www.elsevier.com/locate/ibmb
Soluble proteins from chemosensory organs of Eurycantha
calcarata (Insects, Phasmatodea)
Silvana Marchese
a, Sergio Angeli
a, b, Annapaola Andolfo
c, Andrea Scaloni
c,
Anna Brandazza
a, Mario Mazza
d, Jean-Franc¸ois Picimbon
e, Walter S. Leal
e,
Paolo Pelosi
a,*aDipartimento di Chimica e Biotecnologie Agrarie, University of Pisa, Via S. Michele 4, 56124 Pisa, Italy bScuola Superiore di Studi Universitari e Perfezionamento “S. Anna”, Pisa, Italy
cCentro Internazionale Servizi di Spettrometia di Massa — IABBAM, Consiglio Nazionale delle Ricerche, Naples, Italy dDipartimento di Etologia, Ecologia ed Evoluzione, University of Pisa, Pisa, Italy
eNational Institute of Sericultural and Entomological Science, Tsukuba, Japan
Received 25 January 2000; received in revised form 13 March 2000; accepted 11 April 2000
Abstract
Three related nucleotide sequences, encoding mature proteins of 108–113 amino acids, have been obtained from antennal cDNA of the Phasmid Eurycantha calcarata. Among these, one is also expressed in the tarsi as demonstrated by N-terminal sequence and mass spectrometric analyses of protein samples isolated from both organs. PCR experiments performed with specific primers, showed that this species is also expressed in the mouth organs and in the cuticle, while the other two are antennal specific. All three isoforms are similar to Drosophila OS-D and other proteins reported in several insect orders, but one of them is significantly different from the other two. The best conserved elements are the N-terminal region and the four cysteine residues. Accurate ESMS measurements indicated that all cysteines are involved in two disulphide bonds and ruled out the occurrence of additional post-translational modifications. Polyclonal antibodies, raised against the purified protein, did not react with proteins of the same class expressed in another Phasmid species, Carausius morosus, and in the orthopteran Schistocerca gregaria, nor did antibodies against these proteins recognise those of E. calcarata.2000 Elsevier Science Ltd. All rights reserved.
Keywords: Chemosensory proteins; Odorant-binding proteins; Phasmids; Eurycantha calcarata; sequence analysis; RACE
1. Introduction
Odorant-binding proteins (OBP) are small soluble polypeptides highly concentrated in the fluids (nasal mucus in vertebrates and sensillar lymph in insects) bathing the dendrites of chemosensory neurons (Pelosi 1996, 1998; Pelosi and Maida, 1995; Steinbrecht, 1998). In insects, they are classified as PBPs (pheromone-bind-ing proteins) and GOBPs (general odorant-bind(pheromone-bind-ing proteins), depending on their physiological ligands. Both PBPs and GOBPs have been identified in several species from different orders of insects (Vogt and Riddiford,
* Corresponding author. Tel.: +39-050-571564; fax: + 39-050-574235.
E-mail address: [email protected] (P. Pelosi).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 8 4 - 9
1981; Maida et al., 1993; Krieger et al. 1991, 1996; Dickens et al., 1995; Vogt et al., 1999; Danty et al., 1999; Nagnan-Le Meillour et al., 1996; Ozaki et al., 1995; Raming et al., 1990; Wojtasek et al. 1998, 1999). They share six conserved cysteine residues, connected by three disulphide bridges, as demonstrated in the case of Bombyx mori (Scaloni et al., 1999; Leal et al., 1999), that contribute to a compact structure of the molecule.
pro-teins similar to OS-D from antennae and other chemo-sensory organs of insects of different orders, however, supports the hypothesis that these could represent another class of proteins involved in chemoreception.
