256 (2001) 215–227
www.elsevier.nl / locate / jembe
1 1
Gill Na ,K -ATPase and osmoregulation in the estuarine
crab, Chasmagnathus granulata Dana, 1851 (Decapoda,
Grapsidae)
* Pedro Carvalho Castilho, Isabel Amaral Martins, Adalto Bianchini
˜
´ ˆ ´
Laboratorio de Zoofisiologia, Departamento de Ciencias Fisiologicas, Fundac¸ao Universidade Federal do
Rio Grande, Caixa Postal 474, CEP 96.201-900 Rio Grande, RS, Brazil
Received 5 June 2000; received in revised form 7 September 2000; accepted 26 October 2000
Abstract
1 1
Some kinetic properties of gill Na ,K -ATPase of the estuarine crab, Chasmagnathus
granulata, and its involvement in osmotic adaptation were analyzed. Results suggest the presence 1 1
of different Na ,K -ATPase isoforms in anterior and posterior gills. They have different affinities
1 1 21
for Na , but similar affinity values for K , Mg , ATP and similar enzymatic profiles as a function of temperature of the incubation medium. Ouabain concentrations which inhibit 50% of 1 enzyme activity were also similar in the two types of gills. Enzyme activity and affinity for Na
1 1
are higher in posterior gills than in anterior ones. Furthermore, affinities of Na ,K -ATPase of
1 1
posterior gills for Na and K were similar to or higher than those of gills or other structures involved in the osmoregulation in several euryaline decapod crustaceans. Acclimation to low
1 1
salinity was related to a significant increase in the maximum Na , K -ATPase activity, mainly in posterior gills. On the other hand, crab acclimation to high salinity induced a significant decrease in maximum enzyme activity, both in anterior and posterior gills. These results are in accordance to the osmoregulatory performance showed by C. granulata in diluted media, and point out the major role of posterior gills in the osmoregulation of this species. 2001 Elsevier Science B.V. All rights reserved.
1 1
Keywords: Chasmagnathus granulata; Crustacea; Gill; Hypoosmotic regulation; Na ,K -ATPase
1. Introduction
The estuarine crab, Chasmagnathus granulata, inhabits salt marshes distributed along
*Corresponding author. Tel.: 155-53-233-8655; fax: 155-53-233-8680.
E-mail address: [email protected] (A. Bianchini).
the coast of Southern Brazil, Uruguay and Argentina (Boschi, 1964). In this habitat, it is usually exposed to a broad range of environmental salinities (Castello, 1985). Therefore, ability to cope with salinity changes is essential for the establishment of a population in this habitat. Nery and Santos (1993), studying the ability of C. granulata to regulate carbohydrate metabolism during osmotic stress, reported that this crab tolerates long-term exposure to freshwater and hypersaline medium (40‰). In spite of a seasonal difference in osmoregulatory ability, C. granulata is a good hyper- and hypo-smoregulator, with an isosmotic point in external media of 30‰ salinity (Bromberg, 1992; Miranda, 1994). Also, it was demonstrated that hemolymph osmolality tends to be maintained at the same level after the hyposmotic stress, both in winter and summer (Bromberg et al., 1995).
Sodium and chloride are the major osmotic effectors in the hemolymph of
C.granulata and their concentrations are maintained out of electrochemical equilibrium, both in concentrated and diluted media (Bromberg et al., 1995), as do many other
´
osmoregulating crabs (Mantel and Farmer, 1983; Pequeux, 1995). In C.granulata, gills
1 2
are presumed to be the sites of active transport of Na and Cl , in both concentrated and diluted media. However, the mechanisms of hyper- and hypoosmotic regulation are not well understood.
1 1
The presence of Na ,K -ATPase activity in the gills of estuarine and freshwater decapod crustaceans has been widely reported. Many studies have pointed out a central
1
role of this enzyme in active Na uptake. Studies of the biochemical basis of ion uptake
1 1
have shown that Na ,K -ATPase is present at high specific activities in gills and antennal gland of hyperosmotically regulating species. A larger enzyme activity in salt-transporting gills (posterior pairs) than in respiratory ones (anterior pairs), as well as changes of this activity as a function of the acclimation salinity, have also been reported
´
(Pequeux, 1995).
