256 (2001) 185–198
www.elsevier.nl / locate / jembe
DMSP-consuming bacteria associated with the calanoid
copepod Acartia tonsa (Dana)
*
Kam W. Tang , Pieter T. Visscher, Hans G. Dam
Department of Marine Sciences, University of Connecticut, 1084 Shennecossett Road, Groton, CT 06340,
USA
Received 20 April 2000; received in revised form 4 October 2000; accepted 20 October 2000
Abstract
DMSP-consuming bacteria (DCB) were recovered from the body and fecal pellets of the copepod Acartia tonsa (Dana). The most probable number of DCB associated with starved A.
2 21
tonsa was 9.2310 cells copepod . The abundance of DCB recovered from the copepod body
4
increased to 1.6–2.8310 after the copepod fed on DMSP-containing alga. DCB abundance
4 21
associated with fecal pellets averaged 1.2310 cells pellet . In enrichment cultures, the DCB
21
grew with a doubling time of 1.1–2.9 days, and consumed DMSP at a rate of 4.5–7.5 fmol cell
21
day . The apparent DMSP-to-DMS conversion efficiency was 25–41% for DCB from copepod body, and 99% for DCB from fecal pellets. Our study demonstrated that copepods and their fecal pellets may harbour dense populations of DCB, and that the copepod–bacteria coupling represents a novel mechanism for DMSP consumption in the water column. 2001 Elsevier Science B.V. All rights reserved.
Keywords: Acartia tonsa; DMS; DMSP; Fecal pellets; Most probable number
1. Introduction
The climatologically active sulfur compound, dimethylsulfide (DMS), can be formed by biological and chemical cleavage of dimethylsulfoniopropionate (DMSP), a widely distributed organic sulfur compound in the ocean (Kettle et al., 1999). Although free-living, DMSP-cleaving bacteria are ubiquitous in the marine environment (Diaz et al., 1992; Visscher et al., 1992, 1994; Ledyard and Dacey, 1994), field measurements
˚
*Corresponding author. Present address: Danish Institute for Fisheries Research, Kavelergarden 6, Charlotten-lund DK-2920, Denmark. Tel.: 145-3-396-3421; fax:145-3-396-3434.
E-mail address: [email protected] (K.W. Tang).
usually reveal a weak correlation between DMS and particulate and dissolved DMSP concentrations (Kettle et al., 1999), implicating the existence of other biogeochemical sinks for DMSP and DMS.
DMSP may undergo various microbial degradation pathways without the production of DMS, such as demethylation and demethiolation (Taylor and Gilchrist, 1991; Visscher and Taylor, 1994). Growing evidence shows that non-DMS producing pathways are perhaps more common than DMSP cleavage in the marine environment (Kiene and Service, 1991; Kiene, 1992; Visscher et al., 1992; Ledyard and Dacey,
35
1996). Using radioactive S-DMSP, Kiene et al. (1999) showed that marine bacteria could incorporate a major portion of the DMSP-sulfur into amino acids and proteins. Bacteria that grow solely on DMSP as organic carbon source have also been isolated from various oceanic settings (Diaz et al., 1992; Visscher et al., 1992; Ledyard et al., 1993; Ledyard and Dacey, 1994; Visscher and Taylor, 1994). All these observations point to the importance of bacteria in DMSP dynamics, and the existence of many different microbial–biochemical pathways for DMSP.
DMSP in phytoplankton may also be channeled to micro- and meso-grazers (Wolfe et al., 1994; Kwint et al., 1996; Tang et al., 1999; Tang, 2000a), leading to an uncoupling between the production of DMSP and DMS. Copepods are the dominant mesograzers in the ocean, and they play various roles in determining the fate of oceanic DMSP. Copepods may release dissolved DMSP through grazing (Dacey and Wakeham, 1986) and osmoregulation (Tang et al., 2000a), retain DMSP in biomass (Kwint et al., 1996; Tang et al., 1999, 2000a), or repackage ingested DMSP into fecal material, which may be recycled or exported to deeper waters (Kwint et al., 1996).
represent highly concentrated pockets of DMSP in the water column, and may also act as ‘hot spots’ for microbial DMSP consumption.
In the present study, we quantified DMSP-consuming bacteria (DCB) associated with the body and fecal pellets of the copepod A. tonsa, using the most probable number (MPN) method. We also studied the growth of the DCB on DMSP in enrichment cultures. Our results show that the copepod body and fecal pellets contained dense populations of DCB, whose activity may have important implications for the fate of DMSP in the water column.