Our search for odorant-binding proteins in insect orders other than Lepidoptera first led to the isolation of low molecular weight polypeptides highly expressed in the sensory organs (antennae, tarsi and mouth structures) of several species of Phasmids (Tuccini et al., 1996; Mameli et al., 1996) bearing a significant similarity to
Drosophila OS-D. Members of this class were later
described in the Orthopteran species Schistocerca
grega-ria (Angeli et al., 1999), in three species of Lepidoptera, Cactoblastis cactorum (Maleszka and Stange, 1997), Mamestra brassicae (Bohbot et al., 1998) and Bombyx mori (Picimbon et al., 2000), as well as in the honey bee
(Danty et al., 1998) and in cockroaches (Picimbon and Leal, 1999). Unlike lepidopteran OBPs and their putative homologues of other orders, OS-D-like proteins are well conserved across evolution, with 40–50% of identical residues even between most distant species.
The physiological function of these proteins is still to be identified, and even their role in olfaction has been questioned. A proposed role in carbon dioxide sensing (Maleszka and Stange, 1997) has not received experi-mental evidence. On the other hand, their involvement in chemosensation is well supported by their specific expression in chemosensory organs, such as antennae, tarsi and mouth apparatus (Angeli et al., 1999). More-over, electron microscopy experiments, have clearly shown that in S. gregaria these proteins are highly con-centrated in the lymph of contact sensilla of antennae, tarsi and labial palpi, but are absent in olfactory sensilla (Angeli et al., 1999). A protein similar to Drosophila OS-D is expressed in the ejaculatory bulb of the same species (Dyanov and Dzitoeva, 1995). The presence in this organ of the sex pheromone suggested the idea that such protein could be a carrier for the hydrophobic mol-ecule. This view was supported by recent experiments demonstrating reversible binding of the Drosophila pheromone vaccenyl acetate to the OS-D-like protein of the Lepidopteran species M. brassicae (Bohbot et al., 1998). Another polypeptide of the same class, called p10, has been isolated from the regenerating legs of the cockroach Periplaneta americana (Kitabayashi et al., 1998): the authors suggest a function in the regeneration of limbs during the larval stages.
To provide additional information on the structure of this class of proteins, we have cloned three members of this family from the antennae of the Phasmid Eurycantha
calcarata. A comparison with similar sequences expressed in insects of different orders reveals highly conserved regions, probably involved in a common func-tion.
2. Materials and methods
2.1. Materials
2.1.1. Insects
Individuals of E. calcarata were reared on fresh bram-ble and used for the experiments at their adult stage. Tissue samples of Carausius morosus and S. gregaria, used in Western blot experiments, have been made avail-able in previous studies (Tuccini et al., 1996; Angeli et al., 1999)
2.1.2. Chemicals
The reagents and kits used for molecular biology experiments are described in Section 2.2. Oligonucleot-ides for PCR amplification were synthesised by GIBCO Brl and PRIMM, Milan, Italy. Labelled reagents for ligand binding assays (14C-sodium bicarbonate and 3 H-glucose) were obtained from the Radiochemical Center, Amersham Life Science, UK, and had specific activities of 50 mCi/mmole and 26 Ci/mmole, respectively.
Solvents and reagents for amino acid sequence deter-mination were “sequencing grade”. All other reagents were of analytical grade.
2.2. Methods
2.2.1. Purification of protein p14 from the tarsi and the cuticle of E. calcarata
The protein was purified by gel filtration and anion-exchange chromatography on Mono-Q, as previously reported (Mameli et al., 1996). The tarsi of 11 individ-uals afforded about 4 mg of protein, migrated as a single broad band in SDS-PAGE. Samples were subjected to a final chromatographic step on a Vydac C4 column 214TP54 (250×4.6 mm), 5 µm, 300 A˚ pore size (The Separation Group, USA). Proteins were dissolved in 0.1% TFA, loaded onto the column and eluted by a lin-ear gradient from 5 to 70% of acetonitrile in 0.1% TFA over 30 min, at a flow rate of 1 ml/min.
The same procedure was used for the purification of protein p14 from the cuticle.