The present study was undertaken to determine the major kinetic characteristics of the
1 1
Na ,K -ATPase present in anterior and posterior gills of C. granulata, and to verify a possible correlation between enzyme activity and hemolymph osmolality, during acclimation to diluted sea water or sea water.
2. Materials and methods
Adult male crabs in stage C or early D of the intermoult cycle (Drach and Tchernigovtzeff, 1967) were captured at the salt marshes of the Lagoa dos Patos estuary near Rio Grande / RS (Southern Brazil). They were immediately transferred to the laboratory, and maintained in tanks with aerated diluted sea water (2‰ salinity) or sea water (30‰ salinity) and acclimated for at least 30 days. Photoperiod and temperature were fixed at 12-h light / dark and 208C, respectively. Every 2 days during the acclimation period, crabs were fed ground beef.
ice-cold medium containing 0.25 M sucrose and 5 mM EDTA. Homogenization was performed with a glass-teflon Potter homogenizer and 10 0003g pellets were obtained
1 1
´
by differential centrifugation (Pequeux and Chapelle, 1982). Na ,K -ATPase activity was determined following the method previously described (Bianchini and Castilho, 1999). For each assay, 100ml aliquots of the 10 0003g pellet were added to 2.5 ml of
assay medium which contained, for the ‘standard assay’, the following final composition
21
in mmol l 577.0 NaCl; 20.0 KCl; 6.0 MgCl ; 3.0 ATP and 0.1 buffer Tris–HCl at pH2 7.6. It is important to note that the ionic composition of this medium ensured enzyme saturation (see Results). Protein concentration in 10 0003g pellets and P werei determined colorimetrically based on the methods described by Bradford (1976) and Fiske and Subbarow (1925), respectively. Enzyme specific activity was then expressed as mmol P released per mg protein per h.i
Each kinetic or inhibition study was performed on gills isolated from five crabs acclimated to 2‰ salinity. This acclimation salinity was selected considering the lower limit of salinity tolerance of the species (Miranda, 1994). The optimum time of reaction was determined performing assays during 15, 30, 45 or 60 min. In order to determine the effect of protein content in homogenates and temperature of incubation medium on the maximum enzyme activity, different 10 0003g pellets containing protein
concen-21
trations ranging from 0.044 to 0.992 mg ml and different incubation temperatures (10, 20, 30 and 408C) were assayed. The pH effect was tested by performing enzymatic assays at pH 3.6, 5.6, 7.6 and 9.6. The pH of the reaction media was adjusted with Trizma buffer (Sigma Co., St. Louis, MO). Data from experiments of incubation time, and of protein content in 10 0003g pellets, were subjected to regression analysis, while
those from temperature and pH of incubation media were subjected to two-way ANOVA followed by Newman–Keuls test.
1 1 21
The effect of different Na , K , Mg or ATP concentrations on maximum enzyme activity was analyzed by replacing them in the ‘standard assay’ medium by choline
1 1 21
chloride. Km values for Na , K , Mg , and ATP were estimated by means of non-linear regression analysis (one-site binding).
In order to study the enzyme inhibition by ouabain, the total ATPase activity was also determined. Comparing this activity with that obtained in the ‘standard assay’, the
1 1
maximum Na ,K -ATPase activity was calculated and these values were then consid-ered as 100% in the inhibition assays with ouabain. The final concentrations of ouabain
25 25 24 24 23 22 21
tested were: 10 , 3.3310 , 10 , 3.3310 , 10 , and 10 mol l . Estimation and comparison of IC50for ouabain were performed after data adjustment to a sigmoidal dose–response curve using the GraphPad Prism software (GraphPad Software, Inc.).
