Enhanced tolerance of transgenic
Brassica juncea
to choline
confirms successful expression of the bacterial
codA
gene
K.V.S.K. Prasad, P. Sharmila, P. Pardha Saradhi *
,1Plant Physiology and Biotechnology Laboratory,Department of Biosciences,Jamia Millia Islamia,New Delhi110025,India
Received 28 March 2000; received in revised form 13 July 2000; accepted 13 July 2000
Abstract
Brassica junceacv. Pusa Jaikisan was transformed with thecodAgene for choline oxidase fromArthrobacter globiformiswith an aim to introduce glycine betaine biosynthetic pathway, as it lacks any means to synthesize glycine betaine. Western blot analysis revealed the presence of choline oxidase in the protein extract from the codAtransgenic lines, demonstrating that the bacterialcodAgene had been successfully transcribed and translated in transgenic lines. Good activity of choline oxidase indicated its presence in fully functional form in the transformed lines. This was further confirmed by the presence of glycine betaine only in the transformed lines ofB.juncea. The shoots of both wild type and transformed lines were exposed to various concentrations of choline in order to evaluate if the introduction of thecodAgene in any way enhances the potential ofB.junceato tolerate high levels of choline. The growth (in terms of fresh weight and dry weight) of the shoots of transformed lines exposed to high levels of choline was significantly superior to those of wild type. Moreover, the loss in chlorophyll content and the activity of photosystem II in shoots of the transformed lines exposed to high concentration of choline were significantly lower than that observed in wild type. These results showed that shoots of B.junceatransformed with the codAgene, most probably had the potential to readily convert choline to glycine betaine. Therefore, choline tolerance can be used as an efficient marker for the identification of the lines transformed with thecodAgene. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Brassica juncea; Transformation; Choline oxidase; Glycine betaine; Choline toxicity
www.elsevier.com/locate/plantsci
1. Introduction
Glycine betaine, a quaternary ammonium com-pound, is amongst the most effective compatible solutes which accumulates in a wide variety of organisms viz. bacteria, cyanobacteria, algae, ani-mals and higher plants (e.g. members of Chenopo-diaceae and Poaceae) as an adaptive means to tolerate abiotic stresses [1,2]. Glycine betaine is known to protect living systems from salt/osmotic stress by (i) acting as an osmotic solute (in order to maintain cellular water content by regulating its osmotic/water potential); (ii) stabilizing functional units such as oxygen evolving photosystem II complex; (iii) protecting the structure of proteins
including ribulose 1,5-bisphosphate carboxylase/
oxygenase (Rubisco); (iv) maintaining membrane integrity; and (v) acting as a counteracting solute [3 – 9].
Although, a number of higher plants (especially certain halotolerant plants) have glycine betaine biosynthetic pathways, there are several important crop plants such as tomato, potato, rice and mus-tard which lack any means for the synthesis of this compatible solute [10,11]. Having realized the sig-nificance of glycine betaine in enhancing osmotic tolerance, several groups are involved in the intro-duction of biosynthetic pathways for synthesis of glycine betaine into plant species that lack the ability [2,11 – 13] to synthesize glycine betaine.
In angiosperms glycine betaine is synthesized from choline under the combined action of choline monooxygenase and betaine aldehyde dehydroge-* Corresponding author. Tel.: +91-11-6916275.
E-mail address:[email protected] (P. Pardha Saradhi).
1E-mail: [email protected].
nase [14,15]. In certain animals and bacteria (Es
-cherichia coli ) the synthesis of glycine betaine
from choline is mediated by choline dehydroge-nase and betaine aldehyde dehydrogedehydroge-nase. Both choline monooxygenase and choline dehydroge-nase convert choline to betaine aldehyde whereas betaine aldehyde dehydrogenase converts betaine aldehyde to glycine betaine [14,15]. However, in certain bacteria (such as Arthrobacter sp. and
Alcaligenes sp.) choline is converted to glycine
betaine in a single step by choline oxidase [16 – 18].
