www.elsevier.com / locate / bres
Action of carbamazepine on epileptiform activity of the verartidine
model in CA1 neurons
a b ,
*
Sameer A. Otoom , Karim A. Alkadhi
a
Jordan University of Science and Technology, Faculty of Medicine, Department of Pharmacology, Irbid, Jordan
b
Department of Pharmacological and Pharmaceutical Sciences, University of Houston, Houston, TX 77204-5515, USA
Accepted 29 August 2000
Abstract
The veratridine epileptiform model was utilized to assess the antiepileptic effect of Carbamazepine (CBZ) in rat hippocampal CA1 pyramidal neurons using conventional intracellular recording techniques. In the veratridine model, where brain slices are treated with veratridine (0.3 mM), a single intracellular stimulus evokes epileptiform bursting. Additionally, spontaneous epileptiform activity commonly appears on prolonged exposure to veratridine in this model. In this model, therapeutic (7–15 mM) and high (50 mM) concentrations of CBZ inhibited the evoked and spontaneous epileptiform bursting in a concentration- and voltage-dependent manner. At all concentrations tested, CBZ produced inhibition of epileptiform activity without affecting the membrane resting potential or input resistance. However, at 50mM, the drug increased the firing threshold of neurons. These results confirm the suitability of this model for testing sodium channel-dependent antiepileptic agents. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Veratridine; Carbamazepine; Spontaneous bursting; Evoked bursting; Brain slice; Therapeutic; Hippocampus
1. Introduction ment leads to induction of bursting and development of
negative slope resistance [24]. Additionally, veratridine Voltage-dependent sodium channels are involved in the blocks synaptic transmission as indicated by inhibition of pathogenesis of different diseases such as epilepsy, is- the evoked population spike in the CA1 region of the chemic brain damage and cardiac arrhythmias [19]. The hippocampus [15]. We also showed that veratridine evoked voltage-dependent sodium channel is suggested to possess epileptiform activity even when synaptic transmission was a variety of active sites grouped into six major classes that completely blocked with the glutamate receptor antagonist, have been characterized pharmacologically by using a kynurenic acid [24].
number of remarkable toxins that selectively bind to these Carbamazepine (CBZ), which is related structurally to sites [4]. One of these toxins is the lipid-soluble alkaloid, imipramine, has been used for the treatment of focal as veratridine, which binds to site 2 in the channel. We have well as generalized tonic-clonic seizures [14]. The drug is used veratridine to develop a model of epilepsy known as also used in the treatment of trigeminal neuralgia and in the veratridine model. In this model, veratridine induces lithium-resistant manic-depressive patients [6]. Although epileptiform activity in brain slices by modification of the mechanism of action of CBZ is not fully understood, it sodium channel function [15]. Veratridine promotes sodium is widely accepted that the drug produces its antiepileptic channel opening by slowing its inactivation mechanism effect by blocking sodium channels which reduces the and by shifting the voltage-dependence of the activation to ability of neurons to conduct high frequency impulse flow a more hyperpolarized state [9]. We have shown that [12]. Moreover, CBZ is also known to have a variety of veratridine enhances the slowly inactivating sodium cur- other actions including inhibiting the reuptake of norepi-rent in hippocampal pyramidal neurons [1]. This enhance- nephrine, and blocking adenosine and N-methyl-D
-aspar-tate (NMDA) receptors. However, it is not clear if these mechanisms are related to its antiepileptic effect [26].
*Corresponding author. Tel.: 11-713-743-1212; fax: 1
1-713-743-The present study was designed to ascertain both the
1229.
E-mail address: [email protected] (K.A. Alkadhi). usefulness of the veratridine model as a
amplitude of more than 60 mV. Potentials were amplified
2. Materials and methods (Axoclamp-2A, Axon Instruments Inc.) and stored on
video tapes (Video Tape data recorder, A. R. Vetter Co.)
