www.elsevier.com/locate/ibmb
Cytochrome P450 CYP6L1 is specifically expressed in the
reproductive tissues of adult male German cockroaches, Blattella
germanica (L.)
Zhimou Wen
a, Jeffrey G. Scott
a,*aDepartment of Entomology, Comstock Hall, Cornell University, Ithaca, NY14853, USA
Received 7 February 2000; received in revised form 8 June 2000; accepted 12 June 2000
Abstract
A full-length cDNA encoding a new cytochrome P450, CYP6L1, was cloned from German cockroaches, Blattella germanica.
CYP6L1 has an open reading frame of 1509 nucleotides with a deduced protein of 503 amino acids and molecular mass of 57 Kd.
CYP6L1 is most similar to CYP6H1, a putative ecdysone 20-hydroxylase from Locusta migratoria. CYP6L1 mRNA was not detected in embryos nor nymphs, nor in adult females. CYP6L1 mRNA was detected only in the testes and accessory glands of male adult German cockroaches. Given that the testes and accessory glands are the most important components of the reproductive system in male insects, the expression of CYP6L1 mRNA exclusively in these tissues strongly suggests that CYP6L1 has a role in reproduction. Possible substrates for CYP6L1 are discussed. 2001 Elsevier Science Ltd. All rights reserved.
Keywords: Cytochrome P450 monooxygenase; Sex-specific expression; Male reproductive system; Blattella germanica; Insecta
1. Introduction
Cytochrome P450s (P450s) are an important enzy-matic system found in all organisms examined, including bacteria, fungi, plants and animals. Each animal has approximately 80 (Caenorhabditis elegans,
(Consortium, 1998)) to 90 (Drosophila melanogaster, (Adams et al., 2000)) P450s, and some can metabolize numerous substrates (Bernhardt, 1995; Guengerich, 1995; Nebert and Gonzalez, 1987). P450s are named CYP followed by a number, letter and number indicating the family, subfamily and individual isoform (Nelson et al., 1996). In this system, individual P450s with .40% amino acid sequence identity are usually grouped into the same family, and those with.55% identity grouped into the same subfamily. However, there are several exceptions. For example, CYP6A1 and CYP6B2 are
,40% identical, but are both grouped into family 6 because “sequences surrounding the conserved cysteine
* Corresponding author. Tel.:+1-607-255-7340; fax:+ 1-607-255-0939.
E-mail address: [email protected] (J.G. Scott).
0965-1748/01/$ - see front matter2001 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 1 1 6 - 8
residue make it clear that these two genes are evol-utionarily related” (Nelson et al., 1993). Given that this nomenclature system is based on the overall sequence similarity, and that a minor change in a single amino acid can alter substrate specificity (Lindberg and Negi-shi, 1989), substrate preference cannot be assumed for a certain P450 based on its name.
of P450s in herbivore–plant interactions, insecticide resistance, insect development and physiology.
P450s have been implicated in insect reproduction (Hodgson, 1985; Yu and Terriere, 1974). However, no individual insect P450 has yet been associated with reproduction. Herein, we report cloning of the full-length cDNA of the first sex-specific insect P450, CYP6L1, which is exclusively expressed in the reproductive sys-tem of male adult German cockroaches. This finding has important implications for understanding the roles of P450s in insect reproduction and may offer a potential target for the development of novel insect control agents.
2. Materials and methods
2.1. Insects
Two strains of German cockroaches were used: Bay-gon-R (Siegfried and Scott, 1991) and CSMA (Scott and Matsumura, 1981). Cockroaches were reared as described previously (Siegfried and Scott, 1991).
2.2. Dissection
To examine the expression of CYP6L1 in different body regions of male and female adult cockroaches the abdomens were separated from the remainder of the bod-ies on dry ice using forceps. The expression of CYP6L1 in the abdomens of adult male cockroaches was also examined. For this, cockroaches were anesthetized on ice and their wings removed. Then cockroaches were pinned in a Sylgard dish, opened dorsal-ventrally and the testes and/or accessory glands (Cornwell, 1968; Snodgrass, 1937) were quickly removed under a binocu-lar dissection scope at 24°C. The heads and thoraces were then cut off and the dissected abdomens put on dry ice for subsequent RNA isolation.
2.3. RNA isolation
Total RNA was isolated using 5 M guanidine thio-cyanate solution based on the method of Chirgwin et al. (Chirgwin et al., 1979). Messenger RNA was isolated from total RNA using Oligotex suspension (QIAGEN) or directly from tissues using a QuickPrep mRNA puri-fication kit (Pharmacia) as described by the manufac-turers.