Choline is one of the important quaternary ammonium compounds that has been shown to play a role in regulating membrane composition (through synthesis of phosphotidyl choline) and fluidity [19 – 21]. Exogenous application of choline has been shown to (i) promote root in-duction and root growth [22]; (ii) increase growth [22,23]; (iii) increase the level of glycine betaine in tobacco transformed with the CMO gene [2]; and (iv) increase tolerance of certain plants to abiotic stresses [24 – 26]. However, ap-plication of choline in excess (i.e. in toxic levels) causes deleterious effects on cellular metabolism in plants. In general, high concentration of choline has been shown to inhibit the activities of some of the most vital enzymes such as Ru-bisco, glyceraldehyde-3-phosphate dehydrogenase, isocitrate dehydrogenase and malate dehydroge-nase that are associated with photosynthesis and respiration [27,28].
As choline is a precursor for synthesis of glycine betaine, we believe that the introduction of gene(s) that are associated with the synthesis of glycine betaine would enhance the potential of plants (which otherwise do not have any means to synthesize glycine betaine) to withstand toxic levels of choline. In this communication, we are reporting that codA transformed lines of B.
juncea have the potential to perform well, even
in the presence of choline at levels which are otherwise highly toxic to wild type.
2. Materials and methods
Agrobacterium tumefaciens (EHA 101) with a
binary vector designated as pGAH/codA
{consisting of transit peptide sequence of the small subunit of Rubisco from tobacco, the codA
gene from A. globiformis and nopaline synthase terminator ligated into HpaI site of pGAH (with genes for kanamycin and hygromycin resis-tance)} [13] was used for transforming B. juncea. Shoots selected on kanamycin and hygromycin containing medium were multiplied indepen-dently and tested through PCR and Southern analysis in order to ensure the insertion of the
codA gene and to find out number of copies that got integrated in genome of B. juncea cv. Pusa Jaikisan (data not shown). Two of the transgenic lines (viz. 1 and 3) that showed single insert of the codA gene were selected and selfed. The plants obtained by germinating the seeds (from these two transgenic lines) on medium with kanamycin and hygromycin were grown in greenhouse and the seeds obtained by selfing each individual plant were collected separately. The seed lot which showed 100% germination in the presence of kanamycin and hygromycin were considered to be obtained from homozygous plants. Seeds obtained from homozygous plants of transgenic lines (viz. 1 and 3) were used for the present studies.
2.1. SDS-PAGE and Western analysis
Leaf samples (200 – 300 mg fresh weight) were homogenized in a mortar and pestle with 40 mM Tris – HCl (pH 7.2), 5 mM EDTA and 10 mM b-mercaptoethanol. The homogenate was cen-trifuged at 18 000×g for 15 min at 4°C. The clear supernatant thus obtained was used for Western blot analysis. The protein content was quantified as described by Bradford [29].
Suitable aliquots equivalent to 20 mg of protein were subjected to electrophoresis on 10% polyacrylamide gel that contained 0.1% SDS [30]. For Western analysis the proteins were elec-trophoretically transferred on to a polyvinyl difl-uoride membrane (Immobilon™ – N; Millipore Corporation, Bedford, MA, USA). These mem-branes were blocked overnight with 3% bovine serum albumin (BSA). Subsequently, immunolog-ical detection was performed according to the protocol supplied with Vectastain ABC-PO (rab-bit IgG) kit (Vector laboratories, Burlingame, CA) using polyclonal antiserum that was raised in rabbit against pure choline oxidase from A.
globiformis (from Sigma Chemical Co., USA)
2.2. Determination of choline oxidase acti6ity
For measuring the choline oxidase activity shoots were homogenized in phosphate buffer 40 mM (pH 8.0) and the supernatant obtained after centrifuging the homogenate at 18 000×g for 15 min at 4°C was used for measuring the choline oxidase activity according to the protocol of Ikuta et al. [16].
