www.elsevier.com/locate/ibmb
Maintenance of GABA receptor function of small-diameter
cockroach neurons by adenine nucleotides
Gerald B. Watson
a,*, Vincent L. Salgado
a, baDow AgroSciences Discovery Research, 9330 Zionsville Rd, Indianapolis, IN 46268, USA bCurrent address: Aventis CropScience, 2 T.W. Alexander Drive, Research Triangle Park, NC 27615, USA
Received 31 March 2000; accepted 21 June 2000
Abstract
Small diameter (,20µm) neurons from the sixth abdominal ganglion of the American cockroach, Periplaneta americana, were enzymatically isolated and responses to exogenously appliedγ-aminobutyric acid (GABA) were recorded using the whole-cell patch clamp technique.
With a minimal intracellular medium, responses to repeated applications of GABA decreased to zero within a few minutes. The rate of rundown of GABA responses was decreased by the intracellular inclusion of the phosphatase inhibitors microcystin and okadaic acid, suggesting that phosphorylation is necessary for the maintenance of cockroach GABA receptor function.
ATP (5 mM) prevented GABA response rundown. ADP (5 mM) also slowed GABA response rundown, but responses stabilized at a level about half that seen with ATP.
In the presence of protein kinase A inhibitory peptide (PKI), ATP was only as efficacious as ADP in slowing rundown. PKI had no effect on the ability of ADP to slow rundown, suggesting that the β-phosphate of ADP is not involved in PKA-dependent phosphorylation of the GABA receptor.
These results suggest that in cockroach neurons, GABA receptor function is maintained intracellularly by adenine nucleotides, not only by phosphorylation, but also possibly by an interaction with a nucleotide recognition site unrelated to PKA-dependent phosphorylation.2001 Elsevier Science Ltd. All rights reserved.
Keywords:γ-aminobutyric acid (GABA); Cockroach; Neuronal; Phosphorylation; Adenosine 59-triphosphate (ATP); Adenine nucleotides
1. Introduction
Phosphorylation and dephosphorylation are recog-nized as major post-translational processes in the regu-lation of neuronal function (Walaas and Greengard, 1991; Girault, 1993). In fact, phosphorylation is thought to play a significant role in the maintenance of nearly all receptor and ion channel proteins (cf Girault, 1993). The cloning of many vertebrate γ-aminobutyric acid (GABA) receptor subunits has revealed the existence of many consensus sequences for intracellular phosphoryl-ation by protein kinase A, protein kinase C, and protein tyrosine kinase (see review by Sieghart, 1995). Numer-ous reports have demonstrated that vertebrate GABA receptors are modulated by intracellular phosphorylation
* Corresponding author. Fax:+1-317-337-3249.
E-mail address: [email protected] (G.B. Watson).
0965-1748/01/$ - see front matter2001 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 1 2 0 - X
insect GABA receptors, it is likely that insect GABA receptors can be similarly regulated by phosphorylation. In initial attempts to study GABA receptor pharma-cology from small, dissociated American cockroach (Periplaneta americana) neurons, we were unable to maintain responses to GABA over relatively short per-iods of time (e.g. a few minutes). Regardless of the con-centration of GABA applied, the duration of the appli-cation, or the interval between GABA applications, GABA responses progressively declined, eventually reaching undetectable levels. This led us to examine what factors might retard this loss of GABA function in cockroach neurons. In the present report, we detail the influence of adenine and guanine nucleotides on GABA response stability. Portions of this report have previously been presented in abstract form (Watson and Salgado, 1995).
2. Methods
The sixth abdominal ganglia of male American cock-roaches were removed, desheathed, and treated with col-lagenase (Sigma type IA, 1 mg/ml, dissolved in cock-roach bicarbonate saline without Ca2+
) for 1 h. Ganglia were then washed three times with normal cockroach saline and triturated through a series of pipettes with pro-gressively smaller tip diameters in order to dissociate the neuron cell bodies. Neurons were allowed to settle to the bottom of a 30 mm diameter plastic petri dish for a minimum of 30 min before use.
Neurons were constantly perfused with cockroach bicarbonate saline (20°C) containing (in mM): NaCl (210), KCl (3), CaCl2 (5.4), NaH2PO4 (0.5), NaHCO3 (2.1). The whole-cell patch clamp technique (Hamill et al., 1981) was used for recording GABA-induced cur-rents. Whole-cell currents were recorded using a HEKA EPC-9 patch clamp amplifier controlled by Pulse+ Puls-efit software (ALA Scientific Instruments, Westbury, NY). Recording electrodes (3–7 MV) were made from glass micropipettes pulled on a DMZ Universal Puller (Dagan Corp., Minneapolis, MN), and filled with intra-cellular solution containing (in mM): K-gluconate (100), KF (50), MgCl2(10), CaCl2(1), EGTA (11) and HEPES (10), pH 7.2. In many experiments, additional com-pounds were added to the intracellular medium as detailed in the text.