2.2.2. Amino acid sequence analysis
2.2.3. Mass spectrometry
Intact proteins were submitted to ESMS analysis, using a Platform single quadrupole mass spectrometer (Micromass, UK). Samples were dissolved in 1% (v/v) acetic acid and 2–10 µl of the liquid was injected into the mass spectrometer at a flow rate of 10 µl/min. The quadrupole was scanned from m/z 500 to 1800 at 10 s/scan and the spectra were acquired and elaborated using the MassLynx software. Calibration was perfor-med by the multiply charged ions from a separate injec-tion of myoglobin (M.W. 16,951.5 Da). All mass values are reported as average masses.
2.2.4. Cloning and DNA sequencing
2.2.4.1. cDNA synthesis Total RNA was extracted from the tarsi of one male E. calcarata, using the TrizolReagent kit (GIBCO BRL), a modified guani-dine isothiocyanate/phenol procedure, along with the manufacturer’s instructions. One microgram of total RNA was subjected to reverse transcription, using 200 units of the Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase (GIBCO BRL) and 0.5µg of oligo dT12–18 (Sigma) in a 20 µl total volume. The mixture also contained 1 mM of each dNTP (Pharmacia Biotech, Uppsala, Sweden), 75 mM KCl, 3 mM MgCl2, 10 mM DTT and 0.1 mg/ml BSA in 50 mM Tris/HCl, pH 8.3. The reaction mixture was incubated at 42°C for 60 min and the products were directly used for PCR amplifi-cation or stored at 220°C.
2.2.5. Polymerase chain reaction
Aliquots of 0.5 µg of cDNA mixture were amplified in a Bio-Rad Gene Cycler, or a MJ PTC-150 Min-iCycler, using 2.5 units of Thermus aquaticus DNA Polymerase (Promega), or of pfu DNA Polymerase, 1 mM of each dNTP (Pharmacia Biotech, Uppsala, Sweden), 1 µM of each PCR primer, 50 mM KCl, 2.5 mM MgCl2and 0.1 mg/ml BSA in 10 mM Tris/HCl, pH 8.3, containing 0.1% Triton X-100. At the 59 end we used the 17mer degenerated primer ACNAARTAY-GAYAAYGT, designed on the amino acid sequence 7– 11 (–TKYDNV–). At the 39 end a 15–18mer oligo-dT was employed. After a denaturing step at 95°C for 2 min, the reaction was performed for 30 cycles (95°C for 20 s, 48°C for 20 s, 72°C for 1 min), followed by a final step of 7 min at 72°C. The missing codons at the 59end of the genes were determined using the RACE method. The cDNA sample was incubated with Terminal deoxyn-ucleotidyl transferase and dCTP for 10 min at 37°C. The product was then amplified by PCR, using the primer GGCCACGCGTGGACGATC(G)n: at the 59 end, and
one of the two primers (AACTGCATGGTGTCTT ATAAATGT and AAGATGCACGTCGTGAAGACA GGG), complementary to the non-coding regions of pre-viously determined sequences, at the 39 end.
2.2.6. RACE
A RACE (Rapid Amplification of cDNA Ends) strat-egy was applied for determining the sequence at the 59 end and thus obtaining the complete cDNA sequence. Accordingly a polyC was linked to the 59 end and the cDNA was amplified by PCR using an oligo-dG and spe-cific primers, corresponding to already known sequences following the stop codons.
2.2.7. Cloning of PCR products
PCR products were separated on a 2% agarose gel. The most prominent bands, corresponding to sizes of around 400–500 bp, were dissected and DNA was extracted and purified using a gene clean kit (Qiagen), following the manufacturer’s instructions. Amplified DNA was ligated into a pCR-Script (Stratagene) plas-mid. E. coli Epicurian Coli XL-1 Blue MRF Supercom-petent Cells (Stratagene) were transformed with the lig-ation products, using the manufacturer’s protocol and plated. White colonies were assayed for the presence of the insert by PCR, using the plasmid primers M13R and T7. Selected positive clones were grown in liquid LB medium and plasmids were extracted and purified, using the QIAquick Purification Kit (Qiagen). Nucleotide sequences of both strands of the cDNA clones were determined from double stranded plasmid DNA using the Applied Biosystem Dye Deoxy Terminator Cycle Sequencer Kit and an Applied Biosystem 310 auto-mated sequencer.