In order to analyze the osmoregulatory performance and gill enzyme activity during acclimation to diluted and sea water media, 30 crabs were abruptly transferred from 2 to 30‰ salinity (hyperosmotic shock) or from 30 to 2‰ salinity (hyposmotic shock). After 0, 1, 3, 7, 15 and 30 days, hemolymph from five crabs in each treatment was sampled with a 1-ml syringe at the arthrodial membrane at the base of the 4th or 5th pair of pereiopods. Hemolymph osmolality was then determined by freezing point depression using a semi-micro osmometer (Knauer, Berlin, Germany). After this, crabs were cooled, sacrificed, and their gills were dissected, separated and prepared as previously
1 1
Hemolymph osmolality and enzyme activity were subjected to two-way ANOVA followed by Newman–Keuls test.
In all statistical analysis, the significance level adopted was 95% (a 50.05).
3. Results
1 1
The maximum Na ,K -ATPase activity corresponded to 79.9% (3.59 mmol P mgi
21 21 21 21
protein h ) and 86.5% (4.92mmol P mg proteini h ) of the total ATPase activity in anterior and posterior gills, respectively.
1 1
Some kinetic characteristics of the gill Na ,K -ATPase for both anterior and posterior gills of crabs acclimated to 2‰ salinity are given in Figs. 1–4. Maximum enzyme activity was a linear function of incubation time (Fig. 1A), and protein content in the 10 0003g pellets (Fig. 1B), for both gill types. The optimal pH range for
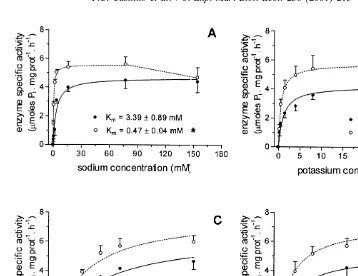
maximum enzyme activity in both anterior and posterior gills was 7.6 (Fig. 2A). In anterior gills, temperature influence on enzyme activity was similar in the range of 10–308C (Q1051.53). On the other hand, its effect on enzyme activity in posterior gills was greater between 10 and 208C (Q1053.46) than between 20 and 308C (Q1051.18). Further, a significant enzyme inhibition was observed at 408C in both gill types (Fig. 2B). Half-saturating concentrations (K ) were estimated for anterior and posterior gillsm
1 21 1 21 21
as follows: Na 53.39 and 0.47 mmol l ; K 51.14 and 0.61 mmol l ; Mg 53.74
21 21
and 2.56 mmol l ; ATP50.84 and 0.65 mmol l , respectively (Fig. 3). The K fori
21
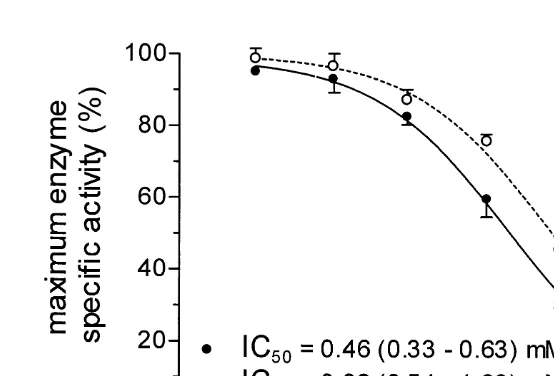
ouabain was estimated as 0.46 and 0.82 mmol l for anterior and posterior gills, respectively (Fig. 4).
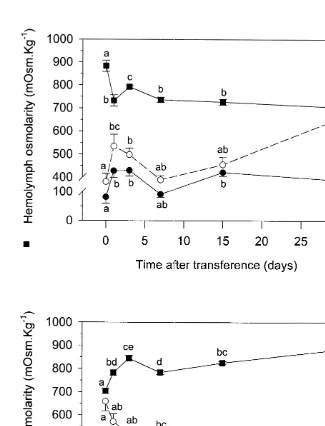
During the hyposmotic shock, hemolymph osmolality decreased rapidly, reaching a
1 1
new steady state after approximately 7 days. The Na ,K -ATPase activity of anterior and posterior gills showed an abrupt increase after 1 day of transference. Another increase in activity was registered, but only in posterior gills, after 15 days of transference (Fig. 5A). On the other hand, hemolymph osmolality increased during the first 3 days of the hyperosmotic shock and a new steady state was attained only 15 days after transference. In this case, enzyme adaptation was also relatively slow. Crabs transferred to high salinity exhibited a gradual decrease in enzyme activity with complete adaptation occurring only 15 days later (Fig. 5B).
