www.elsevier.nlrlocateraqua-online
White spot baculovirus syndrome in the Indian
shrimp Penaeus monodon and P. indicus
P.R. Rajan
a,1, P. Ramasamy
a,1, V. Purushothaman
b,
G.P. Brennan
c,)a
Department of Zoology, Life Sciences Building, UniÕersity of Madras, Chennai 600025, Madras, India b
Department of Microbiology, Madras Veterinary College, TANUVAS, Chennai 600007, Madras, India c
School of Biology and Biochemistry, The Queen’s UniÕersity of Belfast, 97 Lisburn Road, Belfast BT9 7BL
Northern Ireland, UK
Accepted 8 September 1999
Abstract
Ž .
Sporadic occurrences of white spot baculovirus WSBV infections have been reported in shrimp farms throughout the maritime states of India. WSBV presents as a reddish discolouration with white spots on the exoskeleton and epidermis with muscle opacity. Onset of the disease is extremely rapid with mass mortalities. Infected juveniles and sub-adults of Penaeus indicus and
P. monodon become lethargic surface frequently, exhibit loss of balance, with reduced feeding and
Ž .
preening activities. The nuclei of WSBV-infected epithelial hypodermal , septal and secretory cells of the gill filaments exhibit basophilic hypertrophied nuclei with a reduced cytoplasmic volume. Massive tissue disintegration occurred in the ectodermal and mesodermal tissues. The electron-dense nucleoplasm of the gill epithelial cells is mostly replaced with virions. Electron microscopic examination revealed the presence of double-enveloped, non-occluded, rod-shaped virions with a tube-like or branched extension and empty capsids. The numbers of mitochondria,
Ž .
endoplasmic reticulum ER and Golgi were also reduced, as were the numbers of secretory or storage vesicles. WSBV is considered to be the main causative agent responsible for mass mortalities of juveniles and sub-adults in the cultured Indian penaeid shrimp, P. monodon and P.
indicus. WSBV is highly pathogenic and readily transmitted from diseased shrimp to healthy
susceptible shrimp via, contaminated water, faeces and by scavenging on dead infected shrimp. It may affect all stages of shrimp. The spread of the disease from cultured to natural systems and vice versa cannot be dismissed.q2000 Elsevier Science B.V. All rights reserved.
Keywords: White spot baculovirus syndrome; Penaeus monodon; Endoplasmic reticulum
)Corresponding author. E-mail: [email protected] 1
E-mail: [email protected].
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
1. Introduction
Sporadic outbreaks of white spot virus-like infections have been encountered in cultured penaeid shrimp farms throughout the maritime states of India wiping out total stocks. Although the causative agent has not yet been clearly identified in the Indian penaeid shrimp, it was suspected to be systemic ectodermal and mesodermal baculovirus ŽRamasamy et al., 1995; Muralimanohar et al., 1996 . White spot syndrome was initially. reported in several species of shrimp in a number of Asian countries including India,
Ž
Indonesia, Taiwan and Thailand Chou et al., 1995; Wang et al., 1995; Lightner, 1996; Chang et al., 1996; Durand et al., 1996; Wongteerasupaya et al., 1996; Lo et al.,
.
1996a,b . The causative agent is thought to be a non-occluded white spot baculovirus ŽWSBV. ŽInouye et al., 1994; Momoyama et al., 1994; Nakano et al., 1994; Takahashi
.
et al., 1994; Chou et al., 1995; Wang et al., 1995; Durand et al., 1996 and has been reported in both cultured and wild Taiwan shrimp, in fresh water prawn, crabs, copepods
Ž .
and insects Lo et al., 1996b . WSBV is extremely virulent with rapid onset and is now emerging as the single most important threat to the future of the shrimp industry in Asia. The histopathology and characteristics of the WSBV appear similar to that of
Ž . Ž
Systemic mesodermal and ectodermal baculovirus SEMBV Wang et al., 1995; .
Wongteerasupaya et al., 1995; Takahashi et al., 1996; Lo et al., 1996a,b but differ from
Ž . Ž . Ž
Monodon baculovirus MBV and Baculovirus mid-gut necrosis BMN Sano et al., .
1984; Mari et al., 1993; Wongteerasupaya et al., 1995 . Although the clinical signs of Ž
WSBV have been described in Indian shrimp Anonymous, 1995; Karunasagar et al., .
