EMBRYOGENESIS EFFECTIVENESS FROM YOUNG
LEAVES, MATURE ZYGOTIC EMBRYO AND IMMATURE
FEMALE FLOWER EXPLANTS
MONDJELI CONSTANTIN
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
This is to declare that the thesis titled « Oil Palm (Elaeis guineensis Jacq.) var. Tenera In Vitro Embryogenesis Effectiveness from Young Leaves, Mature Zygotic Embryo and Immature Female Flower Explants » is the result of my personal work under the direction of the supervising committee and has never been presented in any form where ever. Any other source of information that has been mentioned in this thesis from published or unpublished works of other authors has been acknowledged in the text and included in the reference chapter.
Bogor, December 2011
Mondjeli Constantin
MONDJELI CONSTANTIN. 2011. Keefektifan Embriogenesis in Vitro Kelapa Sawit (Elaeis guineensis Jacq. var. Tenera)
dari Eksplan
Daun Muda, Embrio Zigotik Matang dan Bunga Betina Immature. Dibawah bimbingan NI MADE ARMINI WIENDI dan ADE WACHJAR.Tiga percobaan terpisah terkait sumber eksplan kelapa sawit varietas Tenera meliputi daun muda, embrio zigotik matang dan bunga betina immature, yang diambil dari pohon induk berumur 9 dan 13 tahun, telah dilakukan untuk menguji responnya terhadap produksi embrio somatik. Pada berbagai komposisi media, eksplan-eksplan ini ditanam pada media dasar MS dan Euwens yang mengandung kombinasi ZPT NAA (107.41µM) dan 2,4-D (0, 22.62, 45.24 atau 67.86 µM) dan media dasar MS dengan konsentrasi ZPT tinggi picloram 450µM atau 2,4-D 450µM mengunakan 0,03% (w/v) arang aktif untuk menginduksi kalus embriogenik. Persentase oksidasi berkisar antara 24 – 100% untuk eksplan daun dan antara 0 – 46.67% pada eksplan embrio zigotik, sementara pada eksplan bunga 100%. Tingkat kontaminasi berkisar antara 1.11 sampai 100 % pada eksplan daun, 0 – 35.56% pada eksplan embrio zigotik dan 8.33 - 41.67% pada eksplan bunga betina. Pada 28 minggu setelah penanaman, didapatkan kalus globular yang kompak dan putih dari daun nomor 5 (L5). Media MS dengan kombinasi ZPT NAA (107.41µM) dan 2,4-D (67.86 µM) merupakan media yang optimal untuk induksi kalus embriogenik (3.63%), sementara media MS dengan NAA (107.41µM) menghasilkan persentase pembentukan kalus tertinggi (30.56%). Pada 36 minggu setelah penanaman, didapatkan embrioid langsung dari daun nomor 6 (L6) pada media MS yang mengandung kombinasi ZPT NAA (107.41µM) dan 2,4-D (45.24 µM). Pada eksplan embrio zigotik dewasa, hasil kalus embriogenik terbaik (7,90%) didapatkan pada media Euwens yang mengandung NAA (107.41µM) setelah 28 minggu. Persentase pertumbuhan tunas tertinggi (38.33%) juga didapatkan pada media yang sama (NAA 107.41µM). Pada eksplan bunga betina immature, tidak dijumpai kalogenesis dalam kurun waktu penelitian ini. Setelah 4 kali subkultur berturut turut pada media yang sama dengan konsentrasi zat pengatur tumbuh yang menurun bertahap, kalus embriogenik dan embrioid tidak dapat berkembang dalam kurun waktu penelitian ini, dari eksplan daun muda dan embrio zygotic matang.
MONDJELI CONSTANTIN. 2011. Oil Palm (Elaeis guineensis Jacq.) var. Tenera in Vitro Embryogenesis Effectiveness from Young Leaves, Mature Zygotic Embryo and Immature Female Flower Explants. Under the supervision of NI MADE ARMINI
WIENDI and ADE WACHJAR.
Oil palm (Elaeis guineensis Jacq.) is an important commercial crop for all the producer countries. It is the world‟s leading vegetable oil crop. In the last decade, the interest in palm oil as biofuel was eventually caused constraints on worldwide supply for edible palm oil. In addition, low production and lack of conventional seeds and seedlings are always observed from the potential producer countries. More than one hundred million of tissue culture plantlets are needed per year. Indonesia is the largest world producer country with about 23 million tons of palm oil per year. The study was carried out to find in short term the optimum medium for callus induction and somatic embryo formation from young leaves, mature zygotic embryo and immature female flower explants of oil palm (E.guineensis Jacq.) var.Tenera. Three different independent experiments related to explant sources of oil palm (Elaeis guineensis Jacq.) var. Tenera include young leaf, mature zygotic embryo and immature female flower explants isolated from 9 and 13 years old trees were investigated on
achieved onto Eeuwens media containing NAA (107.41µM) after 28 weeks. Highest
percentage (38.33%) of direct shoot development was also obtained from the same media NAA (107.41µM). For female flower explant no callogenesis was observed during the study period. After 4 subsequent subcultures onto the same medium with gradually reduction of auxin concentration, the embryogenic callus and embryoid cells from young leaves and mature zygotic embryo explants, failed to develop during the time require for this study.