2.4. cDNA synthesis
Superscript II (GIBCO/BRL) was used to synthesize the first strand cDNA following the manufacturer’s instructions. For the first strand cDNA synthesis, C3PT
(Danielson and Fogleman, 1997) (Table 1) was used as primer and |500 ng mRNA from abdomens of male adult Baygon-R strain of German cockroaches as tem-plate. After RNAase H (2 units) treatment at 37°C for 30 min, the first strand cDNA was isolated using a QIA-quick PCR purification kit (QIAGEN) to remove the pri-mers and short cDNA (eluted with 100 µl H2O). The purified cDNA was used as a template for 39 and 59
RACEs (Frohman et al., 1988) as described below.
2.5. 39RACE
The first strand cDNA (5µl) was used as a template. A degenerate primer based on the P450 heme signature motif GPRNCIG (Danielson et al., 1999) and a C3 primer (Danielson and Fogleman, 1997) (Table 1) were used to amplify putative 39 cDNA sequences of P450 genes by PCR. The PCR reaction (100 µl) was heated to 95°C for 3 min, followed by 35 cycles of amplifi-cation (95°C, 50°C and 72°C sequentially each for 30 s) and a final extension at 72°C for 10 min. After 1.5% agarose gel fractionation of the PCR products, a region corresponding to about 300–700 bp based on DNA size markers (100 bp ladder, GIBCO/BRL) was cut and the DNA purified using a QIAquick gel extraction kit (QIAGEN). The purified PCR products were cloned into pCR2.1 vector (Invitrogen) following the manufac-turer’s instructions. Plasmids were isolated from positive clones (white colonies) and the inserts sequenced by the DNA Sequencing Facility at Cornell University. Putative P450s were identified based on BLAST analysis (Altschul et al., 1997) using the deduced amino acid sequences. The inserts of six clones (MCH1, MCH6, MCH11, MCH45, MCH46 and MCH75) had identical sequences with a length of 371 bp excluding poly (A). The gene these sequences represented was named MCHA.
2.6. 59RACE
To obtain the 59sequence of MCHA, the purified first strand cDNA was tailed with dCTP by terminal deoxyn-ucleotidyl transferase (GIBCO/BRL) following the manufacturer’s instructions with the following modifi-cation. Instead of using the manufacturer’s 5× reaction buffer, we used a 5× tailing buffer (500 mM sodium cacodylate, 1 mM β-mercaptoethanol, 20 mM magnes-ium chloride, pH 7.2) as suggested by P. Danielson (University of Denver, Colorado). The tailed cDNA was purified using a PCR purification kit as described above (eluted with 100µl H2O) and 5µl was used as template for 59 RACE.
An MCHA specific antisense primer MCHAA1 (Table 1 and Fig. 1) was designed based on the 39
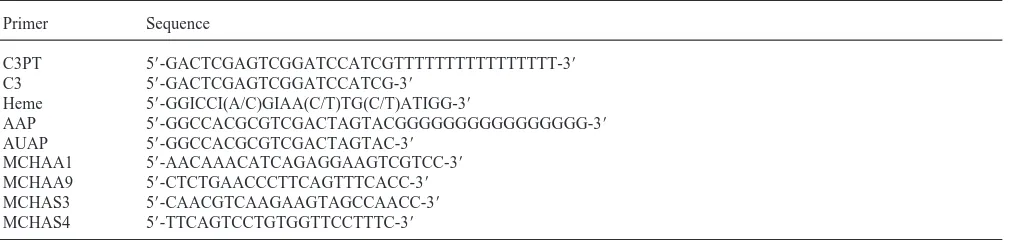
Table 1
Primers used for cloning of CYP6L1 cDNA
Primer Sequence
C3PT 59-GACTCGAGTCGGATCCATCGTTTTTTTTTTTTTTTT-39
C3 59-GACTCGAGTCGGATCCATCG-39
Heme 59-GGICCI(A/C)GIAA(C/T)TG(C/T)ATIGG-39
AAP 59-GGCCACGCGTCGACTAGTACGGGGGGGGGGGGGGGG-39
AUAP 59-GGCCACGCGTCGACTAGTAC-39
MCHAA1 59-AACAAACATCAGAGGAAGTCGTCC-39
MCHAA9 59-CTCTGAACCCTTCAGTTTCACC-39
MCHAS3 59-CAACGTCAAGAAGTAGCCAACC-39
MCHAS4 59-TTCAGTCCTGTGGTTCCTTTC-39
(100 µl reaction) used MCHAA1/AAP (GIBCO, Table 1) as the primer set and dCTP tailed first strand cDNA (see above) as template. The conditions for the first round PCR were 95°C for 3 min followed by 32 cycles in the order of 95°C for 1.5 min, 50°C for 1.5 min and 72°C for 4 min. The PCR reaction had a final extension for 15 min at 72°C. After electrophoresis (1.5% agarose gel) of the first round PCR products, a region corre-sponding to 1–2 kb was cut and the PCR products pur-ified (eluted with 100 µl H2O). The purified first round PCR products (5 µl) then served as templates for the second round PCR using MCHAA1/AUAP (GIBCO, Table 1) as the primer set. Products of the second round PCR (conditions were the same with that of the first round PCR, except the annealing temperature was raised to 55°C and cycles increased to 35) corresponding to a region of 1–2 kb on agarose gel was again purified and cloned into pCR 2.1 vector. Positive clones were screened by colony PCR (Gussow and Clackson, 1989) using single putatively positive colonies (white colonies) as templates and heme primer/MCHAA1 as the primer set. The insert lengths of those positive clones were further checked by colony PCR using MCHAA1/AUAP as the primer set. Plasmids were isolated from two clones (A11 and A180) with the longest inserts and the inserts were sequenced. The newly obtained sequence information from A11 and A180 was used to design pri-mers for another round of 59 RACE. After two rounds of 59 RACE, the 59 sequence beyond the translational start codon was obtained. All the adjacent sequences obtained by 39and 59RACEs had at least 150 bp overlap with each other. The putative full-length MCHA sequence MCHAMER was obtained by integrating over-lapping sequences from the 39 RACEs and 59 RACEs.