1997 , no systematic histopathological, experimental infection nor ultrastructural studies of the WSBV virions have been described. Additionally, there have been conflicting
Ž
reports on the size and shape of the virus and the size of the genome Wongteerasupaya .
et al., 1995, 1996; Lo et al., 1996a . Some of the WSBV isolates were considered as
Ž Ž . . Ž
PmNOBII Penaeus monodon, non-occluded baculovirus NOB II Wongteerasupaya .
et al., 1995, 1996; Takahashi et al., 1996 while other isolates were described as
Ž .
PmNOBIII Wang et al., 1995; Karunsagar and Karunasagar, 1997 . Therefore, a need for further critical studies is apparent to determine the size, shape and the clinical–patho-logical signs of WSBV in both naturally and experimentally infected Indian shrimp.
2. Materials and methods
WSBV-infected juveniles and sub-adults of P. monodon and P. indicus were Ž
collected from a number of aquaculture farms located in Andhra Pradesh Gudur, Kota
. Ž
and Nellore and in Tamil Nadu Chidambaram, Muthupettai, Pattukottai Sirkhazi and
. Ž .
Tuticorin India Fig. 1 , between 1994 and 1999. Tissues from live, moribund WSBV-infected shrimp were prepared for light microscopy and transmission electron mi-croscopy. The tissues selected for investigation were the gills, lymphoid organ, hep-atopancreas, heart, stomach, mid-gut, hind-gut and nerve cord. For light microscopy,
Ž .
Fig. 1. South India map showing samplingU
-sites of WSBV-infected shrimp.
were cut and mounted on glass slides, stained with Harris’s haematoxylin and eosin and examined for pathological changes.
The specimens used for viral isolation and experimental infections were obtained from field studies in a shrimp farm in Tuticorin, Tamil Nadu, where an outbreak of WSBV had been detected in July 1997. Live, moribund WSBV-infected shrimp were collected, transported in dry ice and stored at y408C until required for viral isolation and experimental infection.
2.1. Viral isolation and purification
WSBV-infected tissues of P. monodon were ground into powder in the presence of
Ž .
liquid nitrogen. The virus was isolated using the method of Wang et al. 1995 . The viral Ž
pellet was resuspended in TE buffer 10 mM Tris – HCl and 1 mM
w x.
ethylenediaminetetra-acetic acid EDTA , pH 8.0 and stored at y408C until required for the confirmation of Rivers postulates.
2.2. Experimental-infection
To prove Rivers’ postulate, the following experiments were designed. Juveniles and sub-adults of P. monodon, P. indicus, P. semisulcatus and Metapenaeus dobsoni were
Ž .
collected from Muttukadu estuary, Chennai Madras and transported to the laboratory in aerated sea water. Brooders of P. indicus were collected from the Kovalam coast, Tamil Nadu, post-larval P. monodon were collected from a hatchery in Tamil Nadu and transported to the laboratory. All specimens were maintained in the laboratory in filtered
Ž . Ž .
seawater 28 to 32 ppt at an ambient room temperature 308C and fed with autoclaved pellet feed. After an acclimatization period of 48 h, with no obvious signs of infection or mortalities, the shrimp were experimentally infected with WSBV using a number of different procedures and observed up to 20 days for signs of post-infection morbidity, mortality and mode of transmission. The procedures employed are shown below.
Ž .1 Healthy shrimp at different stages of growth were fed a single meal of minced tissues of WSBV-infected P. monodon and subsequently fed with pellet feed.
Ž .2 WSBV-infected tissues 25 mg of P. monodon gnathothorax were homoge-Ž . Ž . nized in 80 ml Leibovitz L15medium, centrifuged and the supernatant filtered through a 0.45-mm Sartorius filter and the tissue filtrate was inoculated into filtered, sea water tanks containing different growth stages of healthy shrimp.
Ž .3 Faecal pellets from WSBV-infected shrimp were collected and inoculated into uninfected shrimp in tanks containing P. monodon, P. indicus and M. dobsoni.
Ž .
Fig. 2. Sixty-day-old WSBV-infected P. monodon, collected from Chidambaram. Note the white spots w on the carapace and abdominal segments. Scale bars2.2 cm.
Ž . Ž .