2.3. Quantification of glycine betaine and choline
Levels of glycine betaine and choline in leaves of both wild type and transformed plants were deter-mined using the1H-NMR spectroscopy as per the
procedure of Wall et al. [32]. Leaf tissue (4 g fresh weight) was powdered in a mortar using liquid nitrogen. The powder was suspended in 25 ml of 1.0 N H2SO4 and incubated at 25°C for 2 h. Cell
debris was removed by centrifugation at 1000×g
for 10 min and the quaternary ammonium com-pounds (including choline) were recovered from the supernatant by periodide precipitation method [32]. The resultant periodide adducts were col-lected by centrifugation at 1000×g for 30 min and dissolved in 0.5 ml of CD3OD (E-Merck,
Germany) containing 0.5 mM 2-methyl-2-propanol (E-Merck, Germany) as an internal stan-dard. This solution was transferred to the NMR tube and1H-NMR spectrum was recorded at 25°C
with a NMR spectrometer (DRX 00 Bruker, Karl-sruhe, Germany) with a pulse time of 5 ms and an acquisition time of 4 s. Peak identities were confirmed with the help of authentic standards (Sigma Chemical Co., USA). Quantification of glycine betaine and choline was achieved by com-paring integrated peak intensities against standard curves. In order to further confirm that the peaks correspond to these quaternary ammonium com-pounds, spiking experiments were also performed through addition of glycine betaine as well as choline to the periodide adducts. 1H-NMR
re-sponse positions (d) for N-methyl protons of glycine betaine and choline from the plant extracts were compared with that of the standards.
2.4. Measurement of chlorophyll
Plant material (1 g fresh weight) was homoge-nized in a mortar and pestle using 5 ml of chilled 80% acetone. The homogenate was centrifuged at
10 000×g at 4°C for 10 min. The absorbance of the supernatant was measured at 646, 663 and 750 nm, respectively, and chlorophyll content was cal-culated as per the method of Arnon et al. [33].
2.5. Choline tolerance experiments
2.5.1. Effect on growth
To assess the tolerance to high levels of exoge-nously applied choline, shoots (measuring 3 cm long bearing a minimum of four leaves) of wild type and both transformed lines were transferred to half-strength MS medium [34] supplemented with choline chloride (filter sterilized) at concen-trations ranging from 0 to 30 mM. The cultures were incubated at 25°C under cool white fluores-cent tubes (Philips India Ltd.) having a light inten-sity of 120 (mmol m−2 s−1) with 16/8-h day/night
cycle for 20 days. Changes in fresh weight, dry weight and chlorophyll content of the shoots were measured.
2.5.2. Effect on photosynthesis
The leaves from shoots grown on different con-centrations of choline chloride for 20 days were used for isolation of thylakoid membranes. Freshly harvested leaves were dipped in ice cold isolation buffer (pH 7.8) containing 400 mM su-crose, 10 mM NaCl, and 20 mM Tricine and incubated in dark. After 30 min the leaves were taken out and homogenized in the pre-chilled mor-tar and pestle in ice-cold isolation buffer in dark. The homogenate was filtered through four layers of miracloth and the filtrate was centrifuged at 5000×gfor 10 min at 4°C. The pellet was washed and resuspended in a small volume of suspension buffer (pH 7.5) containing 100 mM sucrose, 10 mM NaCl, 2 mM MgCl2 and 20 mM HEPES.
Chlorophyll content of the thylakoid was esti-mated according to the method of Arnon et al. [33].
Photochemical activities of the isolated thy-lakoids were assayed polarographically with a Clark type oxygen electrode [35]. For the assay of Photosystem (PS) II activity the reaction mixture consisted of 500 mM p-benzoquinone along with the suspension buffer. The thylakoids equivalent to 20 mg chlorophyll was used for each assay. All the measurements were carried out at 2592°C using saturating white light (500 mmol m−2
Photosystem II activity was also measured as the ratio of variable to maximum fluorescence (Fv/Fm) by a fluorometer (PAM 2000, Walz,
Effel-trich, Germany) in the pulse amplitude modula-tion mode. The leaves (intact) from the shoots exposed to various treatments were dark adapted in order to ensure that all the components of photosystem II are in an oxidized state (i.e. photo-system II centers are fully open) for 30 min prior to the measurement of Fv/Fm. Original (Fo) and
maximal (Fm) fluorescence yields were measured
with weak modulated red light (B0.5 mmol m−2
s−1) with 0.8 s pulse of saturating light (\6.8
mmol m−2 s−1 PAR), using the data acquisition
software (DA 2000; Walz) attached to the instru-ment. The variable fluorescence yield (Fv) was
defined as Fm−Fo. The photosystem II activity
was measured as Fv/Fm ratio [36].