2.1. Electrophysiology for later analysis.
Ohm’s law was used to calculate membrane input Electrophysiological experiments were performed on resistance, which was determined by passing a hyper-brain slices from male Sprague–Dawley rats (Harlan polarizing current (0.2 nA, 200 ms duration) through the Sprague–Dawley Inc, Indianapolis) weighing 150–200 g. stimulating electrode and then measuring the potential The rats were housed in cages (no more than six rats / cage) changes across the membrane. The firing threshold is at standard 12-h light / 12-h dark cycle. The rat was quickly measured by determining the intracellular threshold current decapitated with a small animal guillotine. The skull bone intensity required for evoking an action potential or a was removed by a small bone ronjours and the dura was burst.
carefully cut with small scissors. A stainless steel spatula
was used to lift the brain out of the skull. The brain was 2.2. Drugs and chemicals gently transferred to a petri-dish filled with ice-cold
oxygenated (95% O , 5% CO ) artificial cerebrospinal2 2 All the chemicals were obtained from Sigma Chemical fluid (ACSF). The brain was divided midsagittally into two Company. Veratridine was dissolved in 0.1 mM HCl. The hemispheres. From each hemisphere, a transverse block final concentration of CBZ was prepared by adding a containing the hippocampal tissue was dissected. This calculated amount of stock solution to ACSF. Application block was affixed onto a slicer stage using cyanoacrylate of the drugs to the hippocampal slices was performed glue. Transverse slices (four to five slices from each through a superfusion system by switching a three-way hemisphere) of 500 mm thickness were cut using a stopcock from the flask that contains the ACSF to the flask vibroslice (Campden Instruments Ltd., London, UK). containing the appropriate drug solution. The composition Before placement in the recording chamber, the slices were of the ACSF used in the hippocampal slice preparation was allowed to recover at room temperature for 3–4 h in a (mM): NaCl, 127; CaCl , 2.5; KCl, 4.7; MgCl , 1.2;2 2
beaker filled with ACSF solution continuously gassed with NaHCO , 22 and NaH PO , 1.2; Glucose, 11.0. The pH of3 2 4
95% O –5% CO2 2 mixture. The recording chamber, this solution was kept at 7.4. fashioned from sylastic gel, consisted of a circular bath of
low volume (,1 ml) with an inlet connected to a 2.3. Statistical analysis polyethylene tube that delivered the superfusate and an
outlet for the drainage of the solution by gravity. This Data were expressed as mean6S.E.M. Statistical analy-chamber was designed to immobilize the brain slice by sis was performed on computer (GB Stat 3.0) using one-holding it between two nylon nets in order to obtain stable way analysis of variance (ANOVA) or paired t-test. P electrophysiological recordings. Solution reached the value of,0.05 was considered statistically significant. circular bath through a polyethylene tube that passed
through a water jacket connected to a heater circulator
(Lauda C3, model T-1). The temperature of the ACSF was 3. Results
thus maintained at 32618C. A fiber-optic illuminator
illuminated from below the chamber. The entire recording Single action potentials were evoked by brief intracellu-system was housed in a grounded Faraday cage on a lar current pulses (20 ms, 0.2–0.5 nA) in CA1 neurons. Micro-G air suspension table. After the recovery period, a The veratridine model was generated by superfusing a single slice was transferred to the recording chamber and brain slice with 0.3mM veratridine which changed evoked, trapped between the two nylon nets. The submerged brain single action potentials into bursts within 30 min. Superfu-slice was superfused with warm, continuously oxygenated sion of CBZ on bursting cells in the veratridine model ACSF. Intracellular recordings were performed using produced a concentration-dependent inhibition of the fre-microelectrodes filled with 4 M potassium acetate and had quency of epileptiform discharge (number of action po-a tip resistpo-ance of 80–120 MV. The microelectrodes used tentials in a burst, Fig. 1A). Calculated from concen-in these experiments were made from capillary tubes (1.0 tration–response curve, the concentration of CBZ that
mm, Kwik-fil, WPI) pulled by an electrode puller produced a 50% reduction in the number of action
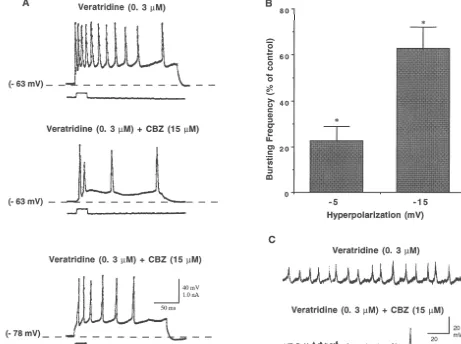
Fig. 1. (A) Concentration-dependent inhibition of epileptiform discharge by CBZ. Bursting frequency (number of action potentials evoked by a single intracellular current pulse (0.5 nA / 20 ms) is expressed as a percentage of that in the presence of veratridine (0.3mM) alone. Bars represent the means from six neurons. Error lines are S.E.M., * indicates significant difference from the control (one way ANOVA, P,0.05, n56). (B) Traces from a representative experiment. Injecting a DC current evoked a single action potential. The same current evoked epileptiform activity after treatment of the neuron with 0.3
mM veratridine. Application of 15 mM CBZ inhibited the burst. The bursting was reversed after washing CBZ with veratridine-containing ACSF. Calibration bars apply to all traces. (C) CBZ significantly (paired t-test, P,0.05, n57) increased the intracellular activation threshold.