Fig. 3. a,b One hundred-day-old P. monodon collected from Tuticorin, showing white spots w on the
Ž . Ž .
carapace a and abdominal segments b . Scale bars1.7 cm.
Ž .
of the second abdominal segment of healthy P. monodon and P. indicus. Uninfected controls were maintained in filtered seawater.
Experimentally infected shrimp, which died or became moribund during the 20 day trial, were removed from the tanks and fixed in Davidson’s fixative for histological studies, the surviving shrimp were stored aty408C for further studies.
3. Results
In infected P. monodon and P. indicus, WSBV presents as a reddish body coloura-tion with characteristic white spots on the exoskeleton and epidermis with muscle
Ž .
opacity Figs. 2–4 . The epidermal cells of the stomach, gills, cuticular epidermis, lymphoid organ and heart of P. monodon and P. indicus exhibited hypertrophied nuclei
Ž .
during the early stages of viral infection Figs. 5–9 . The cell cytoplasm was still visible but became noticeably thinner as viral development progressed until it was fully transparent surrounding the nucleus. In the middle stage of virus development, the infected cells displayed hypertrophied nuclei with marginated basophilic chromatin. As infection progressed, the nucleus became enlarged and the staining reaction changed from acidophilic to basophilic. Eventually the nuclei disintegrated, leaving a large vacant area. Massive tissue disintegration was observed in the gills, stomach, lymphoid organ, heart, mid-gut, hind-gut and nervous tissues.
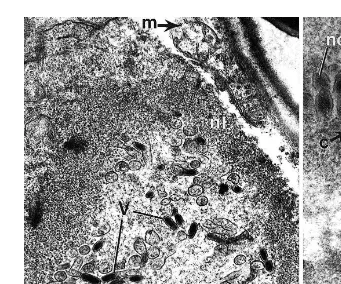
The hepatopancreas remained free of infection. Electron microscopic examination of white spot-diseased shrimp revealed the occurrence of a non-occluded, rod-shaped, double-enveloped WSBV some of which possessed a tube-like, or branched extension
Ž .
and empty capsids Figs. 10–15 . The virions measured 154"2 by 373"5 nm and
Ž . Ž
nucleocapsids 111"1.5 by 293"7 nm ns100 . WSBV-infected epithelial hypoder-.
mal cells, septal cells and secretory cells of the gill filaments exhibited hypertrophied nuclei with a reduced cytoplasmic volume. The numbers of mitochondria, endoplasmic
Ž .
reticulum ER and Golgi were also reduced, as were the numbers of secretory or
Fig. 5. Light photomicrograph of 60-day-old P. monodon collected from Sirkhazki, showing WSBV infected
Ž .
gill epithelial cells. Note an hypertrophied nucleus hn and considerable damage and degeneration of the gill
Ž .
tissue with numerous large vesicles unlabelled arrows . Scale bars15mm.
Ž . Ž .
Fig. 6. P. monodon, 30 day old, collected from Pattukotai, showing infected hn and uninfected un hypertrophied nuclei in the gill epithelial tissue. Degeneration of the gill epithelium is indicated by the unlabelled arrows. Scale bars10mm.
Fig. 7. P. monodon, 100 day old, collected from Tuticorin showing WSBV-infected lymphoid tissue
Ž .
exhibiting nuclear hypertrophy hn in the stromal matrix cells. Scale bars20mm.
Ž .
Fig. 8. Enlarged light photomicrograph image showing hypertrophied nuclei hn in the nerve cells of 100-day-old WSBV-infected P. monodon collected from Tuticorin. Scale bars20mm.
Fig. 9. Section of spongy connective tissue from the stomach of 100-day-old P. monodon, collected from
Ž .
Gudur, showing WSBV-infected cells containing hypertrophied nuclei hn and the nature of the associated
Ž .
storage vesicles. The hypertrophied nuclei lacked heterochromatin and contained en-veloped virions and empty capsids. The electron-dense nucleoplasm of the gill epithelial cells was almost totally replaced with virions. The nuclear envelope was disorganized,
Ž .
appeared thick and consisted of fibrous elements and numerous pores Figs. 10–14 .
Ž .
Shrimp examined from the various locations in Andra Pradesh and Tamil Nadu displayed similar histopathological characteristics of infection.