2.7. Cloning of full-length cDNA
Two sense primers upstream of the start codon (MCHAS3 and MCHAS4, Table 1 and Fig. 1) and one antisense primer downstream of the stop codon (MCHAA9, Table 1 and Fig. 1) were designed based on the sequence of MCHAMER. The first round PCR
products using first strand cDNA as template and MCHAS4/MCHAA9 as primer set were used as tem-plates to perform the second round PCR with MCHAS3/MCHAA9 as primer set. The conditions for the first round PCR were 95°C to denature for 3 min followed by 32 cycles in the order of 95°C for 1 min, 50°C for 1 min and 72°C for 2 min. The PCR reaction ended with a final extension for 15 min at 72°C. The conditions for the second round PCR were the same as the first round PCR, except that the cycles were 35 instead of 32. A 1.6 kb band on agarose gel was purified and cloned into pCR2.1 vector. A positive clone (FA13) was identified by colony PCR and its insert entirely sequenced.
2.8. Northern blots
Northern blot analysis of CYP6L1 mRNA expression was performed as described by Sambrook et al. (Sambrook et al., 1989). Briefly, 10µg of total RNA was fractionated on 1% denaturing formaldehyde agarose gel containing ethidium bromide. After washing in distilled water for about 3–4 h with several changes, the RNA was transferred to a GeneScreen Plus hybridization transfer membrane (NENLife Science Products, Inc.). Equal loading was monitored by comparing the density of the 18S ribosomal RNA (rRNA) band (Savonet et al., 1997; Spiess and Ivell, 1998) on the agarose gel before transfer and/or on the membrane after transfer under UV. A 1.6 kb CYP6L1 fragment was amplified by PCR (using plasmids isolated from FA13 as templates and MCHAS3/MCHAA9 as the primer set), labeled with [α -32P] dCTP using the RadPrame labeling system
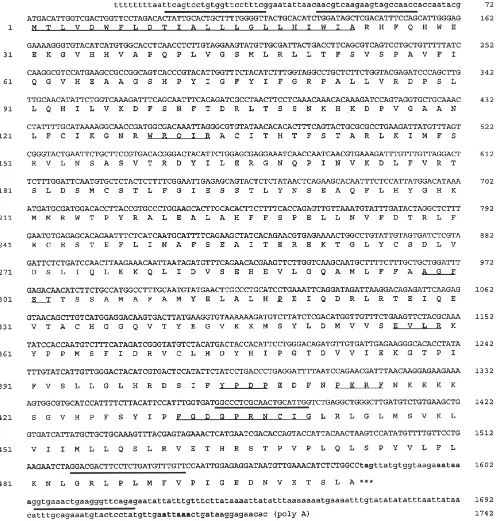
Fig. 1. CYP6L1 full-length cDNA and deduced amino acid sequence. Amino acids (AA) are numbered on the left and nucleotides (NT), on the right. Primers (Table 1) are thickly underlined and conserved regions thinly underlined. Putative polyadenylation signals are indicated by bold font.