3. Results and discussion
Successful transformation of the B. juncea cv. Pusa Jaikisan (an important oil crop of India) with codA gene encoding for choline oxidase was achieved. The integration and the copy number of thecodA gene in the transformed lines were deter-mined through PCR and Southern analysis [37].
3.1. Expression of the codA gene
The expression of the integrated codA gene in both the transgenic lines selected for the present investigations was confirmed through immunoblot analysis. Western blot analysis with antiserum raised against pure choline oxidase, indicated the presence of a 60 kDa polypeptide, corresponding to the authentic choline oxidase from A. globi
-formis (Sigma Chemical Co.), in the soluble
frac-tion from the leaves of both the transgenic lines (Fig. 1). No immunoreactive polypeptide was ob-served in the soluble fraction from wild type plants (Fig. 1). Unlike that observed by Hayashi et al. [13] in Arabidopsis thaliana transformed with the
codA gene, no precursor choline oxidase (corre-sponding to a molecular weight 70 kDa) was noted in the transgenic B. juncea indicating that the codA gene is not only transcribed and trans-lated but also rapidly processed.
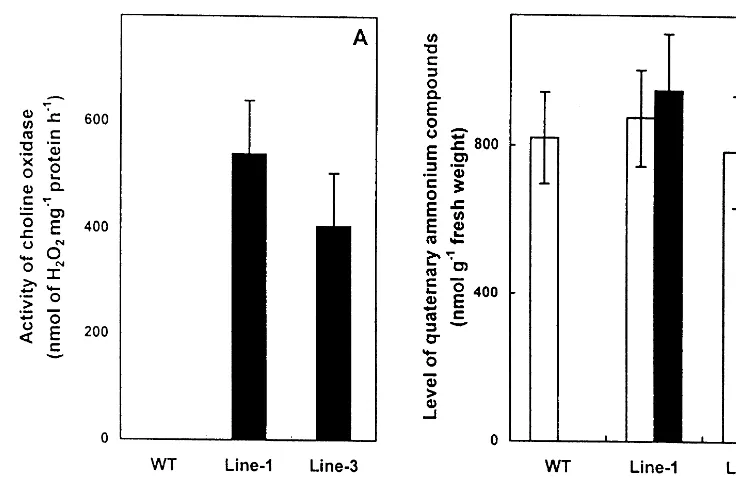
As evident from Fig. 2A, both the transgenic lines showed choline oxidase activity. No choline oxidase activity was detected in the wild type. However, a significant variation in the choline oxidase activity was observed between the two transgenic lines. Similar variation was observed in the intensities of the bands observed in im-munoblot analysis (Fig. 1). The variation in the choline oxidase activity could be due to differen-tial expression of the transgene depending on posi-tion of its integraposi-tion in the genome of B. juncea. Similar differential expression of several transge-nes was noted in other systems [38 – 41].
3.2. Le6els of glycine betaine and choline
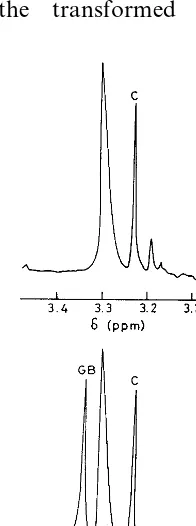
Introduction of the codA gene resulted in the synthesis of biologically active choline oxidase in the transformed plants (Fig. 2). Thus, it was im-portant to estimate the level of glycine betaine, as this provides a direct clue regarding the capacity of this enzyme to convert choline to glycine be-taine under in vivo conditions. 1H-NMR analysis
confirmed the absence of glycine betaine in the wild type genotype of B. juncea, clearly demon-strating that it lacks any means to synthesize glycine betaine (Figs. 2 and 3A). As expected, both the transformed lines showed the presence of glycine betaine (Figs. 2 and 3B) indicating that these transgenic lines had potential to synthesize this compatible solute. However, choline level in Fig. 1. Immunoblot analysis of choline oxidase in the soluble
Fig. 2. Choline oxidase activity (A) and the levels of glycine betaine and choline (B) in wild type (WT), transgenic line-1 (Line-1) and transgenic line-3 (Line-3) ofB.juncea. The values are the average of results of three independent experiments, each with a minimum of three replicates.
the shoots of transgenic lines did not differ signifi-cantly from that of wild type (Fig. 2B).