Studies carried out on a shrimp farm located in Tuticorin, Tamil Nadu, revealed an outbreak of white spot disease confirmed by the appearance of dead shrimp in the feed-check trays and at the margins of the pond, an area frequented by shrimp and from which birds gathered moribund or dead shrimp. The gut of these animals appeared empty. The numbers of dead shrimp increased on a daily basis and mass mortalities were encountered. Examination of the infected stocks revealed the presence of white spots on the exoskeleton with reddish body colouration and muscle opacity. Further histological examination of the infected tissues showed hypertrophied nuclei, marginated chromatin and widespread focal necrosis. The presence of virions in the infected tissues was confirmed by transmission electron microscopy.
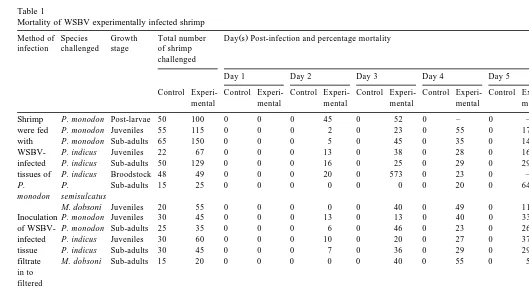
The results of the experimental infections of WSBV are listed in Table 1. Characteris-tic white spots appeared on the inner surface of the carapace and somites, which were numerous and more prominent in P. monodon. In P. indicus, it was necessary to remove the carapace to confirm infection. On examination, the various species and stages of shrimp exhibited the characteristic histopathological signs of WSBV infection. Intramuscular injection was the most rapid route of infection in P. monodon and P.
indicus. The infected shrimp become lethargic with reduced feeding and preening
activities. As the infection progressed, the shrimp stopped feeding, surfaced frequently and exhibited a loss of balance. The cannibalistic behaviour of some shrimp feeding on parts of WSBV-infected siblings was also observed. Organ disintegration and cell lysis occurred as severity of infection increased. A mortality rate of 100% occurred within 1 week post-infection. The controls remained infection-free.
4. Discussion
The occurrence of WSBV has been shown to be the causative agent responsible for mass mortalities in juveniles and sub-adults of the cultured Indian penaeid shrimp, P.
Ž .
Fig. 10. Section through a WSBV-infected gill epithelial cell hn from 100-day-old P. monodon. Free virions
Ž .v are enclosed by a thick layer the nuclear membrane nl . Mitochondria were absent. Bacterial cells b areŽ . Ž .
present in the cytoplasm of the infected cell. Scale bars0.5mm.
Fig. 11. Transmission electron micrograph of WSBV-infected gill epithelial cells, from a 100-day-old P.
Ž . Ž .
indicus, collected from Tuticorin. Hypertrophied nuclei hn contain virions v and there is degeneration of
Ž .
the nuclear membrane, nucleoplasm and cell membrane unlabelled arrows , and a thickening of the nuclear
Ž .
membrane nl . Scale bars1mm.
Fig. 12. P. indicus, 100 day old, collected from Tuticorin. WSBV-infected gill epithelial cell containing
Ž . Ž .
tube-like, sometimes branched capsule envelopes c and virions v . Scale bars0.5mm.
Fig. 13. WSBV-infected gill epithelial cell of 100-day-old P. indicus, collected from Chidambaram, showing a
Ž . Ž . Ž .
cross-sectional view Cv of virions in their envelope capsule e , and nuclear capsids nc . Also present are
Ž . Ž . Ž .
Fig. 14. Transmission electron micrograph of WSBV-infected gill epithelial cells of 100-day-old P. indicus,
Ž .
collected from Chidambaram. The nuclear membrane has degenerated however, a thick nuclear membrane nl ,
Ž . Ž .
encloses the virions v . Mitochondria m . Scale bars0.5mm.
Fig. 15. Transmission electron micrograph of a portion of a WSBV-infected gill epithelial cell from a
Ž .