Cells used in this study were typically less than 20
µm in diameter and usually had few, if any, remaining processes. Whole-cell GABA-induced currents were initiated by a 100 ms pressure pulse of 1024 M GABA from a patch pipette positioned approximately 30–50µm from the neuron using a Picospritzer II pressure pulse system (General Valve Corp., Fairfield, NJ). GABA was dissolved in normal cockroach bicarbonate saline. Hold-ing potential was2100 mV. Data were stored and
ana-lyzed by a personal computer, using Pulse+PulseFit analysis software, and GABA responses were measured at peak current.
GABA, adenosine 59-triphosphate (ATP, potassium salt), adenosine 59-O-(3-thiotriphosphate) (ATPγS, tetra-lithium salt), adenosine 59-diphosphate (ADP, potassium salt), β,γ-imidoadenosine 59-triphosphate (AMP-PNP, lithium salt), collagenase (type IA), and microcystin LR were obtained from Sigma Chemical Co. (St Louis, MO). Okadaic acid was obtained from Research Bio-chemicals, Inc. (Natick, MA).
Data are expressed as percent of the initial response to GABA (mean ±SEM where applicable). Each graph point represents the mean response of $4 neurons, except where indicated. When warranted, statistical comparisons were made using an unpaired Student’s t-test.
3. Results
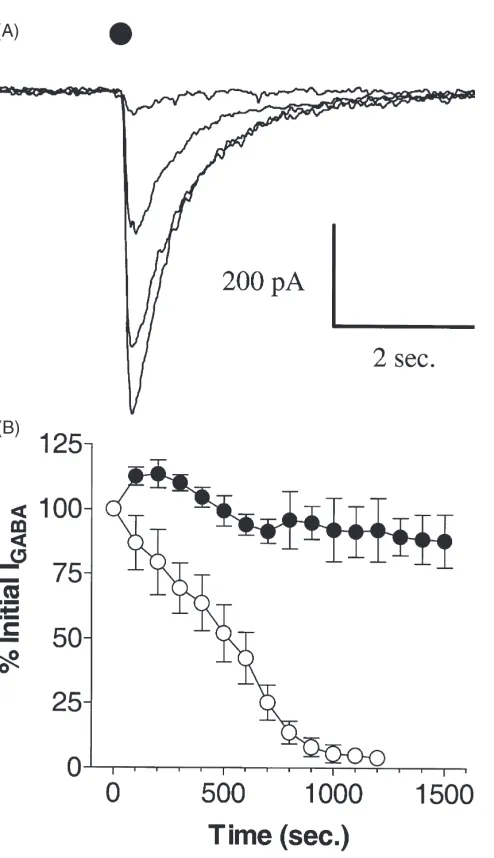
Pulses of 100µM GABA at 100 second intervals led to a progressive decline in GABA response amplitude (Fig. 1). Within a relatively short period of time (10–15 min), GABA responses were often below the limits of detection. When ATP was included in the intracellular medium, however, rundown was slowed [Fig. 1(B)]. In the presence of 5 mM ATP internally (ATPi), GABA responses 20 min after the initial application of GABA were not significantly different from the initial GABA response [Fig. 1(B)]. This effect was concentration-dependent, with 1 and 2 mM ATPiblocking GABA run-down less effectively than 5 mM ATP, and 0.5 mM ATPi providing no block of rundown (Fig. 2).
The slowing of GABA response rundown by ATP suggested that phosphorylation might be involved in the regulation of GABA receptor function. Therefore, we next examined the effects on GABA response rundown of factors that might be expected to alter phosphoryl-ation. Addition of the protein phosphatase inhibitor oka-daic acid (1.2 µM) to the intracellular medium, in the absence of ATPi delayed, but did not block GABA response rundown (Fig. 3). Similar results were obtained with another protein phosphatase inhibitor, microcystin LR (0.5µM, data not shown).
non-Fig. 1. (A) Current traces of every third GABA response during puls-ing at 100 s intervals shows the progressive decline in current ampli-tude. Dot shows the time of GABA (1024M) application. (B) Plot of
peak GABA responses over time in the absence of added internal ATP (s, n=5) and the presence of 5 mM ATP (P, n=5).