resulted in recurrence of bursting (Fig. 1B). The drug, at CBZ (15 mM) produced inhibition of the evoked dis-all concentrations used, had no significant effect on the charge. Hyperpolarization of the neuronal membrane by 5 membrane resting potential or input resistance (not shown, or 15 mV through intracellular injection of negative one way ANOVA, P.0.05) but significantly increased the currents reversed the inhibitory effect of CBZ revealing the firing threshold of neurons only at 50 mM (Fig. 1C). veratridine bursting (Fig. 2A). Fig. 2B summarizes the The inhibitory effect of CBZ was tested for sensitivity to normalized bursting frequency of CBZ in response to changes in membrane potential. In four experiments, after membrane hyperpolarization. There was a significant volt-the development of epileptiform activity, superfusion of age-dependent return of the veratridine-induced bursting
Table 1
a
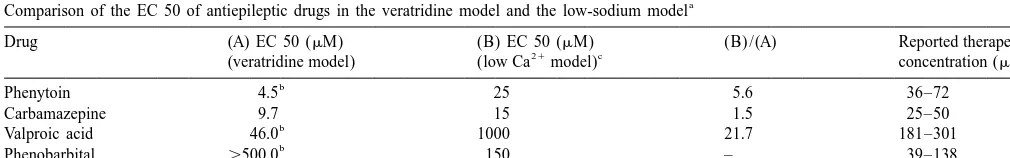
Comparison of the EC 50 of antiepileptic drugs in the veratridine model and the low-sodium model
Drug (A) EC 50 (mM) (B) EC 50 (mM) (B) /(A) Reported therapeutic
21 c d
(veratridine model) (low Ca model) concentration (mM)
b
Phenytoin 4.5 25 5.6 36–72
Carbamazepine 9.7 15 1.5 25–50
b
Valproic acid 46.0 1000 21.7 181–301
b
Phenobarbital .500.0 150 – 39–138
b
Ethosuximide ** 1000 – 284–709
a
At 500mM, phenobarbital produced about 40% inhibition of epileptiform discharge. ** Produced no inhibition even at 10 mM.
b
From Ref. [17].
c
From Ref. [20].
d
Fig. 2. Voltage-dependent inhibition of bursting by CBZ. (A) Traces from a single neuron recorded in the presence of veratridine (0.3mM, upper trace). At the resting membrane potential (263 mV), CBZ (15mM, n54) induced inhibition 60 min after superfusion (middle trace). The CBZ-induced inhibition was reversed when the neuronal membrane was hyperpolarized (15 mV) by intracellular injection of anodal current (lower trace). Dashed lines represent membrane potentials; calibration bars apply to all traces. (B) Voltage-dependent reversal of CBZ-induced inhibition of bursting. Bursting frequency (number of action potentials evoked by a single intracellular current pulse of 0.5 nA / 20 ms) increased after hyperpolarization of the membrane by 5 and 15 mV in the presence of CBZ (15mM). Bars represent the means of six neurons. Vertical lines are S.E.M., * indicates significant difference from veratridine (control) bursting frequency (one way ANOVA, P,0.05). (C) One of three experiments where the effect of CBZ on veratridine-induced spontaneous bursting was tested. Spontaneous bursting activity appeared 45–60 min after treatment with 0.3mM veratridine (upper trace). The application of CBZ (15
mM) blocked this epileptic activity (lower trace). The chart recorder truncated the spikes. Calibration bars apply to both traces.
frequency with hyperpolarization (one way ANOVA, P, inhibiting sodium channel function are potently effective in
0.05). blocking epileptiform activity in this model (Table 1).