2.9. Southern blots
Genomic DNA was isolated from the abdomens of male adult German cockroaches of Baygon-R strain using standard methods (Sambrook et al., 1989). South-ern blots were performed by standard methods (Sambrook et al., 1989) with some modifications. Four
agarose gel containing ethidium bromide. After electro-phoresis, the gel was placed in 0.25 M HCl with gentle shaking until 10 min after the dyes had changed color. After being rinsed in H2O, the gel was denatured in a solution containing 1.5 M NaCl and 0.5 M NaOH with gentle shaking for 30 min. The gel was then rinsed with H2O and neutralized in a solution containing 1.5 M NaCl, 0.5 M Tris–HCl (pH 7.2) and 0.001 M EDTA. After rinsing in H2O, the DNA was transferred to a GeneScreen Plusmembrane (NENLife Science Pro-ducts, Inc.). Probe preparation, membrane hybridization and film exposure were performed exactly as described in Section 2.8. Southern analyses were repeated three times.
3. Results
3.1. cDNA cloning and characterization
The full-length cDNA sequence (accession #: AF227531) of MCHA is shown in Fig. 1. MCHA was named CYP6L1 by the P450 Nomenclature Committee.
CYP6L1 has an open reading frame of 1509 nucleotides
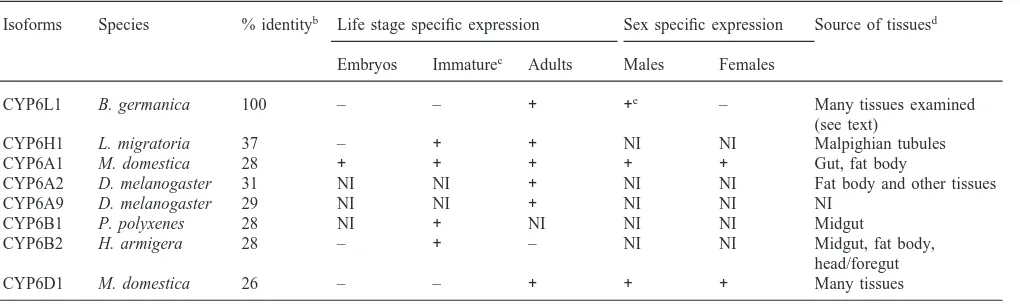
with a deduced protein of 503 amino acids and a molecu-lar mass of 57 kDa. A BLAST search (Altschul et al., 1997) with the deduced amino acid sequence shows that CYP6L1 is most similar to the members of P450 family 6 from insects. A comparison of CYP6L1 with some members of family 6 is shown in Table 2. CYP6L1 has the highest percent amino acid identity (37%) with CYP6H1, a putative ecdysone 20-hydroxylase from the grasshopper, Locusta migratoria (Winter et al., 1999).
CYP6L1 is a typical microsomal P450 and its deduced
Table 2
Comparison of CYP6L1 from B. germanica with other related P450sa
Isoforms Species % identityb Life stage specific expression Sex specific expression Source of tissuesd
Embryos Immaturec Adults Males Females
CYP6L1 B. germanica 100 – – + +e – Many tissues examined
(see text)
CYP6H1 L. migratoria 37 – + + NI NI Malpighian tubules
CYP6A1 M. domestica 28 + + + + + Gut, fat body
CYP6A2 D. melanogaster 31 NI NI + NI NI Fat body and other tissues
CYP6A9 D. melanogaster 29 NI NI + NI NI NI
CYP6B1 P. polyxenes 28 NI + NI NI NI Midgut
CYP6B2 H. armigera 28 – + – NI NI Midgut, fat body,
head/foregut
CYP6D1 M. domestica 26 – – + + + Many tissues
aReferences: CYP6A1 (Carino et al., 1992, 1994); CYP6A2 (Brun et al., 1996); CYP6A9 (Maitra et al., 1996); CYP6B1 (Cohen et al., 1992); CYP6B2 (Ranasinghe et al., 1997; Xiao-Ping and Hobbs, 1995); CYP6D1 (Scott, 1999) and CYP6H1 (Winter et al., 1999) NI=not investigated.
b The per cent amino acid identity was calculated based on the Clustal method of MEGALIGN program (DNASTAR Inc., Madison, Wisconsin). cNymphs for B. germanica and L. migratoria, larvae for all others.
d Tissues where the expression was investigated or from which the cDNA was cloned. eSpecifically in testes and accessory glands (see text).
amino acid sequence shares a number of common characteristics with other members of the P450 super-family. The N-terminal region is strongly hydrophobic, with hydrophobic residues accounting for|70% (16 out of the first 23 amino acid residues), which is important for membrane-anchoring (Wachenfeldt and Johnson, 1995). The sequence FGDGPRNCIG at positions 431– 440 matches the signature motif FxxGxxxCxG, which is the heme-binding region and is highly conserved among P450s. AGFET at positions 298–302 is highly conserved (consensus (A/G)GxxT) within the I helix where T302 may function as a proton donor and/or an acid-base cata-lyst in oxygen scission (Wachenfeldt and Johnson, 1995). The charge pair at positions 356–359 is highly conserved (EVLR; consensus ExxR) within the K helix which is assumed to be hydrogen-bonded to the ‘meander’, a region N-terminus to the heme-binding region (Peterson and Graham-Lorence, 1995). Other conserved regions or residues include: WRQIR (consensus WxxxR) at positions 129–133 in the C helix, P318exactly 16 residues from T302and YPDP (consensus (aromatic)xx(P/D)) at positions 404–407 followed five residues later by PERF at positions 412–415 (Nelson, 1998).