100-day-old P. monodon, collected from Sirkhazki, showing virions, nucleo-capsids nc and the capsule
Ž .
envelope c . Scale bars0.25mm.
monodon and P. indicus and both ultrastructural and experimental evidence is presented
to support this finding. Mass mortalities caused by WSBV were reported in the cultured Ž
shrimp, P. japonicus from Japan Inouye et al., 1994; Nakano et al., 1994; Takahashi et
. Ž . Ž .
al., 1994 , Taiwan Chou et al., 1995 and Thailand Wongteerasupaya et al., 1995 . In the early stages of infection, some shrimp were observed cannibalizing dead infected shrimp thus promoting the spread of WSBV in the population. Infection in P.
monodon was readily recognized by the presence of numerous white spots on the
carapace, while in P. indicus, it was necessary to remove the carapace to confirm Ž
infection, usually accompanied by a reddish body colouration, Wang et al., 1995; .
Chang et al., 1996; Wongteerasupaya et al., 1996; Lo et al., 1996b .
While WSBV infects the ecto- and meso-dermal tissues, it was absent in the Ž
hepatopancreatic cells, suggesting it is similar to SEMBV infection Wongteerasupaya et .
()
Method of Species Growth Total number Day s Post-infection and percentage mortality Percentage of
infection challenged stage of shrimp Mortality
challenged
Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7
Control Control Control Control Control Control Control Control Control
Experi-mental mental mental mental mental mental mental mental mental
a
range of tissues in the penaeid shrimp as well as other crustaceans, whereas MBV,
Ž .
BMNV and PBV are tissue and host specific. WSBV PmNOBIII has been described
Ž . Ž .
from P. monodon by Wang et al. 1995 , Chang et al. 1996 , Karunsagar and
Ž . Ž . Ž .
Karunasagar 1997 and Karunasagar et al. 1997 while Wongteerasupaya et al. 1996
Ž .
reported the occurrence of WSBV PmNOBII infection in ecto- and meso-derm-derived tissues from P. monodon as well as P. chinensis, P. indicus, P. japonicus, P.
merguiensis, P. monodon, and P.Õannamei obtained from China, Thailand, Indonesia
and India. This suggests that WSBV is a substantial pathogen for a range of penaeid, crustacean and insect hosts with a wide geographic distribution.
Histopathological studies of WSBV-infected P. monodon and P. indicus revealed the occurrence of basophilic hypertrophied nuclei with a reduced volume of eosinophilic cytoplasm in the infected cells. Occlusion bodies have previously been reported in
Ž .
WSBV-infected hypertrophied nuclei Wang et al., 1995 but were not reported here. The extensive tissue damage observed in the infected hosts may be sufficient to reduce the physiological and functional efficiencies of the hosts, and thus give rise to the characteristic clinical signs of WSBV epizootics in shrimp. Histopathological observa-tions on experimentally infected tissues reflect the findings of those observed in
Ž .
naturally infected shrimp, and agrees with the findings of Chang et al. 1996 who reported nuclear hypertrophy, cell lysis and tissue degeneration, in experimentally infected P. monodon.
The hypodermal cells of the gills of WSBV-infected shrimp contained reduced numbers of mitochondria, ER, Golgi complex and secretory or storage vesicles in the cytoplasm. The nucleus showed hypertrophy, the nucleoplasm was less electron-dense and a thick nuclear envelope consisted of fibrous elements, numerous pores and enveloped virions. This is the first description of the ultrastructural pathology of WSBV-infected Indian shrimp.
From the gross morphological, ultrastructural and histopathological information, the viral infection was identified as white spot syndrome-associated baculovirus and classi-fied as a member of the genus NOB of the subfamily Nudibaculovirinae of
Baculoviri-Ž
dae and the isolate represents PmNOB Francki et al., 1991; Lightner, 1993; Wang et .
al., 1995; Wongteerasupaya et al., 1995 . A tube-like projection was observed extending from one end of the WSBV virions, the function and significance of which is unknown.
Ž .
Wang et al. 1995 have also described a tail-like projection from the virions isolated from P. monodon. Additionally, they have described the capsid to possess parallel cross-striations composed of rings of subunits in a stacked series.
Ž .
Chou et al. 1998 also showed, using shrimp infectivity tests of WSBV, that moralities reached 100% within 4–6 days and that WSBV was readily transmitted across different penaeid shrimp. It is still uncertain whether WSBV exists in latent forms in larvae or brood stocks and which factors make them virulent or non-virulent. The control shrimp remained healthy for more than 20 days.
Environmental factors may contribute to epizootics of WSBV particularly during the monsoon season with associated changes in salinity, heavy surface run-off and turbidity ŽAnonymous, 1995; Karunsagar and Karunasagar, 1997 . Stress is another contributing.