4. Discussion and conclusions
Chasmagnathus granulata is an estuarine crab that maintains the osmotic and ionic
concentrations of the hemolymph more or less independent of the medium. Like other
1 1
crabs, it is thought to be primarily dependent on the gill Na ,K -ATPase activity for active salt transport, in order to maintain a hyperosmotic hemolymph in diluted media. In fact, as reported for other crustaceans species (D’Orazio and Holliday, 1985;
1 1
1 1
Fig. 1. Characteristics of Chasmagnathus granulata gill Na ,K -ATPase. (A) Enzyme-specific activity as a function of the time of incubation. Data are means (61 S.E.) of five crabs. (B) Enzyme-specific activity as a function of protein content in the gill 10 0003g pellet. (d) Anterior gills; (s) posterior gills.
1 1
gills. In this case, the enzyme activity dependent on Na and K corresponded to about
1 1
80% of the total ATPase activity. Further, the maximum specific Na ,K -ATPase activity values registered for both anterior and posterior gills were similar to those reported for gills or other structures involved in osmoregulation of several crustacean
´
1 1
Fig. 2. Characteristics of Chasmagnathus granulata gill Na ,K -ATPase. (A) The effect of the pH on the specific-enzyme activity. (B) The effect of the temperature of incubation on the specific-enzyme activity. (d) Anterior gills; (s) posterior gills. Data are means (61 S.E.) of five crabs. Different letters indicate significant different mean values for the same gill type (P,0.05).
1988; Morris and Edwards, 1995; Corotto and Holliday, 1996; Bianchini and Castilho, 1999; Lucu and Devescovi, 1999).
1 1 21
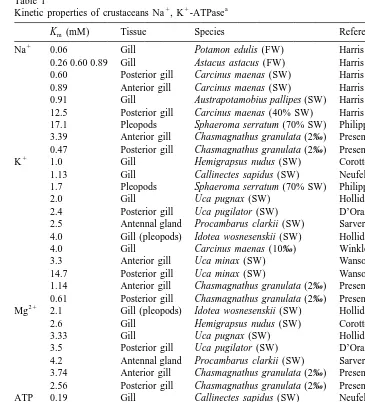
Km values for substrate (ATP) and ions (Na , K and Mg ) reported for gill
1 1
Na ,K -ATPase of C. granulata are generally similar to those for enzymes present in some structures involved in osmoregulation in other crustaceans (Table 1).
1 1 1
1 1 1
Fig. 3. Characteristics of Chasmagnathus granulata gill Na ,K -ATPase. (A) The effect of Na concentration
1 21
on the specific-enzyme activity. (B) The effect of K on the specific-enzyme activity. (C) The effect of Mg concentration on the specific-enzyme activity. (D) The effect of ATP concentration on the specific-enzyme activity. (d) Anterior gills; (s) posterior gills. Data are means (61 S.E.) of five crabs. * Indicates significant different Kmvalues (P,0.05).
of C. granulata was similar to those reported for crustaceans acclimated to highly diluted media, as for example Astacus astacus. Furthermore, this affinity was higher than that of anterior gills of C. granulata, or even of other species acclimated to concentrated media (Table 1). These results indicate that posterior gills in this crab are, as in other
´
species (Pequeux, 1995), likely the main site of active transport of NaCl in diluted
1
media. The enzyme affinity for Na in posterior gills is higher than that reported for posterior gills of Carcinus maenas acclimated to diluted media (40% sea water). These results are in accordance with the salinity tolerance showed by these crab species. C.
granulata tolerates, for long periods of time, salinities as low as 0‰ (Bromberg, 1992;
Miranda, 1994) while C. maenas tolerates dilutions of up to 1 / 3 sea water (Schoffeniels and Gilles, 1970; Gilles, 1974).