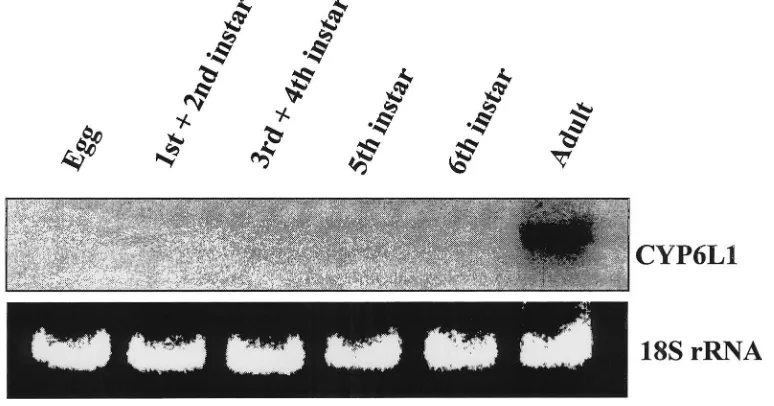
3.2. Expression in adults
Fig. 2. Life stage-specific expression of CYP6L1. Total RNA was prepared from approximately 1 g of cockroaches (mixed sexes) for each life stage and 10µg of total RNA was loaded for each lane. Northern hybridization with the CYP6L1 cDNA probe is shown on the top panel. RNA loading was standardized by ethidium bromide staining of 18S rRNA (bottom panel).
3.3. Expression in adult male abdomens
CYP6L1 mRNA expression was examined in the abdomens (A) versus the remainder of the bodies (H+T) in adult males or females. CYP6L1 mRNA was detected in the abdomens of males, but not in the abdomens of females nor in the remainder of the bodies of males or females (Fig. 3).
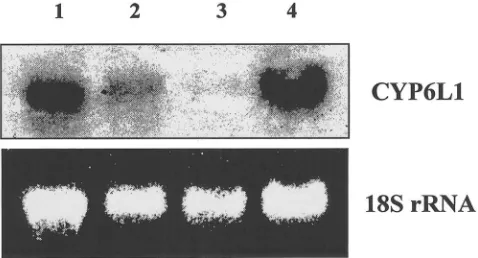
3.4. Expression in reproductive tissues
Given that CYP6L1 mRNA was expressed exclus-ively in the abdomens of adult males, expression in the
Fig. 4. Tissue-specific expression of CYP6L1. Total RNA was pre-pared from abdomens of 15 male adult German cockroaches excluding certain tissues. Ten micrograms of total RNA was loaded for each lane. Lane 1: Abdomens with testes removed; Lane 2: Abdomens with accessory glands removed; Lane 3: Abdomens with both testes and accessory glands removed; Lane 4: Whole abdomens. Northern hybridization with the CYP6L1 cDNA probe is shown on the top panel. RNA loading was standardized by ethidium bromide staining of 18S rRNA (bottom panel).
RNA from guts or abdomen remains (excluding testes, accessory glands and guts) (results not shown). For unknown reasons, we were unable to isolate enough RNA from dissected accessory glands for analysis.
3.5. Southern blots
Genomic DNA digested with BamHI, NotI, SmaI, and
XhoI was used in a Southern blot to evaluate the
speci-ficity of our CYP6L1 probe. A single band was observed in all cases (data not shown), suggesting that the probe was specific for CYP6L1. The relatively large fragments that were detected (ranging from approximately 10–20 kb) make it impossible to conclude whether CYP6L1 exists as a single copy gene or not.
4. Discussion
Although expression patterns for most insect P450s are not yet clear, available information shows that insect P450s have great diversity in their expression patterns with regard to life stages and tissues (Scott et al., 1998) (Table 2). CYP6L1 is unique because it is the first sex-specific insect P450 reported. Furthermore, CYP6L1 stands out because, although a number of mammalian P450s are expressed at different levels in males and females (Waxman and Chang, 1995), CYP6L1 is the first P450 found to be specifically expressed in the testes and accessory glands of the male reproductive system (Table 2).