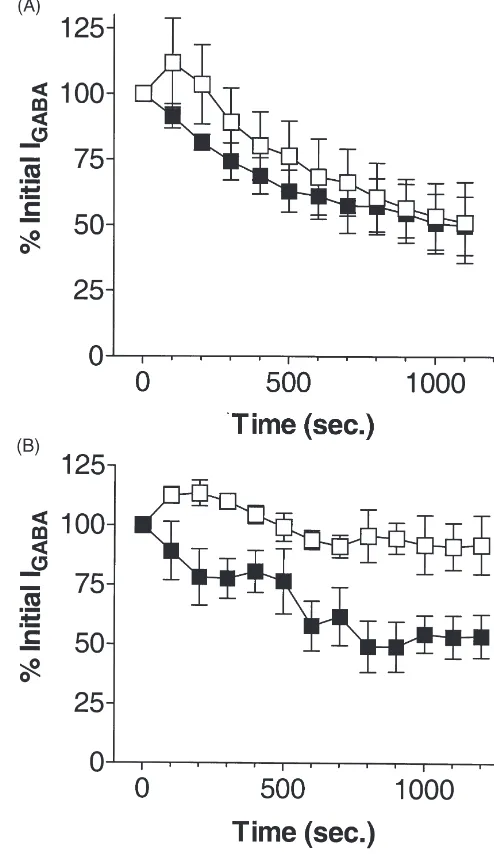
hydrolyzable ATP analog, AMP–PNP (5 mM) had little effect on GABA response rundown [Fig. 4(B)].
The ability of 5 mM ADP to block GABA response rundown was not affected by the addition of 45µM pro-tein kinase A inhibitory peptide (PKI, ‘Walsh peptide’) to the intracellular solution [Fig. 5(A)]. This concen-tration of PKI did, however, impair the ability of 5 mM ATP to block GABA response rundown [Fig. 5(B)]. Rundown in the presence of 5 mM ATP/ PKI resembled that observed in the presence of 5 mM ADP alone [Fig. 5(B)].
Guanine nucleotides did not block GABA response rundown. Neither GTP (5 mM) nor GDP (5 mM) pre-vented the loss of GABA responses, in the absence of ATPI(data not shown).
Fig. 2. The rundown of GABA-induced currents was slowed by the inclusion of ATP in the intracellular medium. Data are expressed as the average GABA response from cockroach neurons after 20 min (1200 s) of whole-cell recording. For each point, n$4, except where indicated.
Fig. 3. In the absence of added ATP, the phosphatase inhibitor, oka-daic acid (P, 1.2µM, added to the intracellular medium) slowed the rate of GABA response rundown relative to control responses (s). For each point, n$4. *P,0.05.
4. Discussion
Fig. 4. (A) GABA response rundown was nearly completely blocked 5 mM ATPγS (s). For comparison, data for 5 mM ATP, replotted from Fig. 1(B), are also shown (P). (B) ADP (s, 5 mM) was less effective than ATP (P, 5 mM, data replotted from Fig. 1(B) for comparison) in blocking GABA response rundown. AMP–PNP (j, 5 mM) did not deter GABA response rundown. For each point, n$4.
referred to as rundown, and is due, at least in part, to the loss of normal intracellular factors as they are replaced by the intracellular dialysate. We therefore sought to determine what important factors needed to be added to the intracellular medium in order to restore GABA receptor function.
Vertebrate and insect GABA receptors share many pharmacological properties (see reviews by Lummis, 1990; Sattelle et al., 1991). Since the phosphorylation state of the vertebrate GABA receptor is known to powerfully influence receptor function, we felt that it was reasonable to hypothesize that phosphorylation
Fig. 5. (A) GABA responses in the presence of 5 mM ADP (j, data replotted from Fig.4(B)) were unaffected by the inclusion of protein kinase A inhibitory peptide (h, PKI, 45 µM). (B) GABA responses in the presence of 5 mM ATP (h, data replotted from Fig. 1(B)) were partially inhibited by PKI (j, 45µM). For each point, n$4.
might exert a similar influence upon insect GABA recep-tor function.
conjunction with the ATP studies discussed below, sup-port a role for phosphorylation in the maintenance of cockroach GABA receptor function.