1
The Km values for K recorded in the posterior gills of C. granulata acclimated to 2‰, are the lowest reported in the literature (Table 1). Thus, the high affinity of
1 1 1 1
posterior gill Na ,K -ATPase of C. granulata for Na and K could be the major biochemical basis for the high tolerance to low salinity of this crab.
21 1 1
1 1
Fig. 4. Characteristics of Chasmagnathus granulata gill Na ,K -ATPase. The effect of ouabain concentration on the specific-enzyme activity. (d) Anterior gills; (s) posterior gills. Data are means (61 S.E.) of five crabs. Mean IC50values with 95% confidence limits in parentheses are provided for anterior and posterior gills in the lower left portion of the figure.
both anterior and posterior gills of C. granulata, is strongly influenced by the co-factor 21
and substrate concentrations in the reaction medium. Enzyme affinities for Mg and ATP in C. granulata are similar to those described for gill or other structures involved in osmoregulation in other crustacean species, except for Uca minax, which has a lower affinity for ATP (Table 1).
1 1
Concerning the effects of temperature and pH on maximum Na ,K -ATPase activity in gills of C. granulata, it was observed that temperature effect is more pronounced in posterior gills, and in the range of 10–208C. This range of temperature is in accordance with that frequently occurring during the year in the Lagoa dos Patos estuary (Castello, 1985) where C. granulata is a typical member of the community (Capitoli et al., 1978). On the other hand, no differences between anterior and posterior gills were registered in enzymatic responses as a function of changes in pH of the incubation media. In this
1 1
case, the Na ,K -ATPase of both gill types was significantly inhibited by acidic pH.
1
This inhibition could be associated with the effect of the H on the tertiary structure of the protein, leading to a reduction in the substrate affinity, as observed for other proteins (Stryer, 1988). Thus, an intracellular acidification could lead to an inhibition of the
1 1
Na ,K -ATPase in the gills of C. granulata, and cause an impairment of the ionic and osmotic balances, mainly in diluted media. In fact, it was reported that the lethal time for crabs exposed at pH 4.0 was higher in crabs acclimated to 20‰ than in those acclimated to 5‰ (Miranda and Bianchini, 1992).
The K values for ouabain observed in anterior and posterior gills of C. granulata arei similar to those reported for other crustaceans (Towle, 1984; Holliday, 1985; Corotto and Holliday, 1996; Lucu and Devescovi, 1999). They are also in accordance with the
1 1
1 1
Fig. 5. Time course of changes in gill Na ,K -ATPase enzyme-specific activity and hemolymph osmolality in
Chasmagnathus granulata during salinity acclimation. (A) Crabs were transferred from 30 to 2‰. (B) Crabs
were transferred from 2 to 30‰. Solid squares values representing hemolymph osmolality (j) are referred to 1 1
Table 1
1 1 a Kinetic properties of crustaceans Na , K -ATPase
Km (mM) Tissue Species Reference
1
Na 0.06 Gill Potamon edulis (FW) Harris and Bayliss (1988) 0.26 0.60 0.89 Gill Astacus astacus (FW) Harris and Bayliss (1988) 0.60 Posterior gill Carcinus maenas (SW) Harris and Bayliss (1988) 0.89 Anterior gill Carcinus maenas (SW) Harris and Bayliss (1988) 0.91 Gill Austrapotamobius pallipes (SW) Harris and Bayliss (1988) 12.5 Posterior gill Carcinus maenas (40% SW) Harris and Bayliss (1988) 17.1 Pleopods Sphaeroma serratum (70% SW) Philippot et al. (1972) 3.39 Anterior gill Chasmagnathus granulata (2‰) Present study 0.47 Posterior gill Chasmagnathus granulata (2‰) Present study 1
K 1.0 Gill Hemigrapsus nudus (SW) Corotto and Holliday (1996) 1.13 Gill Callinectes sapidus (SW) Neufeld et al. (1980) 1.7 Pleopods Sphaeroma serratum (70% SW) Philippot et al. (1972) 2.0 Gill Uca pugnax (SW) Holliday (1985)