The effect of CBZ was also tested on spontaneous However, antiepileptic drugs that do not inhibit sodium bursting which commonly develops in the veratridine channel function, such as phenobarbital and ethosuximide, model. Superfusion of CBZ (15mM) blocked spontaneous are ineffective in blocking the epileptiform activity in this bursting indicating sensitivity of this kind of epileptiform model [17].
activity to CBZ (Fig. 2C). Various studies indicate the involvement of sodium
channels in the mechanism of action of CBZ. Voltage-clamp experiments in Myxicola giant axon [21] and
4. Discussion Xenopus myelinated nerve [22] showed that CBZ inhibited
The antiepileptic profile of CBZ was studied in different phenytoin and valproic acid, the present results show models of epilepsy. The drug inhibited spontaneous long epileptiform activity in the veratridine model to be sensi-bursting induced by the potassium channel blocker 4- tive to inhibition with therapeutic concentrations of CBZ. aminopyridine in rat hippocampal slices [25]. It was also The inhibition is concentration- and voltage-dependent and known to partially decrease the duration of the excitatory occurs without affecting the resting potential or input postsynaptic potentials (EPSP) in the CA1 region of the resistance of the neuronal membrane. The veratridine hippocampus. In the magnesium-free model of epilepsy, model is a more specific preparation, than the low calcium activity was inhibited by CBZ in a dose-dependent manner model for screening of potential antiepileptic drugs, espe-[18]. The drug also suppressed EPSP induced by activation cially those with sodium channel blocking property. of NMDA receptors at concentrations that did not affect
synaptic transmission [7]. Epileptiform activity induced in
1 21
rat hippocampal slices by elevating K and lowering Ca References
21
and Mg was inhibited by CBZ at clinically relevant
doses [11]. Moreover, CBZ inhibited penicillin-induced [1] K.A. Alkadhi, L.M. Tian, Veratridine-enhanced persistent sodium after-discharge in immature rat CA3 pyramidal cells [23]. current induces bursting in CA1 pyramidal neurons, Neuroscience
71 (1996) 625–632.
When comparing the effect of phenytoin, valproic acid,
[2] C.M. Armstrong, G. Cota, Calcium ion as a cofactor in Na channel
CBZ, phenobarbital, ethosuximide and midazolam on
gating, Proc. Natl. Acad. Sci. USA 88 (1991) 6528–6531.
spontaneous bursts, CBZ was found to be the most
[3] D. Ashton, R. Willems, E. de Prins, A. Wauquier, Field-potential
effective in blocking epileptiform activity in the low assay of antiepileptic drugs in the hippocampal slice, Epilepsia 29
calcium model [8,20]. (1988) 321–329.
The veratridine model shares a number of characteristics [4] W.A. Catterall, Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes, Annu. Rev. Pharmacol. Toxicol.
with the low calcium model: (a) both models involve
20 (1980) 15–43.
modification of sodium channels [2,10]; (b) evoked as well
[5] D.A. Coulter, Antiepileptic drug cellular mechanisms of action:
as spontaneous bursting can be produced in both models; where does lamotrigine fit in?, J. Child Neurol. 12 (Suppl 1) (1997) and (c) both are synaptic-independent. The veratridine S2–S9.
model, however, seems to be more specific than the low [6] C.L. Faingold, R.A. Browning, Mechanisms of anticonvulsant drug action I. Drugs primarily used for generalized tonic-clonic and
calcium model for ascertaining whether the antiepileptic
partial epilepsies, Eur. J. Pediatr. 146 (1987) 2–7.
effect of a drug is due to sodium channel block. Unlike in
[7] P.W. Gean, C.C. Huang, J.R. Kuo, J.H. Lin, P.L. Yi, J.J. Tsai,
the low calcium model, in the veratridine model the most Analysis of carbamazepine’s anticonvulsant actions in hippocampal effective antiepileptic drug is phenytoin (Table 1) whose and amygdaloid slices of the rat, Chin. J. Physiol. 36 (1993)
mechanism of antiepileptic action is widely accepted as 199–204.
[8] U. Heinemann, S. Franceschetti, B. Hamon, A. Konnerth, Y. Yaari,
due to sodium channel inhibition [5]. Additionally, the EC
Effects of anticonvulsants on spontaneous epileptiform activity
50s of these drugs are invariably smaller in the veratridine
which develops in the absence of chemical synaptic transmission in
model than those reported in the low calcium model (Table hippocampal slices, Brain Res. 325 (1985) 349–352.