2. Material and methods
2.1. MPN determination
The calanoid copepod Acartia tonsa (Dana) was collected from eastern Long Island Sound (418189260N, 728039270W). Twenty individuals (CV to female) were placed into each of four 500-ml bottles: one ‘no food’ bottle contained only 0.2-mm filtered Instant Ocean artificial seawater (ASW) of a salinity 30‰; three ‘with food’ bottles contained
4 21
2310 ml of Tetraselmis impellucida (McLachlan et Parke) PLY429 prepared with the same ASW used in the ‘no food’ bottle. The collection of copepods and the maintenance of algal culture are described in detail by Tang (2000a). All bottles were
0
fastened to a spinning plankton wheel (2 rpm) and kept at 20 C in dark. After 24 h, five individuals from the ‘no food’ bottle were transferred to 1 ml of autoclaved ASW in a sterile vial and homogenized with a sterile pestle. The homogenate was then washed with autoclaved ASW into a sterile test tube (final volume52 ml), and was labeled as inoculum 4. Copepods from the three ‘with food’ bottles were pooled together. Five copepods were immediately removed, rinsed in autoclaved ASW and homogenized (inoculum 1). Another 25 copepods were rinsed and transferred to an alcohol-cleaned mini-trap with |5 ml of autoclaved ASW for collecting fecal pellets. The mini-trap was
a pellet collection device after the design by Small et al. (1979). The use of a large number of copepods in a small volume of water enabled the collection of enough fecal pellets in a short period of time. After 2 h, the copepods were removed from the mini-trap and five copepods were removed for homogenization (inoculum 2). Fecal pellets were allowed to settle inside the mini-trap; excess water was removed with the sterile pipet and all the fecal pellets were rinsed into a sterile test tube (final volume55 ml). The test tube was shaken vigorously on a vortex for several minutes to homogenize the fecal material (inoculum 3). Aliquots for determination of MPN of suspended DCB were taken from the upper portion of the homogenate. Even if the homogenization was not 100% efficient, this procedure would have likely resulted in an underestimation of the bacterial abundance due to particle settlement in the homogenate.
filtered seawater (pH 7.0) with 1 mM DMSP (University of Groningen, The
Nether-21 lands; .98% purity) as the organic carbon source. Nine dilution levels between 10
29
(most concentrated) and 10 (most diluted) were prepared. Each dilution level consisted of three tubes (5-ml sterile snap-cap test tube, Fisher Scientific), with a final volume of 3 ml in each tube. The tubes were loosely capped and maintained at |308C
in dark to prevent growth of phototrophs. The chosen pH and temperature are commonly used as optimal conditions for culturing marine mesophilic bacteria (Austin, 1992; Madigan et al., 1997). After 15 days, DMSP in the tubes was replenished by spiking with 50 mmol of DMSP (final concentration517 mM). After 7 weeks, all tubes were examined, and the presence or absence of bacteria was confirmed with acridine orange staining (Hobbie et al., 1977). MPNs of DCB in the original homogenates, with 95% confidence intervals, were determined according to DeMan (1975).
2.2. Growth kinetics of DMSP-consuming bacteria
Since we were interested in DMSP consumption rates by the bacterial community instead of individual strains, we prepared enrichment cultures with the MPN inocula of
21
the 10 dilution level. This dilution level conceivably represented the more diverse and active DCB recovered from the copepod bodies and fecal pellets in the MPN experiment. It should be emphasized that the enrichment cultures provided merely an estimate of the growth and DMSP-consumption characteristics of the DCB, since the DCB may behave differently when they are attached to the copepods and fecal pellets than when they are in suspensions (e.g. Bright and Fletcher, 1983; Diab and Shilo, 1988). One ml from each of the four MPN inocula was transferred to a sterile glass test tube with 40 ml 50% marine mineral medium (see above) and 5 mM DMSP (pH 7.0). The test tubes were loosely capped to allow gaseous exchange, and were maintained at 298C in the dark. After 1 week, enrichments were set up by transferring 5 ml from each test tube to a glass Erlenmeyer flask with 200 ml 50% marine mineral medium and 5 mM DMSP (pH 7.6). The four enrichments were labelled corresponding to the original four MPN inocula (see Section 2.1.). All enrichments were incubated aerobically in the dark at 298C. Aliquots from each enrichment were taken for optical density (OD) measurements at 580 nm (daily) and acridine orange (AO) cell counts (day 0, 1, 2 and 5). OD was converted to cell density based on the linear relationship between AO cell
2
counts and OD measurements (r 50.97, 0.97, 0.94 and 0.99 for enrichment 1, 2, 3 and 4, respectively).
The detection limit of the system was 0.4 pmol DMSP per ml injection, which would be equivalent to 2 nM DMSP per sample.