2.4 Posterior gill Uca pugilator (SW) D’Orazio and Holliday (1985) 2.5 Antennal gland Procambarus clarkii (SW) Sarver et al. (1994) 4.0 Gill (pleopods) Idotea wosnesenskii (SW) Holliday (1988) 4.0 Gill Carcinus maenas (10‰) Winkler (1986) 3.3 Anterior gill Uca minax (SW) Wanson et al. (1984) 14.7 Posterior gill Uca minax (SW) Wanson et al. (1984) 1.14 Anterior gill Chasmagnathus granulata (2‰) Present study 0.61 Posterior gill Chasmagnathus granulata (2‰) Present study 21
Mg 2.1 Gill (pleopods) Idotea wosnesenskii (SW) Holliday (1988)
2.6 Gill Hemigrapsus nudus (SW) Corotto and Holliday (1996) 3.33 Gill Uca pugnax (SW) Holliday (1985)
3.5 Posterior gill Uca pugilator (SW) D’Orazio and Holliday (1985) 4.2 Antennal gland Procambarus clarkii (SW) Sarver et al. (1994) 3.74 Anterior gill Chasmagnathus granulata (2‰) Present study 2.56 Posterior gill Chasmagnathus granulata (2‰) Present study ATP 0.19 Gill Callinectes sapidus (SW) Neufeld et al. (1980)
0.48 Posterior gill Uca pugilator (SW) D’Orazio and Holliday (1985) 0.56 Gill Uca pugnax (SW) Holliday (1985)
0.60 Gill Hemigrapsus nudus (SW) Corotto and Holliday (1996) 0.9 Gill (pleopods) Idotea wosnesenskii (SW) Holliday (1988)
1.0 Antennal gland Procambarus clarkii (FW) Sarver et al. (1994) 3.49 Anterior gill Uca minax (SW) Wanson et al. (1984) 1.61 Posterior gill Uca minax (SW) Wanson et al. (1984) 0.84 Anterior gill Chasmagnathus granulata (2‰) Present study 0.65 Posterior gill Chasmagnathus granulata (2‰) Present study a
SW, sea water; FW, fresh water.
1 1
Na ,K -ATPase from insects and chelicerates have higher sensitivity to ouabain (Ki(10 mM) than those from crustaceans tissues (Ki(100mM). Values registered for C. granulata are also in accordance with those reported by Neufeld et al. (1980), which
1 1
demonstrated that the Na ,K -ATPase from vertebrates are much more sensitive to ouabain than those from brachyura crustaceans.
1
Based on the kinetic data presented in this study, mainly those related to Na affinity,
1 1
C. granulata acclimated to diluted media can be suggested. Further, one should also
1 1 1
consider the possible role of intracellular Na as a modulator of the Na ,K -ATPase
1
activity. Analyzing the enzyme kinetics as a function of Na concentration in the reaction medium, it can be observed that enzyme activity increased until it reached a
21 21
maximum at 16 mmol l . This activity remained unchanged until 77 mmol l , for both anterior and posterior gills. However, in posterior gills, an enzyme inhibition was
21
observed at 154 mmol l . These results are in accordance with those reported in the literature, which indicate that maximum enzyme activity occurs in the range of 75–100
21 1 21
mmol l Na , inhibition occurring at concentrations higher than 100 mmol l (Siebers ´
et al., 1982; Pequeux et al., 1984; Harris and Bayliss, 1988). Thus, high intracellular
1
concentration of Na could be a modulator of the enzyme activity in the posterior gills
1
of C. granulata. This hypothesis is supported by the fact that Na intracellular
21
concentrations in these gills are 35 and 130 mmol l for crabs acclimated to 0 and 20‰, situations under which C. granulata is strongly and weakly hyperosmotically regulating (Bianchini and Pereira, 1993).
1 1
Finally, a significant increase in the maximum Na ,K -ATPase activity was observed, mainly in posterior gills, after crab acclimation to low salinity. On the other hand, crab acclimation to high salinity induced a significant decrease in maximum enzyme activity, both in anterior and posterior gills. These results are in accordance with the osmoregulatory performance of C. granulata in diluted media (Nery and Santos, 1993; Bromberg et al., 1995), and point out the major role of posterior gills in the
´
osmoregulation of this species, as described for other crab species (Pequeux, 1995).
