sharpe, W.E., B.R. Swistock and D.R. DeWalle 1993. A greenhouse study of northern red oak seedlings growth on two forest soils at different stages of acidification. Water Air Soil Pollut. 66:121--133. Sokal, R.P. and F.J. Rohlf. 1981. Biometry. W.H. Freeman and
Com-pany, New York, 859 p.
Tennant, D. 1975. A test of a modified line intersect method of estimating root length. J. Ecol. 63:995--1001.
Toumey, J.W. 1929. Initial root habit in American trees and its bearing on regeneration. Proc. 4th Intl. Bot. Congr. 1:713--725.
Vogt, K.A., E.E. Moore, D.J. Vogt, M.J. Redlin and R.L. Edmonds. 1983. Conifer fine root and mycorrhizal biomass within the forest of Douglas-fir stands of different ages and site productivities. Can. J. For. Res. 13:429--437.
Walters, R.S. and L.R. Auchmoody. 1993. Factors limiting northern red oak reproduction in Pennsylvania. USDA For, Serv. GTR NC-161, pp 271--280.