2.2.8. Preparation of polyclonal antibodies
Antisera were obtained by injecting an adult rabbit subcutaneously and intramuscularly with 400µg of pur-ified protein (E. calcarata CSP, C. morosus p19 and S.
gregaria CSP), followed by two additional injections of
250µg after 18 and 30 days. The protein was emulsified with an equal volume of Freund’s complete adjuvant for the first injection and of incomplete adjuvant for further injections. Animals were bled 10 days after the last injection and the serum was partially purified by precipi-tation in 45% ammonium sulphate.
2.3. Western blot
3. Results
3.1. Cloning and sequencing of CSP of E. calcarata
On the basis of the N-terminal amino acid sequence previously reported for protein p14 of E. calcarata (Mameli et al., 1996), we designed a degenerated primer to amplify specific nucleotide sequences encoding such a protein. In order to minimise the degeneracy of the oligonucleotide, we selected the sequence corresponding to amino acids 7–11. This part of the sequence is also conserved in similar proteins of other species of Phas-mids (Mameli et al., 1996), Orthoptera (Angeli et al., 1999) and Blattoidea (Picimbon and Leal, 1999). The amplification products of both antennal and tarsi cDNA showed a major band of around 550 bp, that was purified and cloned, as described in Section 2.
Sequencing of a number of clones, obtained from the antennal sample, afforded three distinct sequences that were each confirmed in at least five clones obtained from two different PCR experiments. In contrast, the sample obtained from the tarsi afforded only one sequence, that was confirmed in eight different clones from three PCR amplification products. This polynucleotide, identical to the one obtained from the antennae, encodes for a pro-tein, whose N-terminus matches the amino acid sequence determined on the protein extracted from both parts of the body (Mameli et al., 1996).
While the complete sequence of one of the proteins expressed was reconstructed by combining the cDNA derived sequence with information obtained by direct Edman degradation of the protein, the N-terminus of the other two polypeptides expressed in the antennae remained unknown. To obtain such information, the RACE technique was applied to a sample of antennal cDNA, using specific primers and the protocol reported in Section 2. The full length cDNA and amino acid sequences are reported in Fig. 1. As reported in our pre-vious paper (Angeli et al., 1999), we adopted the general name of CSP (ChemoSensory Protein) for a protein of this class, followed by the initials of the species and a serial number.
3.2. Post-translation modifications
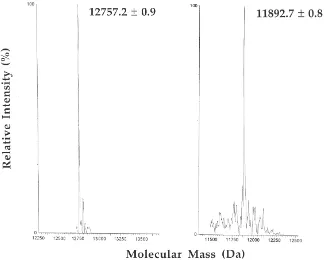
To verify the cDNA sequence of CSP-ec1 and obtain information on post-translational modifications of the polypeptide, a sample of the protein, purified from the tarsi, as previously reported (Mameli et al., 1996), was subjected to electrospray mass spectrometry analysis after fractionation by HPLC. Two chromatographic peaks were isolated (data not shown) relative to polypep-tides with molecular masses of 12,757.2±0.9 Da and 11,893.7±0.8 Da, respectively (Fig. 2). These values were associated to truncated forms of the protein
CSP-ec1, namely fragments 1-109 and 9-109, respectively. In
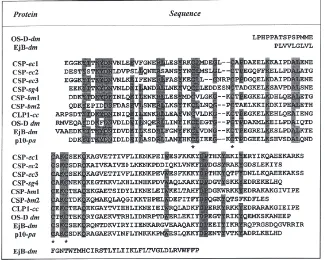
Fig. 1. Nucleotide and derived amino acid sequences of clones: (A)
eury 5B encoding protein ec1, (B) eury 1B encoding protein CSP-ec2, and (C) eury 6B encoding protein CSP-ec3. The signal peptides
and the polyadenylation signals are underlined. The two stop triplets are indicated by asterisks.