3.3. Tolerance to exogenous choline
Choline is ubiquitously present in higher organ-isms. However, its presence at higher levels is toxic to the plants [27,28,41]. As choline oxidase readily converts choline to glycine betaine, the trans-formed plants expressing the gene for choline ox-idase may be able to survive in the presence of choline even at concentrations that are otherwise toxic to the wild type plant. This hypothesis was tested with the shoots of both wild type and transgenic lines, as described in materials and methods (Fig. 4).
Fresh weight and dry weight of the shoots of wild genotype in the presence of choline at concen-trations up to 10 mM were marginally higher than those grown in the absence of choline (Fig. 5A and B). However, at concentrations above 15 mM a sharp decline in both fresh as well as dry weight of the shoots of wild type was observed. In con-trast, the fresh and dry weight of the shoots of both the transgenic lines were significantly higher in the medium with choline at concentration upto 15 mM than those grown in the absence of choline. Even in the presence of choline at
concen-trations as high as 30 mM, no significant alter-ation in the growth of shoots of transgenic line-1 was observed in comparison to those grown in the absence of choline (Fig. 5). Although, the trans-genic line-3 also showed significant enhancement in tolerance to choline, its shoots showed some signs of toxicity in the presence of 30 mM choline. As evident from Figs. 4 and 6A, the chlorophyll content in the shoots of wild type rapidly declined (over the control) with an increase in the concen-tration of choline in the medium. In contrast, the shoots of both the transformed lines showed about 20% increase in chlorophyll content in the pres-ence of 10 mM choline over those grown in its absence. However, a decline in chlorophyll content in the shoots of transformed lines was observed in medium supplemented with choline at concentra-tions above 20 mM (Fig. 6A). In contrast, to over 90% decline in the chlorophyll content of the shoots of wild genotype, only about 20% decline was observed in both the transformed lines in the presence of 30 mM choline. These results sug-gested that the transformation of B. juncea with
codA gene encoding choline oxidase for glycine betaine synthesis impart tolerance to choline toxicity.
activity (measured in terms of mmol O2 evolved
mg−1 Chl h−1 as well as F
v/Fm ratio) in the
shoots of wild type and transgenic genotypes grown on medium with various levels of choline. In general, irrespective of the concentration of choline in the medium, the PS II activity in shoots of both the transgenic lines was signifi-cantly higher than that of wild genotype.
In spite of choline being an important cellular metabolite, exogenous application of choline even at concentrations as low as 5 mM, caused a significant reduction in the PS II activity in the shoots of wild type. The extent of decline in PS II activity in shoots of wild type plants was more prominent with an increase in the concen-tration of choline in the medium. Unlike wild type, the PS II activity in shoots of both the transformed lines remained unaffected in the presence of choline upto 15 mM. However, the shoots of the transformed lines grown on
medium with choline at concentrations above 20 mM showed significantly lower PS II activity than control (0 mM grown shoots). But the de-cline in PS II activity in shoots of transformed lines exposed to higher levels of choline was sig-nificantly less than that in shoots of wild type (Fig. 6B and C). For instance, upon exposure to 30 mM choline the loss in PS II activity in thy-lakoids from shoots of wild type was over 96%, in contrast to about 25 and 35% in the trans-formed lines 1 and 3, respectively (Fig. 6B).
These results clearly demonstrated that the transformed genotypes unlike wild type have in-herent potential to detoxify the toxic levels of choline. This is apparently due to the presence of functional choline oxidase in the transformed lines that is able to readily convert toxic levels of choline into glycine betaine. Lilius et al. [42] reported that plants transformed with the bacte-rial betA gene (which encodes for choline dehy-drogenase) performed better than wild type in the presence of 10 mM choline, but higher con-centrations (20 and 30 mM) of choline caused a sharp decline in growth of even transformed plants. However, Nuccio et al. [2] studied the effect of exogenous choline on tobacco plants transformed with CMO gene encoding spinach choline monooxygenase only upto concentration of 5 mM. On the contrary, during the present investigations the transformed lines of B. juncea
could tolerate choline even upto 30 mM. These results clearly indicated that the transformed plants of B. juncea have enhanced capacity to tolerate toxic levels of choline as compared to their wild type counterpart. Further, transforma-tion of crop plants with the gene encoding for choline oxidase seems to be far superior in im-parting choline tolerance than those for choline dehydrogenase or choline monooxygenase.