3. Results
All four types of homogenate from A. tonsa contained DMSP-consuming bacteria (DCB) (Table 1). Bacteria were absent in the highest dilution in the MPN experiment, which is a prerequisite for the MPN method to be applicable (DeMan, 1975). The MPN of DCB in the body of A. tonsa that had fed and A. tonsa that had voided guts, and in
4 21
fecal pellets ranged from 1.6 to 8310 DCB copepod , and were not significantly 2 different from each other (P.0.05). Starved A. tonsa from the field had |9310 DCB
21
copepod , significantly less than the other three groups (P,0.05). Cells in enrichment
8 21
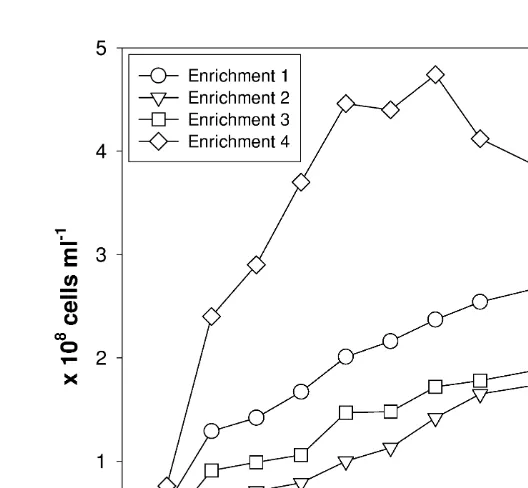
1 reached a plateau of |3310 ml after 12 days. The final cell densities in
8 21
enrichments 2 and 3 were similarly low, at |2310 ml at day 12. Cells in
8 21
enrichment 4 grew to a maximum of 4.7310 ml after 7 days, then decreased to
8 21
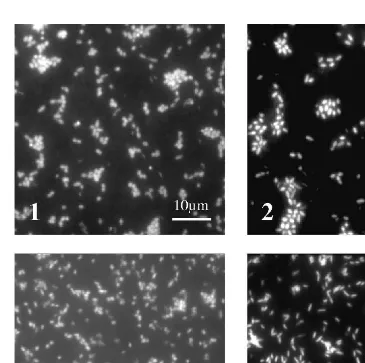
3310 ml at the end of the experiment (Fig. 1). We estimated the average doubling time (t ) in the enrichment cultures assuming an exponential growth between the initiald cell density and the maximum cell density. The doubling times for enrichments 1, 2 and 3 were similar, between 2.4 and 2.9 days (Table 2). Enrichment 4 had a shorter doubling time of 1.1 days. Microscopy revealed that the composition of bacteria was slightly different among the enrichments (Fig. 2). Enrichments 1, 2 and 3 were predominantly composed of thick rods and coccoids; Enrichment 4 was predominantly composed of thin rods.
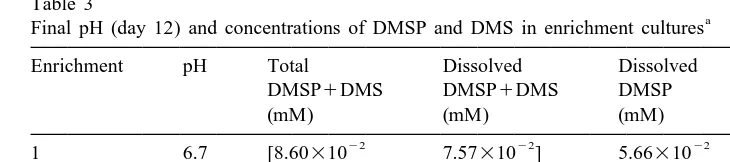
Almost all of the initial DMSP (5 mM) was consumed in enrichment 4 after 12 days, with ,0.3mM (| 0%) remained in the medium. Enrichments 1 and 3 contained ,0.1
mM final DMSP concentration after 12 days, equivalent to ,2% of the initial DMSP. 0.5 mM, or 10% of the initial DMSP remained in enrichment 2 after 12 days (Table 3). In enrichments 1, 2 and 3, total DMSP1DMS concentrations were not different from dissolved DMSP1DMS concentrations, but significantly higher than dissolved DMSP concentrations (one-way ANOVA, P,0.05). This indicates that accumulation of DMSP in bacteria was negligible, and some DMSP had been converted to DMS. In enrichment 4, total DMSP1DMS concentration was significantly higher than dissolved DMSP1
Table 1
Most probable number (MPN) of DMSP-consuming bacteria (DCB) from Acartia tonsa (CV to female) (* Normalized to number of copepods used in 2-h defecation)
21
Inoculum Homogenate type MPN of DCB copepod Mean 95% C.I.
4 4
1 Copepods after feeding 1.6310 0.8–8.4310
4 4
2 Copepods after defecation 2.8310 0.8–11.2310
5 5
*
3 Fecal pellets 0.8310 0.4–4.2310
3 3
Fig. 1. Growth curves for enrichment cultures. (1) Copepods after feeding; (2) copepods after defecation; (3) fecal pellets; (4) starved copepods.
DMS and dissolved DMS concentrations (one-way ANOVA, P,0.05), suggesting an accumulation of DMSP in bacteria, and a negligible net production of DMS.