Ž .
factor leading to mass mortalities Lo et al., 1996b . WSBV has a widespread occur-rence over a range of hosts, some of which may act as vectors in natural and cultured
Ž .
systems. Supamattaya et al. 1998 have shown that crustaceans, commonly found in shrimp culture ponds, may act as viral reservoirs and aid transmission of WSBV. The interaction between cultured penaeid shrimp and other presumed reservoir hosts, known to inhabit shrimp farms, estuaries and coastal waters are of great interest and
understand-Ž .
ing them may help in the management of the disease Lo et al., 1996b . Untreated source water contaminated with possible vectors may be one route of entry for the virus in culture systems. However, whether similar routes of infection exist in the Indian situation needs to be examined and investigated at the ultrastructural and molecular levels.
The current study is the first report describing the morphological and ultrastructural changes in WSBV-infected Indian P. monodon and P. indicus. The release of hatchery-reared WSBV-infected post-larvae by farmers, naturalists or even by chance, into the environment to replenish natural stocks must be avoided because it may have a negative feed back impact in addition to increasing the spread of the disease from cultured systems to natural coastal systems.
References
Anonymous, 1995. SEMBV — an emerging viral threat to cultured shrimp in Asia. CP Shrimp News 3, 2–3. Bell, T.A., Lightner, D.V., 1988. A Handbook of Normal Penaeid Shrimp Histology. World Aqua. Soc., Baton
Rouge, pp. 1–114.
Chang, P.S., Lo, C.F., Wang, Y.C., Kou, G.H., 1996. Identification of white spot syndrome associated
Ž .
baculovirus WSBV target organs in the shrimp Penaeus monodon by in situ hybridization. Dis. Aquat. Org. 27, 131–139.
Chou, H.Y., Huang, C.Y., Wang, C.H., Chiang, H.C., Lo, C.F., 1995. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis. Aquat. Org. 23, 165–173. Chou, H.Y., Haung, C.Y., Lo, F.C., Kou, G.H., 1998. Studies on transmission of white spot syndrome
Ž .
associated baculovirus WSBV in Penaeus monodon and P. japonicus via waterborne contact and oral ingestion. Aquaculture 164, 263–276.
Durand, S., Lightner, D.V., Nunan, L.M., Redman, R.M., Mari, J., Bonami, J.R., 1996. Application of gene
Ž .
probes as diagnostic tools for white spot baculovirus WSBV of penaeid shrimp. Dis. Aquat. Org. 27, 59–66.
Ž .
Francki, R.I.B., Fauquet, C.M., Knudson, D.L., Brown, F. Eds. , 1991. Classification and nomenclature of viruses. Arch. Virol., Suppl., 2, pp. 117–123.
Inouye, K., Miwa, S., Oseko, N., Nakano, H., Kimura, T., 1994. Mass mortalities of cultured kuruma shrimp
Penaeus japonicus, in Japan in 1993: electron microscopic evidence of the causative virus. Fish Pathol. 29,
Karunsagar, I., Karunasagar, I., 1997. Strategies for management of white spot disease in shrimp. Souvenir of the National Aquaculture week 24th–7th Feb., 1997. Aquaculture Foundation of India, Neelankarai, Chennai, India. pp. 37–39.
Karunasagar, I., Otta, S.K., Karunasagar, I., 1997. Histopathological and bacterial study of white spot syndrome of Penaeus monodon along the west coast of India. Aquaculture 153, 9–13.
Ž .
Lightner, D.V., 1993. Diseases of cultured penaeid shrimp. In: McVey, J. Ed. , CRC Handbook of Mariculture, Vol. 1, Crustacean Aquaculture, 2nd edn. CRC Press, Boca Raton. pp. 393–486.
Ž .
Lightner, D.V. Ed. , 1996. A Handbook of Pathology and Diagnostic Procedures for Diseases of Penaeid Shrimp. World Aquaculture Soc., Baton Rouge.
Lo, C.F., Leu, J.H., Ho, C.H., Chen, C.H., Peng, S.E., Chen, Y.T., Chou, C.M., Yeh, P.Y., Huang, C.J., Chou, H.Y., Wang, C.H., Kou, G.H., 1996a. Detection of baculovirus associated with white spot syndrome
ŽWSBV in penaeid shrimp using polymerase chain reaction. Dis. Aquat. Org. 25, 133–141..