Fig. 2. Transformed electrospray mass spectra of the two samples of p14 purified from the tarsi of E. calcarata. The molecular masses were calculated from the multiply charged ion traces. The measured masses agree with those calculated for truncated forms of the protein CSP-ec1, corresponding to peptides 1-109 (left) and 9-109 (right).
3.3. Western blot
A sample of p14 protein, purified from the tarsi of E.
calcarata, was used to raise polyclonal antibodies in a
rabbit, using the protocol reported in Section 2. The crude antiserum was partially purified by 45% ammonium sulphate precipitation and used in Western blot experiments.
Fig. 3 reports the results of cross immunoreactivity performed with proteins p14 from E. calcarata, p19 from C. morosus (Tuccini et al., 1996), p14 from
Schis-tocerca gregaria (Angeli et al., 1999) and their relative
polyclonal antisera. Although the three proteins are
simi-Fig. 3. Western blot analysis of CSPs purified from S. gregaria (S), E. calcarata (E) and C. morosus (C), using the antisera raised against the same proteins. Arrows indicate the proteins utilised for raising antibodies. Molecular weight markers (M) are, from the top: BSA (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (29 kDa), trypsin inhibitor (20 kDa),α-lactalbumin (14 kDa).
lar enough to be assigned to the same class, each of them was stained only by its own relative antiserum.
3.4. Isolation of a CSP from the cuticle of E. calcarata
other two sequences expressed in the antennae both showed differences in this region.
4. Discussion
The sequences reported in this paper, together with OS-D of D. melanogaster and the other similar proteins expressed in other orders of insects (Fig. 4), represent a recently discovered class of soluble proteins expressed in the chemoreception organs of insects (CSPs). While the function of lepidopteran OBPs as pheromone and odour carriers is supported in several cases by ligand-binding data (Vogt and Riddiford, 1981; Du et al., 1994; Feixas et al., 1995; Maida et al., 1993; Maı¨be`che-Coisne et al., 1997; Wojtasek et al., 1999), a role of CSPs in chemical perception can only be proposed on the basis of their histochemical localisation.
Although CSPs and OBPs share a common low mol-ecular mass and an acidic nature, they do not exhibit significant similarities in their amino acid sequences. CSPs present four conserved cysteine residues, which do not match any of the six cysteines of OBPs. In both cases, these amino acids are linked by disulphide bridges, but with a clearly different topology. In the OBPs of B. mori, the three disulphide bonds are inter-laced (1–3, 2–5, 4–6), constraining the proteins into a structure with a reduced flexibility (Scaloni et al., 1999; Leal et al., 1999). In contrast, in the CSPs of S. gregaria
Fig. 4. Alignment of the three CSP of E. calcarata with proteins of the same class identified in other insect species. EjB: ejaculatory bulb; sg:
S. gregaria; ec: E. calcarata; dm: D. melanogaster; cc: C. cactorum; bm: B. mori; pa: P. americana. Residues common to most sequences are
shaded. The four conserved cysteines are marked by asterisks.
(Angeli et al., 1999), and very likely in all proteins of this class, disulphide bridges link adjacent cysteines (1– 2 and 3–4), forming two small loops along the main polypeptide chain. We incidentally observed that a simi-lar arrangement of disulphide bonds is present in thiore-doxin that, however, does not show any further structural similarity with CSPs.