4. Discussion
4.1. DMSP-consuming bacteria from Acartia tonsa
Bacterial colonization of copepod bodies and fecal pellets has been well documented
Table 2
a
Growth and DMSP consumption characteristics of enrichment cultures
Enrichment td E R MPN3R
21 21 21 21
(days) (%) (fmol cell day ) (pmol copepod day )
1 2.79 25 4.53 72.5
2 2.85 41 5.67 159
3 2.37 99 7.48 598*
4 1.11 0 6.65 6.12
a
Labels of enrichments correspond to inocula shown in Table 1. td is doubling time; E is apparent DMSP-to-DMS conversion efficiency from Eq. (1); R is DMSP consumption rate from Eq. (2). MPN3R is
Fig. 2. Acridine orange-stained bacteria in enrichment cultures. (1) Copepods after feeding; (2) copepods after defecation; (3) fecal pellets; (4) starved copepods. Scale bar same for all panels.
(Gowing and Silver, 1983; Nagasawa et al., 1985; Delille and Razouls, 1994; Hansen and Bech, 1996; Hansen et al., 1996). In the study by Kwint et al. (1996), DMSP was lost from the fecal pellets of Eurytemora affinis (calanoid copepod) without an increase in surrounding dissolved DMSP; thus, the authors suggested that the DMSP was consumed by bacteria within the pellets. In the present study, we demonstrated that the body and fecal pellets of the copepod A. tonsa contained bacteria that consumed DMSP. We did not differentiate between bacteria on the copepod body surface and those within the copepod’s gut. In the only other quantitative study of bacteria associated with A.
tonsa, Hansen and Bech (1996) estimated that the bacterial abundance on the copepod
5 21
body surface and gut totaled 2310 copepod (acridine orange cell count), and the
3 21
Table 3
a
Final pH (day 12) and concentrations of DMSP and DMS in enrichment cultures
Enrichment pH Total Dissolved Dissolved % initial
DMSP1DMS DMSP1DMS DMSP DMSP
(mM) (mM) (mM) consumed
Labels of enrichments correspond to inocula shown in Table 1. Each number represents the mean of two samples. For each enrichment culture, numbers in brackets are not statistically different (one-way ANOVA,
P.0.05). Initial DMSP in all enrichment cultures was 5 mM. % initial DMSP consumed was calculated as [52(Total DMSP1DMS)]3100%.
DMSP-consuming bacteria (DCB) associated with starved A. tonsa (inoculum 4) was
2 21
9.2310 copepod ; thus, based on Hansen and Bech (1996), we estimated that ,1% of the total bacteria, or |10% of the culturable bacteria associated with the copepod
body were able to grow on DMSP. Feeding the copepods with DMSP-containing alga significantly increased the abundance of DCB recovered from the copepod body by .10-fold (inocula 1 and 2).
There have been very few studies on the abundance of free-living DCB in marine systems. In the Caribbean Sea, Visscher et al. (1992) estimated that the abundance of
4 5 21
free-living DCB ranged from 2310 to 2310 cells ml . In Tokyo Bay, free-living
3 5 21
DCB abundance was estimated to be 10 –10 cells ml (Niki et al., 1997). Therefore, considering that the natural population density of Acartia tonsa in Long Island Sound is
21
|1 ind l (Tang et al., 2000b), the percentage of total DCB in the water column
associated with copepod bodies is likely negligible. However, since the body volume of
25
A. tonsa is |2.5310 ml, the copepod body is equivalent to a DCB population of
7 21
3.7310 cells ml , higher than the density of free-living DCB (Visscher et al., 1992;
5 6 21
Niki et al., 1997), and free-living bacteria in general (10 –10 ml ; Ducklow, 1999).
8 9
The density of DCB recovered from A. tonsa body increased to 6.4310 to 1.1310
21
cells ml after the copepod fed on DMSP-containing alga. The original algal strain used in the present study was not axenic, and therefore it is possible that some DCB were passed from the food medium to the copepods during feeding. However, other studies have shown that direct consumption of free-living bacteria by planktonic adult
¨
copepods is negligible (Boak and Goulder, 1983; Verity and Paffenhofer, 1996; Overmann et al., 1999). Therefore, it is more likely that only DCB that were attached to the algal cells would have been ingested by the copepods, and the DMSP-rich environments in the copepod bodies (and fecal pellets) favored the growth and establishment of DCB populations (e.g. Lawrence et al., 1993). We did not measure the fecal pellet production rate. If we assume a maximum fecal pellet production rate of 80
21 21 4
copepods defecated in sterile seawater, the fecal DCB should have originated from the ingested food, or the copepod body (e.g. gut flora) (Gowing and Silver, 1983). Based on
27
a pellet volume of 1.88310 ml for A. tonsa feeding on Tetraselmis sp. (Feinberg and 10 Dam, 1998), the estimated DCB density associated with a pellet would be 6310 cells
21
ml , several orders of magnitude higher than that of free-living DCB (Visscher et al., 1992; Niki et al., 1997).