Lo, C.F., Ho, C.H., Penug, S.E., Chen, C.H., Hsu, H.C., Chiu, Y.L., Chang, C.F., Liu, K.F., Su, M.S., Wang,
Ž .
C.H., Kou, G.H., 1996b. White spot syndrome baculovirus WSBV detected in cultured and wild shrimp, crabs and other arthropods. Dis. Aquat. Org. 27, 215–225.
Mari, J., Bonami, J.R., Poulos, B., Lightner, D.V., 1993. Preliminary characterization and partial cloning of
Ž .
the genome of a baculovirus from Penaeus monodon Pm SNPVsMBV . Dis. Aquat. Org. 16, 207–215. Momoyama, K., Hiraoka, M., Nakano, H., Koube, H., Inouye, K., Oseka, N., 1994. Mass mortalities of cultured kuruma shrimp, Penaeus japonicus, in Japan in 1993: histopathological studies. Fish Pathol. 29, 141–148.
Muralimanohar, B., Sundararaj, D., Selvaraj, D., Sheela, P.R.R., Chidambaram, P., Mohan, A.C., Ravishankar, B., 1996. An outbreak of SEMBV and MBV infection in cultured Penaeus monodon in Tamil Nadu. Indian J. Fish. 43, 403–406.
Nakano, H., Koube, H., Umezaea, S., Momoyama, K., Hiraoka, M., Inouye, K., Oseka, N., 1994. Mass mortalities of cultured kuruma shrimp, Penaeus japonicus, in Japan in 1994: epizootiological survey and infection trials. Fish Pathol. 29, 135–139.
Ramasamy, P., Brennan, G.P., Jayakumar, R., 1995. A record and prevalence of Monodon baculovirus from post-larval Penaeus monodon in Madras, India. Aquaculture 130, 129–135.
Sano, T., Nishimura, T., Fukuda, H., Hayashida, T., Momoyama, K., 1984. Baculoviral mid-gut gland necrosis
ŽBMN of Kuruma shrimp. ŽPenaeus japonicus larvae in Japanese intensive culture systems. Helgol..
Meeresunters. 37, 255–264.
Supamattaya, K., Hoffmann, R.W., Boonyaratpalin, S., Kanchanaphum, P., 1998. Experimental transmission
Ž .
of white spot syndrome virus WSSV from black tiger Penaeus monodon to the sand crab Portunnus
pelagicus, mud crab Scylla serrata and krill Acetes sp. Dis. Aquat. Org. 32, 79–85.
Takahashi, Y., Itami, T., Kondom, M., Maeda, M., Fujii, R., Tomonaga, S., Supamattaya, K., Boonyaratpalin,
Ž
S., 1994. Electron microscopic evidence of bacilliform virus infection in Kuruma shrimp Penaeus .
japonicus . Fish Pathol. 29, 121–125.
Takahashi, Y., Itami, T., Maeda, M., Suzuki, N., Kasornchandra, J., Supamattaya, K., Khongpradit, R., Boonyaratpalin, S., Kondo, M., Kawai, K., Kusuda, R., Hirono, Aoki, T., 1996. Polymerase chain reaction
ŽPCR amplification of bacilliform virus RV-PJ DNA baculovirus SEMBV DNA in Penaeus monodon. Ž . Ž .
Fabricius. J. Fish Dis. 19, 399–403.
Wang, C.H., Lo, C.F., Leu, J.H., Chou, C.M., Yeh, P.Y., Chou, H.Y., Tung, M.C., Chang, C.F., Su, M.S., Kou, G.H., 1995. Purification and genomic analysis of Baculovirus associated with white spot syndrome
ŽWSBV of Penaeus monodon. Dis. Aquat. Org. 23, 239–242..
Wongteerasupaya, C., Vickers, J.E., Sriurairatana, S., Nash, G.L., Akarajamorn, A., Boonsaeng, V., Panyim, S., Tassanakajon, A., Withyachumnarnkul, B., Flegel, T.W., 1995. A non-occluded, system baculovirus that occurs in cells of ectodermal and mesodermal origin and causes high mortality in the black tiger prawn
Penaeus monodon. Dis. Aquat. Org. 21, 69–77.