ATP and its thiophosphate analog, ATPγS, blocked cockroach GABA response rundown. This is not entirely surprising since putative insect GABA receptor subunits contain consensus sequences for phosphorylation (ffrench-Constant et al., 1991; Henderson et al., 1993; Harvey et al., 1994). However, the ability of ADP to slow GABA response rundown was unexpected, as ADP is thought to be incapable of serving as a substrate for phosphorylation. Furthermore, the block of rundown of GABA responses by ADP was unaffected by PKI, sug-gesting that if ADP does serve as a substrate for receptor phosphorylation, it is not mediated by PKA. GABA response rundown in the presence of 5 mM ATP/PKI was virtually identical to that seen in the presence of 5 mM ADP alone, suggesting a dual role for ATP itself in GABA receptor maintenance: a PKA-dependent receptor phosphorylation and a PKA-independent mechanism. The PKA-independent mechanism could be an intra-cellular nucleotide regulatory site, unrelated to phos-phorylation, and similar to that proposed by Akaike (1992) for GABA receptors of rat nucleus tractus solitar-ius (NTS) neurons. In NTS neurons, an interaction of the β-phosphate of adenine nucleotides with the GABA receptor appears necessary for maintaining GABA receptor function (Akaike, 1992). Our data support the possibility that a similar interaction between theβ -phos-phate of adenine nucleotides and cockroach GABA receptors might also serve to prevent GABA response rundown.
Millimolar concentrations of adenine nucleotides were required to prevent GABA response rundown in cock-roach neurons. Likewise, millimolar concentrations of ATP are required to prevent GABA response rundown in vertebrate neurons (e.g. review by Stelzer, 1992). These concentrations are probably physiologically relevant, as rat brain neurons have been shown to contain millimolar concentrations of intracellular ATP (cf Lehninger, 1982). Since GTP and GDP did not affect GABA response run-down under the conditions employed in these studies, intracellular mechanisms that regulate GABA receptor function appear to be specific for adenine nucleotides.
The physiological role of cockroach GABA receptor maintenance by adenine nucleotides is not known. How-ever, as with the mammalian GABA receptor, the pres-ence of intracellular regulatory sites suggests that the maintenance of insect GABA receptor function is rather complex, involving both extracellular and intracellular factors.
A recent whole-cell patch-clamp study of the GABA pharmacology of dissociated, embryonic cockroach neu-rons employed intracellular EGTA, but no intracellular ATP (Aydar and Beadle, 1999). No mention of the stab-ility of GABA responses was made, but given the
author’s detailed pharmacological study, it is unlikely that responses were transient in nature. If not, then this might suggest differences between the intracellular regu-lation of adult and embryonic cockroach GABA recep-tors.
There are a number of electrophysiological studies on insect GABA responses where there are distinct differ-ences in GABA pharmacology (e.g. Lees et al., 1987; Hue, 1998). For example, there appear to be both picro-toxin-sensitive and picrotoxin-insensitive receptor sub-types in cockroach (Hue, 1998). Similarly, it is likely that, like their vertebrate counterparts, insect GABA receptor subtypes are differentially regulated by intra-cellular factors. Millar et al. (1994) found that homo-oligomeric Drosophila GABA receptors (Rdl) stably expressed in a Drosophila cell line required the intra-cellular presence of the Ca2+ chelator EGTA (10 mM) to maintain consistent GABA responses. In the present study, intracellular EGTA was held constant at 11 mM and GABA responses were found to nonetheless run-down in the absence of adenine nucleotides. This might suggest that native insect GABA receptors are differen-tially regulated relative to homo-oligomeric Rdl GABA receptors. Further, this suggests that the modulation of GABA responses by adenine nucleotides may involve sites on non-Rdl GABA receptor subunits.
In summary, we have demonstrated that GABA recep-tor function in small diameter cockroach neurons from the sixth abdominal ganglion is maintained by adenine nucleotides. Phosphorylation of the GABA receptor by PKA appears to account for part of this maintenance of function. However, the β-phosphate of adenine nucleo-tides also appears to participate in the maintenance of GABA receptor function by a PKA-independent mech-anism. To the best of our knowledge, this study rep-resents the first demonstration of the regulation of insect GABA receptor function by adenine nucleotides.
Acknowledgements
We would like to thank N. Orr, P. Ripa, A. Schmidt, J. Sheets, and T. Sparks for helpful discussions and com-ments.
References
Akaike, N., 1992. Modulations of GABAA response by intracellular
ATP in rat CNS neurons. In: Yoshida, H., Ui, M. (Eds.), Neuro-transmitter Receptors and Intracellular Signaling. Excerpta Medica, Amsterdam, pp. 19–29.