4.2. DMSP consumption in enrichment cultures
Several aerobic microbial degradation pathways for DMSP have been found (Visscher and Taylor, 1994): cleavage, demethylation and demethiolation. Only the cleavage pathway will yield DMS. There is also evidence that some bacteria may accumulate DMSP intracellularly (Diaz et al., 1992; Wolfe, 1996). In enrichments 1, 2 and 3, dissolved DMSP1DMS concentrations in the filtrate was not significantly different from total DMSP1DMS concentrations; therefore, accumulation of DMSP by bacteria was negligible. Dissolved DMSP concentrations in the purged filtrate were significantly lower (Table 3), indicating that some of the DMSP had been converted to DMS. The apparent DMSP-to-DMS conversion efficiency (E ) in the media was estimated as:
DMSP in purged filtrate ]]]]]]]
S
12D
3100% (1)DMSP1DMS in filtrate
E was equal to 25 and 41% in enrichment 1 and 2, respectively (Table 3). The actual
conversion efficiencies should be higher, since some of the DMS would have escaped from the enrichments, and DMS might have been consumed by the bacteria (Kiene and Bates, 1990; Kiene, 1992; Visscher and Taylor, 1993). Nevertheless, our results indicate that DCB in enrichments 1 and 2 consumed part of the DMSP without DMS production, possibly through demethylation and demethiolation pathways (Taylor and Gilchrist, 1991; Visscher and Taylor, 1994). In enrichment 3, the apparent conversion efficiency was 99% (Table 3), indicating that most of the DMSP was metabolized through cleavage. Net DMS production was negligible in enrichment 4, and a statistically
24
significant amount of DMSP (1.5310 mM) was not in dissolved form (Table 3),
24
indicating that each cell accumulated 4.9310 fmol DMSP at the end of the experiment.
The DMSP consumption in the enrichments during growth from initial to maximum cell density can be described mathematically as:
ST T
t / td
E
dS5 2C R0E
2 dtS0 0
21
where S is initial DMSP concentration (fmol ml0 ) and S is DMSP concentration inT
21 the medium at maximum cell density, C0 is initial cell density (cells ml ), td is doubling time (days), T is time elapsed before reaching maximum cell density (days),
21 21
(S02S ) ln 2T
]]]]
R5 T / td (2)
C t0 d
s
2 21d
21 21
R for the enrichment cultures ranged from 4.5 to 7.5 fmol cell day (Table 2). Note that the consumption rate for enrichment 4 (starved copepods) was slightly overesti-mated since the cell density was in decline when the DMSP concentration in the medium was measured. However, as we will show later, DCB activity associated with fed copepods has more ecological significance in terms of DMSP turnover. Nevertheless, these consumption rates are 1 order of magnitude lower than that of the free-living DMSP-cleaving bacterium strain LFR from the Sargasso Sea (Ledyard and Dacey, 1994), and 2–3 orders of magnitude lower than that of marine sediment bacteria (Visscher et al., 1994) and a bacterium isolated from the Caribbean Sea (Diaz et al., 1992). Such a comparison suggests that DCB associated with copepod bodies and fecal pellets may have a different metabolism than their free-living counterpart.
4.3. Turnover of DMSP in copepod body and fecal pellets
In the present study, we showed that DCB were present on the body and fecal pellets of A. tonsa at densities that are orders of magnitude higher than that reported for free-living DCB (Visscher et al., 1992; Niki et al., 1997). Therefore, the presence of these dense populations of DCB, combined with the high concentrations of DMSP associated with the copepod body and fecal pellets, may represent ‘hot spots’ for DMSP consumption in the water column. We estimated the potential DMSP consumption rate mediated by this copepod–DCB coupling mechanism as MPN3R (Table 2). The
21 21
consumption rate was 6.1 pmol body day for starved A. tonsa (enrichment 4), and
21 21
increased to 73 and 160 pmol body day after the copepod fed on DMSP-containing food (enrichment 1 and 2). In another laboratory study, the body DMSP content of A.
21 4 21
tonsa reached 35–100 pmol copepod after feeding on high density (2310 ml ) of
21
T. impellucida, and 25–70 pmol copepod was lost within 12 h after the food was removed (Tang, 2000a). Those observations yield a DMSP disappearance rate of 50–140
21 21
pmol copepod day , comparable to the DCB consumption rates (enrichment 1 and 2) in the present study. Therefore, consumption by DCB associated with the A. tonsa body could explain the disappearance of DMSP from the copepod body during starvation (Tang, 2000a).