Table 1
Similarity matrix of CSPs from different species of insects. The sequences compared are those of Fig. 4. Identities greater than 45% are in bold type
CSP-ec1
CSP-ec2 44 CSP-ec2
CSP-ec3 77 44 CSP-ec3
CSP-sg4 48 36 47 CSP-sg4
CSP-bm1 43 35 44 36 CSP-bm1
CSP-bm2 38 36 40 33 38 CSP-bm2
p10-pa 51 39 47 56 40 35 p10-pa
EjB-dm 38 34 36 38 34 28 48 EjB-dm
CLP-cc 42 38 40 42 72 32 45 34 CLP-cc
OS-D-dm 42 37 41 40 41 35 50 34 41
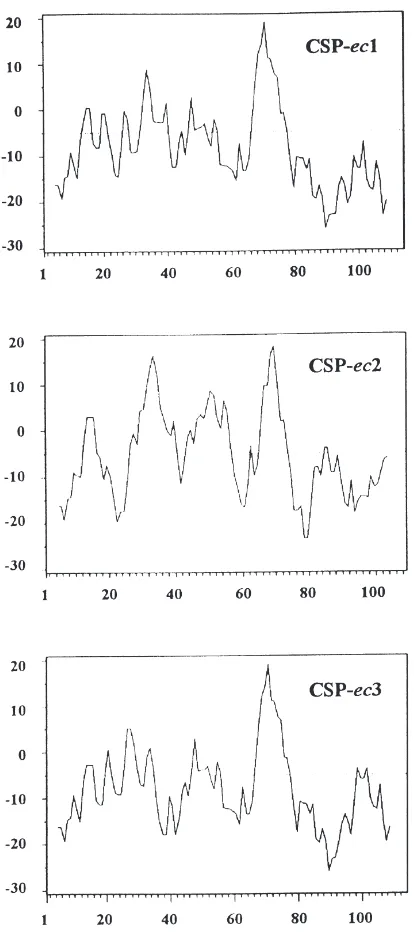
Fig. 5. Hydropathy profiles of CSPs from E. calcarata. The method of Kyte and Doolittle was used with a window size of nine amino acids. Positive values indicate hydrophobicity.
their residues between themselves (Picimbon et al., 2000). If CSPs play some role in the recognition of chemical stim-uli, certainly the presence of different sub-types in the same species represents an essential element of support.
In some species the selective expression of CSPs in chemosensory structures has suggested a specific function in chemosensory mechanisms. The OS-D protein of D.
melanogaster is expressed in a region highly populated by
sensilla coeloconica, responding to carbon dioxide and some other volatile compounds. In the lepidopteran species
C. cactorum, the expression of CLP-1 in the labial palpi,
populated with a single type of sensilla, indicates, accord-ing to the authors, a role in carbon dioxide sensaccord-ing (Maleszka and Stange, 1997). The CSPs of S. gregaria (Angeli et al., 1999), are selectively expressed in the lymph of the contact sensilla of the antennae, tarsi and palpi, but not of olfactory hairs of the same organs. Ligand binding experiments have been performed with the CSPs of S.
gre-garia, using sodium bicarbonate and glucose, but neither
of these two compounds was effective (Angeli et al., 1999). Successful binding, instead, was demonstrated for a protein of this class, expressed in the moth M. brassicae and the sex pheromone of D. melanogaster, vaccenyl acetate (Bohbot et al., 1998). The choice of this ligand was sug-gested by the fact that another protein significantly similar to CSPs is produced by the ejaculatory organ of D.
mel-anogaster. The same organ also secretes the sex
phero-mone, although no evidence that the protein is actually a carrier for the pheromone has been provided. This result seems to indicate that CSPs could be involved in phero-mone perception, in addition to PBPs, as in Lepidoptera, where both classes of proteins are expressed.
The work here described provides the necessary back-ground for heterologous expression of these proteins, which in turn is a prerequisite for a structural investigation by means of X-ray crystallography and NMR, as well as for measuring their affinity to several different potential ligands.
Acknowledgements
Activities for Innovative Biosciences (BRAIN) and by a special coordination fund for promoting science and technology by the Science and Technology Agency, Japan, granted to W.S.L. A two-month visit to Japan (NISES, Tsukuba) by P.P. and a fellowship to J.F.P. were supported by the same funds. The Italian compo-nent was supported by the EEC grant no. BIO4-CT98-0420 to P.P.