Aydar, E., Beadle, D.J., 1999. The pharmacological profile of GABA receptors on cultured insect neurones. J. Insect Physiol. 45, 213– 219.
Chen, Q.X., Stelzer, A., Kay, A.R., Wong, R.K.S., 1990. GABAA
dis-sociated guinea-pig hippocampal neurons. J. Physiol. (L.) 420, 207–221.
Ffrench-Constant, R.H., Mortlock, D.P., Shaffer, C.D., MacIntyre, R.J., Roush, R.T., 1991. Molecular cloning and transformation of cyclodiene resistance in Drosophila: an invertebrate GABAA
recep-tor locus. Proc. Natl. Acad. Sci. USA 88, 7209–7213.
Girault, J.-A., 1993. Protein phosphorylation and dephosphorylation in mammalian central nervous system. Neurochem. Int. 23, 1–25. Gratecos, D., Fischer, E.H., 1974. Adenosine 59-O(3-thiotriphosphate)
in the control of phosphorylase activity. Biochem. Biophys. Res. Commun. 58, 960–967.
Gyenes, M., Farrant, M., Farb, D.H., 1988. ‘Run-down’ ofγ -aminobu-tyric acidAreceptor function during whole-cell recording: a
poss-ible role for phosphorylation. Mol. Pharmacol. 84, 719–722. Hamill, O., Marty, A., Neher, E., Sakmann, B., Sigworth, F.J., 1981.
Improved patch-clamp techniques for high resolution current rec-ording from cell-free membrane patches. Pflu¨g. Arch. Ges. Physiol. 391, 85–100.
Harvey, R.J., Schmitt, B., Hermans-Borgmeyer, I., Gundelfinger, E.D., Betz, H., Darlison, M.G., 1994. Sequence of a Drosophila ligand-gated ion-channel polypeptide with an unusual amino-terminal extracellular domain. J. Neurochem. 62, 2480–2483.
Henderson, J.E., Soderlund, D.M., Knipple, D.C., 1993. Characteriz-ation of a putativeγ-aminobutyric acid (GABA) receptorβsubunit gene from Drosophila melanogaster. Biochem. Biophys. Res. Commun. 193, 474–482.
Hue, B., 1998. A picrotoxin-resistant GABA-gated chloride channel receptor subtype in the cockroach central nervous system. Arch. Insect Biochem. Physiol. 37, 231–238.
Lees, G., Beadle, D.J., Neumann, R., Benson, J.A., 1987. Responses to GABA by isolated insect neuronal somata: pharmacology and modulation by a benzodiazepine and a barbiturate. Brain Res. 401, 267–278.
Lehninger, A.L., 1982. The ATP cycle and cell bioenergetics. In: Lehninger, A.L. (Ed.), Principles of Biochemistry. Worth Pub-lishers, New York, pp. 361–396.
Lummis, S.C.R., 1990. GABA receptors in insects. Comp. Biochem. Physiol. 95, 1–8.
Marty, A., Neher, E., 1983. Tight-seal whole-cell recording. In: Sak-mann, B., Neher, E. (Eds.), Single-Channel Recording. Plenum Press, New York, pp. 107–122.
Millar, N.S., Buckingham, S.D., Sattelle, D.B., 1994. Stable expression of a functional homo-oligomeric Drosophila GABA receptor in a
Drosophila cell line. Proc. R. Soc. Lond. B. 258, 307–314.
Sattelle, D.B., Lummis, S.C.R., Wong, J.F.H., Rauh, J.J., 1991. Phar-macology of insect GABA receptors. Neurochem. Res. 16, 363– 374.
Sieghart, W., 1995. Structure and pharmacology of γ-aminobutyric acidAreceptor subtypes. Pharmacol. Rev. 47, 181–234.
Stelzer, A., 1992. Intracellular regulation of GABAA-receptor function.
In: Narahashi, T. (Ed.), Ion Channels, vol. 3. Plenum Press, New York, pp. 83–136.
Stelzer, A.A., Kay, A.R., Wong, R.K.S., 1988. GABAA-receptor
func-tion in hippocampal cells is maintained by phosphorylafunc-tion factors. Science 241, 330–341.
Walaas, S.V., Greengard, P., 1991. Protein phosphorylation and neu-ronal function. Pharmacol. Rev. 43, 299–349.
Walsh, D.A., Glass, D.B., 1991. Utilization of the inhibitor protein of adenosine cyclic monophosphate-dependent protein kinase, and peptides derived from it, as tools to study adenosine cyclic mono-phosphate-mediated cellular processes. Meth. Enzymol. 201, 304–316.