In the laboratory study by Tang (2000a), A. tonsa ingested 1.5–3.1 nmol DMSP
21 21 4 21
copepod day at a food concentration of 2310 cells ml of T. impellucida, and 88–99% of the ingested DMSP was not retained in the copepod body, presumably lost as fecal pellets. If we assumed that the ingestion rate was proportional to food
21 21 concentration, A. tonsa would have ingested 0.8–1.5 nmol DMSP copepod day in
21 the present study, and the defecation rate would be 0.7–1.5 nmol DMSP copepod
21
day . However, ,2 pmol DMSP in the fecal pellets would remain after 24 h (unpublished data); thus, the DMSP disappearance rate from fecal pellets would be
21 21
0.7–1.5 nmol copepod day . In the present study, DCB recovered from fecal pellets
If one assumes that the copepod feeds and defecates continuously, defecation by A.
21 21
tonsa in 24 h would result in a DMSP consumption rate of 7.2 nmol copepod day , which is higher than the estimated DMSP disappearance rate from fecal pellets. Thus, this analysis indicates that DCB consumption is at least as important as diffusive loss in removing DMSP from the fecal pellets, consistent with the suggestion by Kwint et al. (1996).
4.4. Copepod–bacteria coupling and DMSP dynamics in the water column
Through grazing activity, copepods concentrate seston from the surrounding water into microenvironments in their bodies and fecal pellets, where dense populations of bacteria may exploit the concentrated organic material. This mechanism would be particularly important in shunting phytoplankton DMSP to bacteria during the early phase of a phytoplankton bloom, when the direct release of dissolved DMSP from actively growing phytoplankton tends to be negligible (Keller et al., 1999). In eastern Long Island Sound where A. tonsa was collected for the present study, annual
21
chlorophyll and seston-DMSP concentrations averaged 1.8 mg l and 12 nM, respectively (Tang et al., 2000b). At this ambient chlorophyll level, A. tonsa typically
21 21
ingests 29 ng chl copepod day (Dam et al., 1994). Since the in situ abundance of A.
21
tonsa averages|1 copepod l (Tang et al., 2000b), 1.6% of the seston-chlorophyll, and
an equivalent of 0.2 nM seston-DMSP would pass through the A. tonsa population daily as a result of grazing activity. We use data from enrichment 1, 2 and 3 to estimate how the coupling between A. tonsa and DCB may affect DMSP dynamics in the water column in the presence of DMSP-containing food. The DMSP consumption rate of DCB
21 21
associated with A. tonsa body averaged 116 pmol copepod day , and the DMSP
21 21 consumption by DCB associated with daily defecation was 7.2 nmol copepod day . Thus, the DMSP consumption rate due to the coupling between DCB and A. tonsa in the
21
water column totaled 7.3 nM day , high enough to consume all seston-DMSP that passes through the copepods due to grazing.
4.5. Conclusion
In the present paper, we for the first time demonstrated and quantified the presence of DMSP-consuming bacteria (DCB) associated with the bodies and fecal pellets of A.
tonsa. Copepod bodies and fecal pellets harbour dense populations of DCB relative to
therefore investigate the possible exchange of DCB among the three compartments. In addition, while the body-DMSP of A. tonsa is turned over rapidly (Tang, 2000a), the body-DMSP of another copepod species, Temora longicornis, is relatively stable (Tang et al., 1999, 2000a). Thus, one may expect the DCB abundance and activity associated with T. longicornis to be different. Interspecific variability in the copepod–DCB coupling should be further studied.
Acknowledgements
This study is supported by NSF OCE-9714900 awarded to PTV, NSF OCE-9521907 (CAREER) awarded to HGD, and Sigma Xi GIAR awarded to KWT. We thank D. Rogers for assistance with microscopy, and C. Lamborg for discussion on mathematical analysis. Contribution No. 312 of the Marine Science and Technology Center of the University of Connecticut. [RW]
References
Austin, B. (Ed.), 1992. Marine microbiology, 2nd Edition. Cambridge University Press, Cambridge, pp. 24–27. Boak, A.C., Goulder, R., 1983. Bacterioplankton in the diet of the calanoid copepod Eurytemora sp. in the
Humber Estuary. Mar. Biol. 73, 139–149.
Bright, J.J., Fletcher, M., 1983. Amino acid assimilation and electron transport system activity in attached and free-living marine bacteria. Appl. Environ. Microbiol. 45, 818–825.
Butler, M., Dam, H.G., 1994. Production rates and characteristics of fecal pellets of the copepod Acartia tonsa under simulated phytoplankton bloom conditions: implications for vertical fluxes. Mar. Ecol. Prog. Ser. 114, 81–91.