©
Copy right IPB, 2012 All rights reserved by law.No part or all of this work may be reproduced without citing the source. Copying may be done only on the basis of education, research, scientific writing, reports or reviews; and which should not cause any prejudice to IPB.
OIL PALM (Elaeis guineensis Jacq.) var. Tenera in Vitro
EMBRYOGENESIS EFFECTIVENESS FROM YOUNG LEAVES,
MATURE ZYGOTIC EMBRYO AND IMMATURE FEMALE FLOWER
EXPLANTS
MONDJELI CONSTANTIN
Thesis
Submitted to the Graduate School in partial fulfilment of the requirements for the degree
Master of Science
In Plant Breeding and Biotechnology
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
Title : Oil Palm (Elaeis guineensis Jacq.) var. Tenera In Vitro Embryogenesis Effectiveness from Young Leaves, Mature
Zygotic Embryo and Immature Female Flower Explants
Name : MONDJELI CONSTANTIN
Registration Number : A253098021
Program : Plant Breeding and Biotechnology
Approved by
Supervising Committee
Head Member
Dr. Ir. Ni Made Armini Wiendi, MS Dr. Ir. Ade Wachjar, MS
Endorsed by
Head of Study Program Dean of Graduate School
Dr.Ir. Trikoesoemaningtyas, MSc. Dr. Ir. Dahrul Syah, MSc. Agr.
ACKNOWLEDGEMENTS
Glory to God in the highest and peace to his people on earth. Almighty God and Father, I worship you and give you thanks for all your permanent presence, light, guidance, peace and
protection that I have always received during my studies and specially my research activities in Bogor Agricultural University, without which all my efforts would not have borne any fruits.
I would like to convey my sincere and special thanks to my supervising committee Dr. Ir. Ni Made Armini Wiendi, MS and Dr. Ir. Ade Wachjar, MS for their advice, corrections resourceful instructions and comments during the preparation, execution and presentation of this research work.
Grateful thanks to Bapak Jo Daud Dharsono and Dr. Zok Simon, respectively the Project Leader of Indonesian Palm Oil Board‟s (IPOB) and the former General Manager of the Institute of Agricultural Research for Development (IRAD) Cameroon for their wisdom and full goodwill, without which this project would never have surfaced.
I wish to convey my special thanks to Bapak Lalang Buana of PT SMART‟s V Team Operations and all the members of IPOB mainly Ibu Tyas and Bapak Wasis, to Dr. Koona Paul the Chief of Centre of the Specialised Centre on Oil Palm Research of La Dibamba Cameroon (CEREPAH) and all the members for their constant support and encouragements during my stay in Indonesia.
I want to take this opportunity to thank my family especially the Late Mouadiba
Stanislas who initiated the perfection cult that is expressing in me today; my mother Ango Imelda, my wife Ngassa Nelly, my children Biba Mondjeli Audrey-stephane, Lihan Mondjeli Cecile Santana, Mondjeli Mondjeli Constantin II, my brothers, sisters and cousins for their constant prayers, moral support, sacrifices and encouragement.
I would like to thank the generosity of my classmates of Plant Breeding and Biotechnology 2009 batch especially Yogo Adhi Nugroho, Nur Arifin, Vina Novita, Vitria Pupistasari and Jose Maria Alves who were adopted me rapidly in this new environment of Indonesia. My sincere acknowledgements to all the laboratory staff and technicians of Tissue Culture Laboratory number 2 of IPB, especially Okti Hanayati and Yogo Adhi Nugroho.
AUTOBIOGRAPHY
The author was born in Ebodié, Cameroon on the 10th of March 1972 from father Mouadiba Stanislas and mother Ango Emilda. The author is eighth from a total of twenty five children. The author is a father of two sons and one daughter with one wife.
In year 1995, the author passed the " Baccalauréat D " and was admitted into the Faculty of Agronomy and Agricultural Sciences of University of Dschang through a competitive entrance examination. In May 2001, the author was graduated with a Post Graduate Certificate " Ingenieur Agronome " and was later recruited into the Ministry of Scientific Research and Innovations of Cameroon in 2002.
The author was posted to Barong-bikang Multipurpose Agricultural Research Station as assistant researcher on Perennial Crops (Cocoa and Coffee) from 2002 to 2004. Since 2004, the author has serving in Agronomy and Breeding sections of the Specialised Centre for Oil Palm Research La-Dibamba as researcher.