In summary, the results presented in this com-munication clearly demonstrated the successful expression of the bacterial codA gene in two in-dependent transgenic lines of B. juncea as they showed the presence of functional choline ox-idase and glycine betaine. Moreover, the ability of transgenic lines to photosynthesize and grow well even in the presence of choline at levels that are highly toxic to wild type is probably because of their potential to readily convert choline to glycinbetaine.
Fig. 3. 1H-NMR spectra of quaternary ammonium com-pounds from leaves of wild type (A) and transgenic (B) plants ofB.juncea. Quaternary ammonium compounds were precip-itated as periodides, dissolved in CD3OD and subjected to 1H-NMR spectrometer. 1H-NMR response positions (d) for
Fig. 4. Shoots of wild type, transgenic line-1 and transgenic line-3 ofB.junceaexposed to 0, 5, 10, 20 and 30 mM choline for 10 days. Note chlorosis and leaf burning in the shoots of wild type in the presence of higher levels of choline.
Acknowledgements
We are grateful to Professor Norio Murata for providing Agrobacterium strain (EHA 101) bear-ing binary vector plasmid pGAH/codA. We
Fig. 5. Change in fresh weight (A), dry weight (B) of shoots of wild type and two transgenic lines (viz. 1 and 3) ofB.junceagrown on medium with various concentrations of choline. The initial values of fresh weight, dry weight for wild type and transformed lines were taken as 100%. The initial value of fresh weight of the shoot was 380910 mg and dry weight was 3893 mg in both wild type as well as transgenic lines. The results are the average of data from three independent experiments. () Wild type; () transgenic line-1; ( ) transgenic line -3.
Fig. 6. Change in Chlorophyll content (A) and PS II activity in the leaves of shoots of wild type and transgenic lines ofB.juncea
grown on medium with various concentrations of choline (B and C). PS II activity was measured in terms of oxygen evolution due to the photoreaction H2O pBQ by the thylakoids isolated from the leaves (B) andFv/Fmratio of undetached leaves (after 30 min of dark adaptation) (C). The initial values of oxygen evolution andFv/Fmratio were taken as 100%. The initial value of chlorophyll content was 2.1990.21 mg g−1fresh weight, oxygen evolution by the isolated thylakoids was 210.9918.6mmol O
2 evolved mg−1chl h−1and the initialF
v/Fmratio was 0.8390.015 in both wild type as well as transgenic lines. The results are the average of data obtained from three independent experiments. () Wild type; () transgenic line-1 and ( ) transgenic line-3.
is grateful to the University Grants Commission for providing fellowship. We are thankful to Dr Uma Sharma (National NMR Facility, All India Institute for Medical Sciences, New Delhi) for helping us in determining the levels of glycine betaine and choline through 1H-NMR
spectrophotometer.
References
[1] D. Rhodes, A.D. Hanson, Quaternary ammonium and tertiary sulfonium compounds in higher plants, Ann. Rev. Plant Physiol. Plant Mol. Biol. 44 (1993) 357 – 384.
[2] M.L. Nuccio, B.L. Russel, K.D. Nolte, B. Rathinasaba-pathi, D.A. Gage, A.D. Hanson, The endogenous choline supply limits glycine betaine synthesis in transgenic to-bacco expressing choline monooxygenase, Plant J. 16 (1998) 487 – 496.
[3] S.P. Robinson, G.P. Jones, Accumulation of glycine betaine in chloroplast provides osmotic adjustments dur-ing salt stress, Aust. J. Plant Physiol. 13 (1986) 659 – 668. [4] T. Bernard, M. Ayache, D. Le Rudulier, Restoration of growth and enzymic activities of Escherichia coli Lac-mutant by glycine betaine, C. R. Acad. Sci. III 307 (1988) 99 – 104.
[5] A. Incharoenskdi, T. Takabe, T. Akazawa, Effect of betaine on enzyme activity and subunit interaction of ribulose1-5-bisphosphate carboxylase/oxygenase from
[6] M.M. Santoro, Y. Liu, S.M. Khan, L.X. Hou, D.W. Bolen, Increased thermal stability of proteins in the presence of naturally occurring osmolytes, Biochemistry 31 (1992) 5278 – 5283.