Carman, K.R., 1994. Stimulation of marine free-living and epibiotic bacteria-activity by copepod excretions. FEMS Microb. Ecol. 14, 255–262.
Dacey, J.W.H., Wakeham, S.G., 1986. Oceanic dimethylsulfide: production during zooplankton grazing on phytoplankton. Science 233, 1314–1316.
Dam, H.G., Peterson, W.T., Bellantoni, D.C., 1994. Seasonal feeding and fecundity of the calanoid copepod
Acartia tonsa in Long Island Sound: is omnivory important to egg production? Hydrobiologia 292–293,
191–199.
Delille, D., Razouls, S., 1994. Community structures of heterotrophic bacteria of copepod fecal pellets. J. Plankton Res. 16, 603–615.
DeMan, J.C., 1975. The probability of most probable numbers. Eur. J. Appl. Microbiol. 1, 67–78. Diab, S., Shilo, M., 1988. Effect of adhesion to particles on the survival and activity of Nitrosomonas sp. and
Nitrobacter sp. Arch. Microbiol. 150, 377–393.
Diaz, M.R., Visscher, P.T., Taylor, B.F., 1992. Metabolism of dimethylsulfoniopropionate and glycine betaine by a marine bacterium. FEMS Microbiol. Lett. 96, 61–66.
Ducklow, H.W., 1999. The bacterial component of the oceanic euphotic zone. FEMS Microbiol. Ecol. 30, 1–10.
Feinberg, L.R., Dam, H.G., 1998. Effects of diet on dimensions, density and sinking rates of fecal pellets of the copepod Acartia tonsa. Mar. Ecol. Prog. Ser. 175, 87–96.
Gowing, M.M., Silver, M.W., 1983. Origins and microenvironments of bacteria mediating fecal pellet decomposition in the sea. Mar. Biol. 73, 7–16.
Hansen, B., Fotel, F.L., Jensen, N.J., Madsen, S.D., 1996. Bacteria associated with a marine planktonic copepod in culture. II. Degradation of fecal pellets produced on a diatom, a nanoflagellate or a dinoflagellate diet. J. Plankton Res. 18, 275–288.
Hines, M.E., Visscher, P.T., Devereux, R., 1997. Sulfur cycling. In: Hurst, C.J., Knudsen, G.R., McInerney, M.J., Stetzenbach, L.D., Walter, M.V. (Eds.), Manual of Environmental Microbiology. American Society of Microbiologists, Washington, DC, pp. 324–334.
Hobbie, J.E., Daley, R.J., Jasper, S., 1977. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33, 1225–1228.
Jacobsen, T.R., Azam, F., 1984. Role of bacteria in copepod fecal pellet decomposition: colonization, growth rates and mineralization. Bull. Mar. Sci. 35, 495–502.
Keller, M.D., Korjeff-Bellows, W., 1996. Physiological aspects of the production of dimethyl sulfoniopropion-ate (DMSP) by marine phytoplankton. In: Kiene, R.P., Visscher, P.T., Keller, M.D., Kirst, G.O. (Eds.), Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds. Plenum Press, New York, pp. 131–142.
Keller, M.D., Kiene, R.P., Matrai, P.A., Bellows, W.K., 1999. Production of glycine betaine and di-methylsulfoniopropionate in marine phytoplankton. I. Batch cultures. Mar. Biol. 135, 237–248. Kettle, A.J. et al., 1999. A global database of sea surface dimethylsulfide (DMS) measurements and a
procedure to predict sea surface DMS as a function of latitude, longitude, and month. Global Biogeochem. Cycles 13, 399–444.
Kiene, R.P., 1992. Dynamics of dimethyl sulfide and dimethylsulfoniopropionate in oceanic water samples. Mar. Chem. 37, 29–52.
Kiene, R.P., Bates, T.S., 1990. Biological removal of dimethyl sulfide from sea water. Nature 345, 702–705. Kiene, R.P., Service, S.K., 1991. Decomposition of dissolved DMSP and DMS in estuarine waters: dependence
on temperature and substrate concentration. Mar. Ecol. Prog. Ser. 76, 1–11.
Kiene, R.P., Linn, L.J., Gonzalez, J., Moran, M.A., Bruton, J.A., 1999. Dimethylsulfoniopropionate and methanethiol are important precursors of methionine and protein-sulfur in marine bacterioplankton. Appl. Environ. Microbiol. 65, 4549–4558.
Kwint, R.L.J., Irigoien, X., Kramer, K.J.M., 1996. Copepods and DMSP. In: Kiene, R.P., Visscher, P.T., Keller, M.D., Kirst, G.O. (Eds.), Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds. Plenum Press, New York, pp. 239–252.