Acknowledgements
We thank Dr E.A. Santos for careful reading of the manuscript and DOLES Reagentes e Equipamentos Ltda for material support. A. Bianchini is a research fellow from Brazilian CNPq (Proc. 300536 / 90-9). [SS]
References
1 1 Bianchini, A., Castilho, C., 1999. Effects of zinc exposure on oxygen consumption and gill Na ,K -ATPase
of the estuarine crab Chasmagnathus granulata Dana, 1851 (Decapoda, Grapsidae). Bull. Environ. Contam. Toxicol. 62 (1), 63–69.
˜ ˆ ´
Bianchini, A., Pereira, Z.M., 1993. Volume celular e composic¸ao ionica dos fluıdos extra e intracelular de ˜
Chasmagnathus granulata Dana, 1851 (Crustacea — Decapoda) em diferentes salinidades. In: VIII Reuniao
˜ ´
Anual da Federac¸ao de Sociedades de Biologia Experimental, Caxambu-MG, Brazil, p. 265. ´
Boschi, E.E., 1964. Los crustaceos decapoda brachiura del litoral bonaerense. Bol. Inst. Biol. Mar del Plata 6, 1–76.
Bradford, M.M., 1976. A rapid and sensitive method for quantification of microgram quantities of proteins utilizing the principle of protein binding. Anal. Biochem. 72, 248–254.
ˆ ´
Bromberg, E., 1992. Dinamica osmo e ionorregulatoria de Chasmagnathus granulata Dana, 1851 (Crustacea, ˜
´
Decapoda, Grapsidae) submetido ao estresse hipo e hiperosmotico no inverno e no verao. Master thesis,
˜ ˜
Bromberg, E., Santos, E.A., Bianchini, A., 1995. Osmotic and ionic regulation in Chasmagnathus granulata Dana, 1851 (Decapoda, Grapsidae) during hyposmotic stress. Nauplius 3, 83–99.
˜ ˆ
Capitoli, R.R., Bemvenuti, C.E., Gianuca, N.M., 1978. Estudos da ecologia bentonica na regiao estuarial da
ˆ ˆ
Lagoa dos Patos. I. Comunidades Bentonicas. Atlantica 3, 5–22. ´
Castello, J.P., 1985. La ecologia de los consumidores del estuario de la Lagoa dos Patos, Brasil. In: ˜
Yanez-Arancibia, A. (Ed.), Fish Community Ecology in Estuaries and Coastal Lagoons: Towards an Ecosystem Integration. UNAM Press, Mexico City, p. 654.
Corotto, F.S., Holliday, C.W., 1996. Branchial Na, K-ATPase and osmoregulation in the purple shore crab,
Hemigrapsus nudus (Dana). Comp. Biochem. Physiol. A 113, 361–368.
D’Orazio, S.E., Holliday, C.W., 1985. Gill Na,K-ATPase and osmoregulation in the sand fiddler crab, Uca
pugilator. Physiol. Zool. 58, 364–373.
´ ´
Drach, N., Tchernigovtzeff, C., 1967. Sur la methode de determination des stades d’intermude et son
´ ´ ´
application generale aux crustaces. Vie Milieu 18, 595–607.
Fiske, C.H., Subbarow, Y., 1925. The colorimetric determination of phosphorus. J. Biol. Chem. 66, 375–400.
´ ´ ˆ
Gilles, R., 1974. Metabolisme des acides amines et controle du volume cellulaire. Arch. Int. Physiol. Biochem. 82, 423–589.
1 1
Harris, R.R., Bayliss, D., 1988. Gill (Na 1K )-ATPases in decapod crustaceans: distribution and characteris-1
tics in relation to Na regulation. Comp. Biochem. Physiol. A 90, 303–308.
Holliday, C.W., 1985. Salinity-induced changes in gill Na,K-ATPase in the mud fiddler crab, Uca pugilator. J. Exp. Zool. 233, 199–208.