Substrates for CYP6L1 cannot be predicted based on its sequence similarity with other P450s of family 6. However, gene expression patterns can provide a “strong clue as to its biological role” (DeRisi et al., 1997), as
has been demonstrated for CYP4C7 (Sutherland et al., 1998). Given that testes and accessory glands are the most important components of the reproduction system in male insects, the expression of CYP6L1 exclusively in these tissues suggests that it plays some role in repro-duction. What could this role be?
JHs and ecdysteroids are very important in that they control insect development during larval and pupal stages (nymphal stages in cockroaches) and have gona-dotropic function in the adult stages (Hardie, 1995). Yu and Terriere suggested that insect P450s were involved in reproduction via control of hormone titers (Hodgson, 1985; Yu and Terriere, 1974). Thus, one possible role for CYP6L1 might be the regulation of JHs in the accessory glands and ecdysteroids in the testes. Three lines of reasoning suggest that this is possible. First, insect accessory glands can synthesize juvenile hormone (Borovsky et al., 1994) and testes are an alternative site for ecdysteroid synthesis in several insects (Delbecque et al., 1990). Although the production of hormones by these tissues might be small, the local concentration increase could be significant. Second, insect P450s are involved in metabolism of JH and ecdysteroids (Feyereisen, 1999; Hodgson, 1985). Third, substrates for a given P450 can be structurally unrelated compounds (Andersen et al., 1994, 1997; Guengerich, 1995). There-fore, it appears possible that CYP6L1 could be involved in ecdysteroid synthesis in testes and juvenile hormone synthesis in the accessory glands of male adult Ger-man cockroaches.
Other possible roles for CYP6L1 exist as well. Insect testes and accessory glands produce a complex mixture of compounds which can modify female fecundity, receptivity and/or have other functions (Gillott, 1995). CYP6L1 might be involved in some of these processes. Alternatively, CYP6L1 could be involved in a function yet unknown to us (e.g., regulating titers of an unknown hormone). Further study of CYP6L1 will help clarify the function(s) of this sex-specific P450 and improve our understanding of the male reproductive system in insects. If CYP6L1 is critical for reproduction, this will offer a potential target for the development of novel insect control agents.
Acknowledgements
References
Adams, M.D. et al., 2000. The genome sequence of Drosophila
mel-anogaster. Science 287, 2185–2195.
Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W., Lipman, D.J., 1997. Gappled BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 17.
Andersen, J.F., Utermohlen, J.G., Feyereisen, R., 1994. Expression of house fly CYP6A1 and NADPH-cytochrome P450 reductase in
Escherichia coli and reconstitution of an insecticide-metabolizing
P450 system. Biochemistry 33, 2171–2177.
Andersen, J.F., Walding, J.K., Evans, P.H., Bowers, W.S., Feyereisen, R., 1997. Substrate specificity for the epoxidation of terpenoids and active site topology of house fly cytochrome P450 6A1. Chem. Res. Toxicol. 10, 156–164.
Berenbaum, M.R., 1999. Animal-plant warfare: molecular basis for cytochrome P450-mediated natural adaptation. In: Puga, A., Wal-lace, K.B. (Eds.), Molecular Biology of the Toxic Response. Taylor and Francis, Philadelphia, pp. 553–571.
Bernhardt, R., 1995. Cytochrome P450: structure, function, and gener-ation of reactive oxygen species. Rev. Physiol. Biochem. Pharma-col. 127, 137–221.
Borovsky, D., Carlson, D., Hancock, R.G., Rembold, H., Handel, E.V., 1994. De Novo biosynthesis of juvenile hormone III and I by the accessory glands of the male Mosquito. Insect Biochem. Molec. Biol. 24, 437–444.
Brun, A., Cuany, A., LeMouel, T., Berge, J., Amichot, M., 1996. Inducibility of the Drosophila melanogaster cytochrome P450 gene, CYP6A2, by phenobarbital in insecticide susceptible or resist-ant strains. Insect Biochem. Molec. Biol. 26, 697–703.
Carino, F., Koener, J.F., Plapp, F.W.J., Feyereisen, R., 1992. Expression of the cytochrome P450 gene CYP6A1 in the housefly,
Musca domestica. In: Mullin, C.A., Scott, J.G. (Eds.), Molecular
Mechanisms of Insecticide Resistance: Diversity Among Insects. ACS Symposium Series, vol. 505. American Chemical Society, Washington DC, pp. 31–40.
Carino, F.A., Koener, J.F., Plapp, F.W. Jr., Feyereisen, R., 1994. Constitutive overexpression of the cytochrome P450 gene CYP6A1 in a house fly strain with metabolic resistance to insecticides. Insect Biochem. Molec. Biol. 24, 411–418.
Chirgwin, J.M., Przybyla, A.E., MacDonald, R.J., Rutter, W.J., 1979. Isolation of biologically active ribonucleic acid from sources enriched in ribonucease. Biochemistry 18, 5294–5299.