References
Angeli, S., Ceron, F., Scaloni, A., Monti, M., Monteforti, G., Min-nocci, A., Petacchi, R., Pelosi, P., 1999. Purification, structural characterization, cloning and immunocytochemical localization of chemoreception proteins from Schistocerca gregaria. Eur. J. Biochem. 262, 745–754.
Bohbot, J., Sobrio, F., Lucas, P., Nagnan-Le Meillour, P., 1998. Func-tional characterization of a new class of odorant-binding proteins in the moth Mamestra brassicae. Biochem. Biophys. Res. Com-mun. 253, 489–494.
Danty, E., Arnold, G., Huet, J.-C., Masson, C., Pernollet, J.-C., 1998. Separation, characterization and sexual heterogeneity of multiple putative odorant-binding proteins in the honeybee Apis mellifera L. (Hynenoptera: Apidea). Chem. Senses 23, 83–91.
Danty, E., Briand, L., Michard-Vanhee, C., Perez, V., Arnold, G., Gau-demer, O., Huet, D., Huet, J.C., Ouali, C., Masson, C., Pernollet, J.C., 1999. Cloning and expression of a queen pheromone-binding protein in the honeybee: an olfactory-specific, developmentally regulated protein. J. Neurosci. 17, 7468–7475.
Dickens, J.C., Callahan, F.E., Wergin, W.P., Erba, E.F., 1995. Olfac-tion in a hemimetabolous insect: antennal-specific protein in adult
Lygus lineolaris (Heteroptera: Miridae). J. Insect Physiol. 41 (10),
857–867.
Du, G., Ng, C.S., Prestwich, G.D., 1994. Odorant binding by a phero-mone binding protein-active site mapping by photoaffinity labeling. Biochemistry 33, 4812–4819.
Dyanov, H.M., Dzitoeva, S.G., 1995. Method for attachment of micro-scopic preparations on glass for in situ hybridization, PRINS and
in situ PCR studies. BioTechniques 18, 822–824.
Feixas, J., Prestwich, G.D., Guerrero, A., 1995. Ligand specificity of pheromone-binding proteins of the processionary moth. Eur. J. Biochem. 234, 521–526.
Garibotti, M., Navarrini, A., Pisanelli, A.M., Pelosi, P., 1997. Three odorant-binding proteins from rabbit nasal mucosa. Chem. Senses 22, 383–390.
Kitabayashi, A.N., Arai, T., Kubo, T., Natori, S., 1998. Molecular cloning of cDNA for p10, a novel protein that increases in the regenerating legs of Periplaneta americana (American cockroach). Insect Biochem. Mol. Biol. 28, 785–790.
Krieger, E., Raming, K., Breer, H., 1991. Cloning of genomic and complementary DNA encoding insect pheromone binding proteins: evidence for microdiversity. Biochim. Biophys. Acta 1088, 277– 284.
Krieger, E., Von Nickisch, E., Mameli, M., Pelosi, P., Breer, H., 1996. Binding proteins from the antennae of Bombyx mori. Insect Biochem. Mol. Biol. 26, 297–307.
Kyhse-Andersen, J., 1984. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J. Biochem. Biophys. Methods 10, 203–209.
Leal, W.S., Nikonova, L., Peng, G., 1999. Disulfide structure of the pheromone binding protein from the silkworm moth, Bombyx mori. FEBS Lett. 464, 85–90.
Maı¨be`che-Coisne, M., Sobrio, F., Delaunay, T., Lettere, M., Dubroca, J., Jacquin-Joly, E., Nagnan-Le Meillour, P., 1997. Pheromone binding proteins of the moth Mamestra brassicae: specificity of ligand binding. Insect Biochem. Mol. Biol. 27, 213–221. Maida, R., Steinbrecht, R.A., Ziegelberger, G., Pelosi, P., 1993. The
pheromone-binding protein of Bombix mori: purification, character-isation and immunocytochemical localcharacter-isation. Insect Biochem. Mol. Biol. 23, 243–253.