Holliday, C.W., 1988. Branchial Na, K-ATPase and osmoregulation in the isopod, Idotea wosnesenskii. J. Exp. Biol. 136, 259–272.
1 1
Lucu, C., Devescovi, M., 1999. Osmoregulation and branchial Na ,K -ATPase in the lobster Homarus
gammarus acclimated to diluted seawater. J. Exp. Mar. Biol. Ecol. 234, 291–304.
Mantel, L.H., Farmer, L.L., 1983. Osmotic and ionic regulation. In: Vernberg, G.F.J., Vernberg, W.B. (Eds.). The Biology of Crustacea, Vol. 5. Academic Press, New York, pp. 53–161.
˜ ˆ
Miranda, R.B., 1994. Efeitos da temperatura e da salinidade sobre a tolerancia e a ionorregulac¸ao de ˜
Chasmagnathus granulata Dana, 1851 (Crustacea, Decapoda). Master thesis, Fundac¸ao Universidade do
Rio Grande, Rio Grande—RS, Brazil, 108 pp. ˆ
Miranda, R.B., Bianchini, A., 1992. Tolerancia de Chasmagnathus granulata Dana, 1851 (Crustacea —
´ ˆ ´
Grapsidae) ao estresse acido. In: XXI Encontro Anual de Ciencias Fisiologicas, Porto Alegre—RS, Brazil, p. 90.
1 1
Morris, S., Edwards, T., 1995. Control of osmoregulation via regulation of Na / K -ATPase activity in the amphibious purple shore crab Leptograpsus variegatus. Comp. Biochem. Physiol. C 112, 129–136. Nery, L.E.M., Santos, E.A., 1993. Carbohydrate metabolism during osmoregulation in Chasmagnathus
granulata Dana, 1851 (Crustacea — Decapoda). Comp. Biochem. Physiol. B 106, 747–753.
Neufeld, G.J., Holliday, C.W., Pritchard, J.B., 1980. Salinity adaptation of gill Na,K-ATPase in the blue crab
Callinectes sapidus. J. Exp. Zool. 211, 215–224.
´
Pequeux, A., 1995. Osmotic regulation in crustaceans. J. Crust. Biol. 15 (1), 1–60. 1 1
´
Pequeux, A., Chapelle, S., 1982. Na -K -ATPase activity and phospholipids in two euryhaline crabs related to changes in the environmental salinity. Mar. Biol. Lett. 3, 43–52.
´
Pequeux, A., Marchal, A., Wanson, S., Gilles, R., 1984. Kinetic characteristics and specific activity of gill 1 1
(Na 1K ) ATPase in the euryaline Chinese crab Eriocheir sinensis during salinity acclimation. Mar. Biol. Lett. 5, 35–45.
1 1
Philippot, J., Thuet, M., Thuet, P., 1972. Properties of the (Na -K )-ATPase from pleopods of Sphaeroma
serratum (Fabricius). Comp. Biochem. Physiol. B 41, 231–243.
Sarver, R.G., Flynn, M.A., Holliday, C.W., 1994. Renal Na,K-ATPase and osmoregulation in the crayfish
Procambarus clarkii. Comp. Biochem. Physiol. A 107, 349–356.
Schoffeniels, E., Gilles, R., 1970. Osmoregulation in aquatic arthropods. In: Florkin, M., Scheer, B.T. (Eds.). Chemical Zoology, Vol. V. Academic Press, New York, pp. 255–286.
Siebers, D., Leweck, K., Markus, H., Winkler, A., 1982. Sodium regulation in the shore crab Carcinus maenas as related to ambient salinity. Mar. Biol. 69, 37–43.
´
Stryer, L., 1988. Bioquımica, 3rd Edition. Guanabara Koogan, Rio de Janeiro.
Towle, D.W., 1984. Membrane bound ATPases in arthropod ion transporting tissues. Am. Zool. 24, 177–185.
1 1 1
´
Wanson, S., Pequeux, A., Roer, R., 1984. Na regulation and (Na 1K ) ATPase activity in the euryaline fiddler crab Uca minax (Leconte). Comp. Biochem. Physiol. A 79, 673–678.