[7] C.L. Winzor, D.J. Winzor, L.G. Paleg, G.P. Jones, B.P. Naidu, Rationalization of the effects of compatible so-lutes on protein stability in terms of thermodynamic non ideality, Arch. Biochem. Biophys. 296 (1992) 102 – 107. [8] Y. Zhao, D. Aspinall, L.G. Paleg, Protection of
mem-brane integrity inMedicago sati6aL. by glycine betaine
against the effects of freezing, J. Plant Physiol. 40 (1992) 541 – 543.
[9] G.C. Papageorgiou, N. Murata, The unusually strong stabilizing effects of glycine betaine on the structure and function in the oxygen-evolving photosystem-II com-plex, Photosynth. Res. 44 (1995) 243 – 252.
[10] K.F. McCue, A.D. Hanson, Drought and salt tolerance: towards understanding and application, Trends Biotech-nol. 8 (1990) 358 – 362.
[11] B. Rathinasabapathi, K.F. McCue, D.A. Gage, A.D. Hanson, Metabolic engineering of glycine betaine syn-thesis: plant betaine aldehyde dehydrogenase lacking transit peptide are targeted to tobacco chloroplast where they confer betaine aldehyde resistance, Planta 193 (1994) 155 – 162.
[12] K.-O. Holmstrom, B. Weilin, A. Mandal, I. Kristians-dottir, T.H. Teeri, T. Lamark, A.R. Strom, E.T. Palva, Production of the Escherichia coli betaine-aldehyde de-hydrogenase, an enzyme required for the synthesis of osmoprotectant glycine betaine in transgenic plants, Plant J. 6 (1994) 749 – 758.
[13] H. Hayashi, Alia, L. Mustardy, P. Deshnium, M. Ida, N. Murata, Transformation ofArabidopsis thalianawith
codAgene for choline oxidase; accumulation of glycine betaine and enhanced tolerance to salt and cold stress, Plant J. 12 (1997) 133 – 142.
[14] P. Weigel, E.A. Weretilnyk, A.D. Hanson, Betaine alde-hyde oxidation by spinach chloroplast, Plant Physiol. 82 (1986) 753 – 759.
[15] R. Brouquisse, P. Weigel, D. Rhodes, C.F. Yocum, A.D. Hanson, Evidence for a ferredoxin-dependent choline monooxygenase from spinach chloroplast stroma, Plant Physiol. 108 (1989) 581 – 588.
[16] S. Ikuta, S. Imamura, H. Misaki, Y. Horiuchi, Purifica-tion and characterizaPurifica-tion of choline oxidase from A.
globiformis, J. Biochem. 82 (1977) 1741 – 1749.
[17] K.L. Rozwadowski, G.G. Khachatourians, G. Selvaraj, Choline oxidase, a catabolic enzyme in A. globiformis pascens, facilitates adaptation to osmotic stress in Es
-cherichia coli, J. Bacteriol. 173 (1991) 472 – 478. [18] M. Ohta-Fukuyama, M. Yoshihiro, S. Emi, T. Yamano,
Identification and properties of prosthetic group of choline oxidase from Alcaligenes sp, J. Biochem. 88 (1988) 197 – 203.
[19] I. Horvath, L. Vigh, T. Farakas, The manipulation of polar head group composition of phospholipids in wheat Miranovskaja 808 affects frost tolerance, Planta 151 (1981a) 103 – 108.
[20] I. Horvath, L. Vigh, T. Farakas, L. Horvath, D. Dudits, Effect of choline chloride on fatty acid chain ordering in
membranes of wheat (Triticum aesti6um L.
Mira-novskaja 808), Planta. 153 (1981b) 476 – 480.
[21] M. Singer, Permeability of phosphotidylcholine and phosphotidylethanolamine bilayers, Chem. Phys. Lipids 29 (1981) 253 – 267.