1). In the supposedly synaptic-independent low calcium [9] B. Hille, Modifiers of gating, in: Ionic Channels of Excitable
model, drugs whose mechanism of antiepileptic action is Membranes, 2nd Edition, Sinauer Associates, Sunderland, MA, 1992, 445–470.
known to depend on the presence of synaptic transmission
[10] J.G. Jefferys, H.L. Haas, Synchronized bursting of CA1
hippocam-such the barbiturates, ethosuximide and the
benzodiaze-pal pyramidal cells in the absence of synaptic transmission, Nature
pines, are effective in blocking bursting activity [3,8,20]. 300 (1982) 448–450.
However, the same antiepileptic drugs are not effective in [11] A. Leschinger, J. Stabel, P. Igelmund, U. Heinemann,
Pharmaco-blocking epileptiform discharge in the veratridine model logical and electrographic properties of epileptiform activity induced
1 21 21
by elevated K and lowered Ca and Mg concentration in rat
[17].
hippocampal slices, Exp. Brain Res. 96 (1993) 230–240.
In the low calcium model, valproic acid was found to be
[12] R.L. Macdonald, K.M. Kelly, Mechanisms of action of currently
either active at concentrations much higher than the prescribed and newly developed antiepileptic drugs, Epilepsia 35 therapeutic level [8,20] or completely inactive [3]. In the (1994) S41–50.
veratridine model on the other hand, we found valproic [13] J.O. MacNamara, Drugs effective in the therapy of the epilepsies, in: J.G.A. Hardman, L.E. Limbird (Eds.), The Pharmacological Basis of
acid to be consistently effective at concentrations within
Therapeutics, McGraw-Hill, New York, 1996, pp. 461–486.
the lower limits of the therapeutic plasma levels (Table 1
[14] M.J. McLean, R.L. Macdonald, Carbamazepine and
10,11-epox-[17],). In fact, in the veratridine model, when VPA was ycarbamazepine produce use- and voltage-dependent limitation of used at higher concentrations (500 mM), it produced rapidly firing action potentials of mouse central neurons in cell proepileptic action. Such proepileptic effect of VPA was culture, J. Pharmacol. Exp. Ther. 238 (1986) 727–738.
[15] S. Otoom, L.M. Tian, K.A. Alkadhi, Veratridine-treated brain slices:
not seen in the magnesium-free model, bicuculline model
a cellular model for epileptiform activity, Brain Res. 789 (1998)
[16], or the low calcium model [20]. Thus, the veratridine
150–156.
model can be useful for screening sodium channel-active [16] S.A. Otoom, K.A. Alkadhi, Valproic acid intensifies epileptiform drugs for both antiepileptic and proepileptic effects. activity in the hippocampal pyramidal neurons, Neurosci. Res. 35
Pharmacol. 339 (1989) 613–616. [24] L.M. Tian, S. Otoom, K.A. Alkadhi, Endogenous bursting due to [19] B. Ricard-Mousnier, F. Couraud, Role of voltage-dependent ion altered sodium channel function in rat hippocampal CA1 neurons,
channels in epileptogenesis, Neurophysiol. Clin. 23 (1993) 395– Brain Res. 680 (1995) 164–172.
421. [25] A.E. Watts, J.G. Jefferys, Effects of carbamazepine and baclofen on [20] G.M. Rose, H.R. Olpe, H.L. Haas, Testing of prototype antiepilep- 4-aminopyridine-induced epileptic activity in rat hippocampal slices,
tics in hippocampal slices, Naunyn Schmiedebergs Arch. Pharmacol. Br. J. Pharmacol. 108 (1993) 819–823.
332 (1986) 89–92. [26] M. Willow, E.A. Kuenzel, W.A. Catterall, Inhibition of voltage-[21] C.L. Schauf, F.A. Davis, J. Marder, Effects of carbamazepine on the sensitive sodium channels in neuroblastoma cells and synaptosomes ionic conductances of Myxicola giant axons, J. Pharmacol. Exp. by the anticonvulsant drugs diphenylhydantoin and carbamazepine, Ther. 189 (1974) 538–543. Mol. Pharmacol. 25 (1984) 228–234.