Maleszka, R., Stange, G., 1997. Molecular cloning, by a novel approach, of a cDNA encoding a putative olfactory protein in the labial palps of the moth Cactoblastis cactorum. Gene 202, 39–43. Mameli, M., Tuccini, A., Mazza, M., Petacchi, R., Pelosi, P., 1996. Soluble proteins in chemosensory organs of Phasmids. Insect Biochem. Molec. Biol. 26, 875–882.
McKenna, M.P., Hekmat-Scafe, D.S., Gaines, P., Carlson, J.R., 1994. Putative Drosophila pheromone-binding proteins expressed in a subregion of the olfactory system. J. Biol. Chem. 269, 16340– 16347.
Nagnan-Le Meillour, P., Huet, J.C., Maibeche, M., Pernollet, J.C., Descoins, C., 1996. Purification and characterization of multiple forms of odorant/pheromone binding proteins in the antennae of
Mamestra brassicae (Noctuidae). Insect Biochem. Mol. Biol. 26,
59–67.
Ozaki, M., Morisaki, K., Idei, W., Ozaki, K., Tokunaga, F., 1995. A putative lipophilic stimulant carrier protein commonly found in the taste and olfactory systems. Eur. J. Biochem. 230, 298–308. Pelosi, P., 1996. Perireceptor events in olfaction. J. Neurobiol. 30,
3–19.
Pelosi, P., 1998. Odorant-binding proteins: structural aspects. Ann. N.Y. Acad. Sci. 855, 281–293.
Pelosi, P., Maida, R., 1995. Odorant binding proteins in insects. Comp. Biochem. Physiol. 111B, 503–514.
Picimbon, J.F., Dietrich, K., Angeli, S., Scaloni, A., Krieger, J., Pelosi, P., Breer H., 2000. Purification and molecular cloning of chemo-sensory proteins in Bombyx mori. Arch. Insect Biochem. Physiol. (in press) .
Picimbon, J.F., Leal, W.S., 1999. Olfactory soluble proteins of cock-roaches. Insect Biochem. Mol. Biol. 29, 973–978.
Pikielny, C.W., Hasan, G., Rouyer, F., Rosbach, M., 1994. Members of a family of Drosophila putative odorant-binding proteins are expressed in different subsets of olfactory hairs. Neuron 12, 35–49. Raming, K., Krieger, J., Breer, H., 1990. Primary structure of phero-mone-binding protein from Antheraea pernyi: homologies with other ligand-carrying protein. Comp. Physiol. B 160, 503–509. Scaloni, A., Monti, M., Angeli, S., Pelosi, P., 1999. Structural analysis
and disulfide-bridge pairing of two odorant-binding proteins from
Bombyx mori. Biochem. Biophys. Res. Comm. 266, 386–391.
Steinbrecht, R.A., 1998. Odorant-binding proteins: expression and function. Ann. N.Y. Acad. Sci. 855, 281–293.
Tuccini, A., Maida, R., Rovero, P., Mazza, M., Pelosi, P., 1996. Puta-tive odorant-binding proteins in antennae and legs of Carausius
morosus (Insecta, Phasmatodea). Insect Biochem. Mol. Biol. 26,
19–24.
Vogt, R.G., Callahan, F.E., Rogers, M.E., Dickens, J.C., 1999. Odorant binding protein diversity and distribution among the insect orders, as indicated by LAP, an OBP-related protein of the true bug Lygus
lineolaris. Chem. Senses 5, 481–495.
Vogt, R.G., Riddiford, L.M., 1981. Pheromone binding and inacti-vation by moth antennae. Nature 293, 161–163.
Wojtasek, H., Hansson, B.S., Leal, W.S., 1998. Attracted or repelled? — a matter of two neurons, one pheromone binding pro-tein, and a chiral center. Biochem. Biophys. Res. Commun. 250, 217–222.
Wojtasek, H., Picimbon, J.F., Leal, W.S., 1999. Identification and clon-ing of odorant bindclon-ing proteins from the scarab beetle Phyllopertha