[22] F.S. Che, F. Sato, S.B. Hycon, A. Isogai, Y. Yamada, A. Suzuki, Stimulation of photosynthesis and growth of photoautotrophically cultured plant cells by choline and its analogs, Plant Cell Rep. 12 (1993) 691 – 697. [23] L. Jian, H. Rotian, H. Yinfei, J. Lu, R.T. He, Y.F. He,
Promotive effects of choline chloride on the growth of tobacco plant, J. Guanxi Agric. Univ. 16 (1997) 110 – 114.
[24] Y. Jolivet, F. Larher, J. Hamelin, Osmoregulation in halophytic higher plants: the protective effect of glycine betaine against the heat destabilization of the mem-branes, Plant Sci. 25 (1982) 193 – 201.
[25] M.M. Mansour, E.J. Stadelmann, O.K.T.L. Stadel-mann, Salt acclimation of Triticum aesti6um by choline
chloride: plant growth mineral content, and cell permi-ability, Plant Physiol. Biochem. 31 (1993) 341 – 348. [26] M.G. Guye, L. Vigh, J.M. Wilson, Choline induced
chill-tolerance in mung bean (Vigna radiata L.Wilcz.), Plant Sci. 53 (1987) 223 – 228.
[27] D. Nash, L.G. Paleg, J.T. Wiskich, Effect of proline, betaine, and some other solutes on heat stability of mitochondrial enzymes, Aust. J. Plant Physiol. 9 (1982) 47 – 57.
[28] F.S. Feng, L.G. Ma, Effect of choline chloride on the photosynthesis in wheat, Acta Agriculturae Boreali Sinica 8 (1993) 54 – 57.
[29] M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye-binding, Anal. Biochem. 72 (1976) 248 – 254.
[30] U.K. Laemmli, Cleavage of structural proteins during the assembly of head bacteriophage T4, Nature 227 (1970) 680 – 685.
[31] J. Sambrook, E.J. Fritsch, T. Maniatis, Molecular cloning, in: A Laboratory Manual, second ed, Cold Spring Harbour Laboratory Press, New York, 1989. [32] J.S. Wall, D.D. Christianson, R.J. Dimler, F.R Senti,
Spectrophotometric determination of betaines and other quaternary nitrogen compounds as their periodides, Anal. Chem. 32 (1960) 870 – 874.
[33] D.I. Arnon, B.D. McSwain, H.Y. Tsujimoto, K. Wada, Photochemical activity and components of membrane preparation from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin, Biochim. Biophys. Acta 357 (1974) 231 – 245.
[34] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassay with tobacco tissue cultures, Phys-iol. Plant 15 (1962) 473 – 497.
[35] Alia, P. Pardha Saradhi, P. Mohanty, Effect of sodium chloride on primary photochemical activities in cotyle-donary leaves of B.juncea, Biochemie Und Physiologie der Pflanzen. 188 (1992) 1 – 12.
Caldwell (Eds.), Ecophysiology of Photosynthesis, Springer, Berlin, 1994, pp. 49 – 70.
[37] K.V.S.K. Prasad, Production and characterization of osmotic stress tolerant transformants of B. juncea (L.) Czern with bacterialcodAgene, Ph.D. thesis, Jamia Millia Islamia, New Delhi, 1999.
[38] J.D. Jones, P. Dunsmuir, J. Bedbrook, High level expression of introduced chimeric genes in regener-ated transformed plants, EMBO J. 4 (1985) 2411 – 2418.
[39] J. Stockhaus, P. Eckes, A. Blau, J. Schell, L. Willmitzer, Organ-specific and dosage-dependent expression of leaf/
stem specific from potato after tagging and transfer into
potato and tobacco plants, Nucleic Acids Res. 15 (1987) 3479 – 3491.
[40] F. Prols, P. Meyer, The methylation patterns of the chromosomal integration regions influence activity of transferred DNA in Petunia hybrida, Plant J. 2 (1992) 465 – 475.
[41] S.L.A. Hobbs, T.D. Warkentin, C.M.O. Delong, Trans-gene copy number can be positively or negatively associ-ated with transgene expression, Plant Mol. Biol. 21 (1993) 17 – 26.
[42] G. Lilius, N. Holmberg, L. Bulow, Enhanced NaCl stress tolerance in transgenic tobacco expressing bacterial choline dehydrogenase, Biotechnology 14 (1996) 177 – 180.