Cohen, M.B., Schuler, M.A., Berenbaum, M.R., 1992. A host-inducible cytochrome P-450 from a host-specific caterpillar: mol-ecular cloning and evolution. Proc. Natl. Acad. Sci. USA 89, 10920–10924.
Consortium, C.E., 1998. Genome sequence of the nematode C.
ele-gans: a platform for investigating biology. Science 282, 2012–
2018.
Cornwell, P.B., 1968. The Cockroach. Hutchinson and Co, London. Danielson, P.B., Fogleman, J.C., 1997. Isolation and sequence analysis
of cytochrome P450 12B1: the first mitochondrial insect P450 with homology to 1alpha,25 dihydroxy-D3 24-hydroxylase. Insect Biochem. Molec. Biol. 27, 595–604.
Danielson, P.B., Foster, J.L.M., Cooper, S.K., Fogleman, J.C., 1999. Diversity of expressed cytochrome P450 genes in the adult Medit-erranean fruit fly Ceratitis capitata. Insect Molec. Biol. 8, 149–159. Delbecque, J.P., Weidner, K., Hoffmann, K.H., 1990. Alternative sites for ecdysteroid production in insects. Invertebr. Reprod. Dev. 18, 29–42.
DeRisi, J.L., Iyer, V.R., Brown, P.O., 1997. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278, 680–686.
Dunkov, B.C., Guzov, V.M., Mocelin, G., Shotkoski, F., Brun, A., Amichot, M., Ffrench-Constant, R.H., Feyereisen, R., 1997. The
Drosophila cytochrome P450 gene Cyp6a2: structure, localization,
heterologous expression, and induction by phenobarbital. DNA Cell Biol. 16, 1345–1356.
Feyereisen, R., 1999. Insect P450 enzymes. Ann. Rev. Entomol. 44, 507–533.
Frohman, M.A., Dush, M.K., Martin, G.R., 1988. Rapid production of full-length cDNAs from rare transcripts: amplification using a sin-gle gene-specific oligonucleotide primer. Proc. Natl. Acad. Sci. USA 85, 8998–9002.
Gillott, C., 1995. Insect male mating systems. In: Leather, S.R., Hardie, J. (Eds.), Insect Reproduction. CRC Press, New York, pp. 33–55. Guengerich, F.P., 1995. Human cytochrome P450 enzymes. In: Ortiz de Montellano, P.R. (Ed.), Cytochrome P450: Structure, Mech-anism, and Biochemistry. Plenum Press, New York, pp. 473–535. Gussow, D., Clackson, T., 1989. Direct clone characterization from plaques and colonies by the polymerase chain reaction. Nucleic Acids Res. 17, 4000.
Guzov, V.M., Unnithan, G.C., Chernogolov, A.A., Feyereisen, R., 1998. CYP12A1, a mitochondrial cytochrome P450 from the house fly. Arch. Biochem. Biophys. 359, 231–240.
Hardie, J., 1995. Hormones and reproduction. In: Leather, S.R., Har-die, J. (Eds.), Insect Reproduction. CRC Press, New York, pp. 95–108.
Hodgson, E., 1985. Microsomal mono-oxygenases. In: Kerkut, G.A., Gilbert, L.C. (Eds.), Comprehensive Insect Physiology Biochemis-try and Pharmacology, vol. 11. Pergamon Press, Oxford, pp. 647–712.
Hung, C.-F., Berenbaum, M.R., Schuler, M.A., 1997. Isolation and characterization of CYP6B4, a furanocoumarin-inducible cyto-chrome P450 from a polyphagous caterpillar (Lepidoptera: papilionidae). Insect Biochem. Molec. Biol. 27, 377–385. Lindberg, R.L.P., Negishi, M., 1989. Alteration of mouse cytochrome
P450coh substrate specificity by mutation of a single amino-acid residue. Nature 339, 632–634.
Ma, R., Cohen, M.B., Berenbaum, M.R., Schuler, M.A., 1994. Black swallowtail (Papilio polyxenes) alleles encode cytochrome P450s that selectively metabolize linear furanocoumarins. Arch. Biochem. Biophys. 310, 332–340.
Maitra, S., Dombrowski, S., Waters, L., Ganguly, R., 1996. Three second chromosome-linked clustered Cyp6 genes show differential constitutive and barbital-induced expression in DDT-resistant and susceptible strains of Drosophila melanogaster. Gene 180, 165– 171.
Nebert, D.W., Gonzalez, F.J., 1987. P450 genes: structure evolution and regulation. Ann. Rev. Biochem. 56, 945–993.
Nelson, D.R., 1998. Cytochrome P450 nomenclature. Meth. Molec. Biol. 107, 15–24.