Lawrence, S.G., Ahmad, A., Azam, F., 1993. Fate of particle-bound bacteria ingested by Calanus pacificus. Mar. Ecol. Prog. Ser. 97, 299–307.
Ledyard, K.M., Dacey, J.W.H., 1994. Dimethylsulfide production from dimethylsulfoniopropionate by a marine bacterium. Mar. Ecol. Prog. Ser. 110, 95–103.
Ledyard, K.M., Dacey, J.W.H., 1996. Microbial cycling of DMSP and DMS in coastal and oligotrophic seawater. Limnol. Oceanogr. 41, 33–40.
Ledyard, K.M., DeLong, E.F., Dacey, J.W.H., 1993. Characterization of a DMSP-degrading bacterial isolate from the Sargasso Sea. Arch. Microbiol. 160, 312–318.
Madigan, M.T., Martinko, J.M., Parker, J. (Eds.), 1997. Brock’s Biology of Microorganisms, 8th Edition. Prentice Hall, Englewood Cliffs, NJ, pp. 161–169.
Nagasawa, S., Simidu, U., Nemoto, T., 1985. Scanning electron microscopy investigation of bacterial colonization of the marine copepod Acartia clausi. Mar. Biol. 87, 61–66.
Niki, T., Kunugi, M., Kohata, K., Otsuki, A., 1997. Annual monitoring of DMS-producing bacteria in Tokyo Bay, Japan, in relation to DMSP. Mar. Ecol. Prog. Ser. 156, 17–24.
Overmann, J., Hall, K.J., Northcote, T.G., Beatty, J.T., 1999. Grazing of the copepod Diaptomus connexus on purple sulphur bacteria in a meromictic salt lake. Environ. Microbiol. 1, 213–221.
¨ ¨
Small, L.F., Fowler, S.W., Unlu, M.Y., 1979. Sinking rates of natural copepod fecal pellets. Mar. Biol. 51, 233–241.
Tang, K.W., 2000a. Dynamics of dimethylsulfoniopropionate (DMSP) in a migratory grazer: a laboratory simulation study. J. Exp. Mar. Biol. Ecol. 243, 283–293.
Tang, K.W., 2000b. Role of the estuarine copepod Acartia tonsa in DMSP dynamics. In: ASLO Aquatic Sciences Meeting. Copenhagen, Denmark, Abstract.
Tang, K.W., Fenn, T.D., Visscher, P.T., Dam, H.G., 2000a. Regulation of body dimethylsulfoniopropionate (DMSP) content by the copepod Temora longicornis: a test of four mechanisms. Mar. Biol. 136, 749–757. Tang, K.W., Rogers, D.R., Dam, H.G., Visscher, P.T., 2000b. Seasonal distribution of DMSP among seston, dissolved matter and zooplankton along a transect in the Long Island Sound estuary. Mar. Ecol. Prog. Ser. 206, 1–11.
Taylor, B.F., Gilchrist, D.C., 1991. New routes for the aerobic biodegradation of dimethylsulfoniopropionate. Appl. Environ. Microbiol. 57, 3581–3584.
Urban-Rich, J., 1999. Release of dissolved organic carbon from copepod fecal pellets in the Greenland Sea. J. Exp. Mar. Biol. Ecol. 232, 107–124.
¨
Verity, P.G., Paffenhofer, G.A., 1996. On assessment of prey ingestion by copepods. J. Plankton Res. 18, 1767–1779.
Visscher, P.T., Diaz, M.R., Taylor, B.F., 1992. Enumeration of bacteria which cleave or demethylate dimethylsulfoniopropionate in the Caribbean Sea. Mar. Ecol. Prog. Ser. 89, 293–296.
Visscher, P.T., Kiene, R.P., Taylor, B.F., 1994. Demethylation and cleavage of dimethyl sulfoniopropionate in marine intertidal sediments. FEMS Microbiol. Ecol. 14, 179–190.
Visscher, P.T., Taylor, B.F., 1993. A new mechanism for the aerobic catabolism of dimethyl sulfide. Appl. Environ. Microbiol. 59, 3784–3789.
Visscher, P.T., Taylor, B.F., 1994. Demethylation of dimethylsulfoniopropionate to 3-mercaptopropionate by an aerobic marine bacterium. Appl. Environ. Microbiol. 60, 4617–4619.
Wolfe, G.V., 1996. Accumulation of dissolved DMSP by marine bacteria and its degradation via bacterivory. In: Kiene, R.P., Visscher, P.T., Keller, M.D., Kirst, G.O. (Eds.), Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds. Plenum Press, New York, pp. 277–291.