Nelson, D.R., Kamataki, T., Waxman, D.J., Guengerich, F.P., Estab-rook, R.W., Feyereisen, R., Gonzalez, F.J., Coon, M.J., Gunsalus, I.C., Gotoh, O., Okuda, K., Nebert, D.W., 1993. The P450 super-family: update on new sequences, gene mapping, accession num-bers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 12, 1–51.
Nelson, D.R., Koymans, L., Kamataki, T., Stegeman, J.J., Feyereisen, R., Waxman, D.J., Waterman, M.R., Gotoh, O., Coon, M.J., Estab-rook, R.W., Gunsalus, I.C., Nebert, D.W., 1996. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 6, 1–42.
Peterson, J.A., Graham-Lorence, S., 1995. Bacterial P450s: structural similarities and functional differencies. In: Ortiz De Montellano, P.R. (Ed.), Cytochrome P450: Structure, Mechanism, and Bio-chemistry. Plenum Press, New York, pp. 151–180.
Ranasinghe, C., Headlam, M., Hobbs, A., 1997. Induction of the mRNA for CYP6B2, a pyrethroid inducible cytochrome P450, in
Helicoverpa armigera (Hubner) by dietary monoterpenes. Arch.
Insect Biochem. Physiol. 34, 99–109.
Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, New York.
Savonet, V., Maenhaut, C., Miot, F., Pirson, I., 1997. Pitfalls in the use of several ‘housekeeping’ genes as standards for quantitation of RNA: the example of thyroid cells. Analyt. Biochem. 247, 165–167.
Scott, J.G., 1999. Molecular basis of insecticide resistance: cyto-chromes P450. Insect Biochem. Molec. Biol. 29, 757–777. Scott, J.G., Liu, N., Wen, Z., 1998. Insect cytochromes P450: diversity,
insecticide resistance and tolerance to plant toxins. Comp. Biochem. Physiol. 121C, 147–155.
Scott, J.G., Matsumura, F., 1981. Characteristics of a DDT-induced case of cross-resistance to permethrin in Blattella germanica. Pes-tic. Biochem. Physiol. 16, 21–27.
Siegfried, B.D., Scott, J.G., 1991. Mechanisms responsible for pro-poxur resistance in the German cockroach Blattella germanica (L). Pestic. Sci. 33, 133–146.
Snodgrass, R.E., 1937. The male genitalia of Orthopteroid insects. Smithsonian Misc. Coll. 96, 1–107.
Spiess, A.-N., Ivell, R., 1998. Normalization of RNA hybridization signals by means of SYBR Green II-stained 28S or 18S ribosomal RNA and phosphor imager. BioTechniques 26, 46–50.
Sutherland, T.D., Unnithan, G.C., Andersen, J.F., Evans, P.H., Murata-liev, M.B., Szabo, L.Z., Mash, E.A., Bowers, W.S., Feyereisen, R., 1998. A cytochrome P450 terpenoid hydroxylase linked to the suppression of insect juvenile hormone synthesis. Proc. Natl. Acad. Sci. USA 95, 12884–12889.
Wachenfeldt, C.V., Johnson, E.F., 1995. Structure of eukaryotic cyto-chrome P450 enzymes. In: Ortiz de Montellano, P.R. (Ed.), Cyto-chrome P450: Structure, Mechanism, and Biochemistry. Plenum Press, New York, pp. 182–223.
Waxman, D., Chang, T.K.H., 1995. Hormonal regulation of liver cyto-chrome P450 enzymes. In: Ortiz de Montellano, P.R. (Ed.), Cyto-chrome P450: Structure, Mechanism, and Biochemistry. Plenum Press, New York, pp. 391–417.
Wheelock, G.D., Scott, J.G., 1992. The role of cytochrome P450lpr in deltamethrin metabolism by pyrethroid resistant and susceptible strains of house flies. Pestic. Biochem. Physiol. 43, 67–77. Winter, J., Bilbe, G., Richener, H., Sehringer, B., Kayser, H., 1999.
Cloning of a cDNA encoding a novel cytochrome P450 from the insect Locusta migratoria: CYP6H1, a putative ecdysone 20-hydroxylase. Biochem. Biophys. Res. Comm. 259, 305–310. Xiao-Ping, W., Hobbs, A.A., 1995. Isolation and sequence analysis of
a cDNA clone for a pyrethroid inducible cytochrome P450 from
Helicoverpa armigera. Insect Biochem. Molec. Biol. 25, 1001–
1009.
Yu, S.J., Terriere, L.C., 1974. A possible role for microsomal oxidases in metamorphosis and reproduction in the house fly. J. Insect Phy-siol. 20, 1901–1912.