In August 2009, the author obtained from the Bogor Agricultural University, the opportunity to further his studies on Plant Breeding and Biotechnology at the Graduate School with funding from the Indonesia Palm Oil Board and the Government of Cameroon.
TABLE OF CONTENTS
Pages
LIST OF TABLES……… iv
LIST OF FIGURES………. v
LIST OF APPENDICES……….. vi
INTRODUCTION……….……… 1
Background……… 1
Aim of Research……… 3
Hypothesis………. 4
LITERATURE REVIEW……… 5
Origin and History of Oil Palm (Elaeis guineensisJacq)……….. 5
Taxonomy of Oil Palm (Elaeis guineensisJacq)……… 7
Botany of Oil Palm (Elaeis guineensisJacq)……… 7
Plant Tissue Culture………..………... 12
History and Definition……… 12
Medium of Tissue Culture……….. 13
Plant Growth Regulators………. 14
Zygotic Embryo Culture……….…. 15
Ovary Culture………..………. 15 Tissue Culture problems and Preventions……….... 15
Genetic Changes in Culture……… 15
Blackening or Browning……… 16
Embryogenesis………... 16
Tissue Culture Field in Plant Breeding………..… 17
Tissue Culture and Oil Palm improvement………..……….. 19
MATERIALS AND METHODS……….. 22
Location and Time……… 22
Plant Materials and Equipments……….... 22
Media Preparation……… 22
Sterilization Compounds……….. 25
Laboratory Equipments and Tools…………...……… 25
Methods……… 25
Experiment 1. Callus and Somatic Embryos Induction from Young Leaf Explant... 25
Palm Tree Sampling and Explant Processing……… 25
Callogenesis Mixing Medium (Combination)...……… 26
Environmental Culture Conditions……… 27
Embryogenesis Mixing Medium……… 27
Culture conditions of Embryos……….... 27
Subculture‟s Flow Chart………..……….…………. 28
Experiment 2. Induction of Somatic Embryo from Mature Zygotic Embryo Explant ………...……… 28
Explant Sampling and Sterilization Process……… 28
Callogenesis Mixing Medium (Combination)..……….………. 29
Environmental Culture Conditions……….………. 29
Embryogenesis Mixing Medium………. 30
Experiment 3.Induction of Somatic Embryo from Immature Female Flower Explant ...……… 30
Explant Sampling and Sterilization Process……… 30
Callogenesis Mixing Medium (Combination)..……….. 31
Environmental Culture Conditions………..……… 31
Data Analysis……….. 31
CONCLUSIONS ………..……… 45
REFERENCES……….… 46
LIST OF TABLES
Number Pages
1. Agroecological factors affecting growth and production of Oil Palm………… 12 2. Murashige and Skoog (1962) Medium composition……….. 23
3. Eeuwens and Blake (1976) Medium Composition………. 24 4. Percentage of contamination of oil palm leaves explant per medium composition
and rank of leaf after 36 weeks of culture (%) ……….… 32 5. Influence of various medium composition (NAA and 2,4-D) and young leaves
rank of oil palm (E. guineensis) cv. Tenera spear on percentage of oxidized
calli after 4 subcultures.……….…… 33
6. Influence of various medium composition (NAA and 2,4-D) and young leaf rank of oil palm (E. guineensis) cv. Tenera spear on the percentage of
explant bearing callus after 39 weeks ……….……….. 34
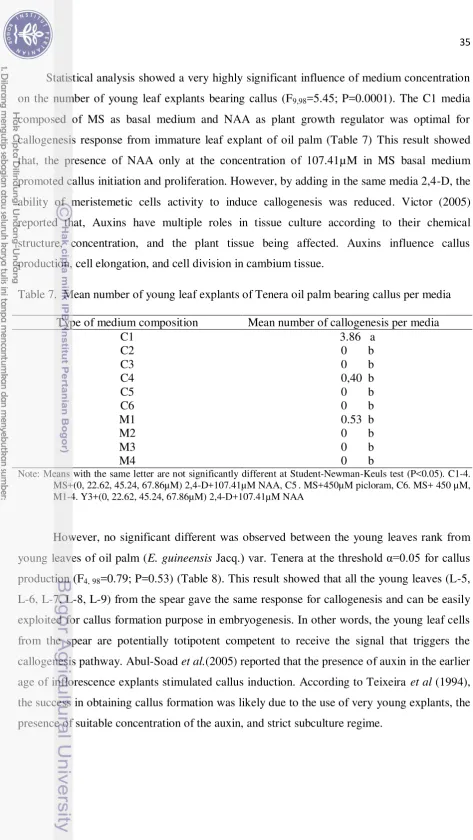
7. Mean number of young leaf explants of Tenera oil palm bearing callus per
media ……... 35
8 Effect of the spear leaf rank of oil palm var. Tenera on callogenesis after
39 weeks in medium ………. 36
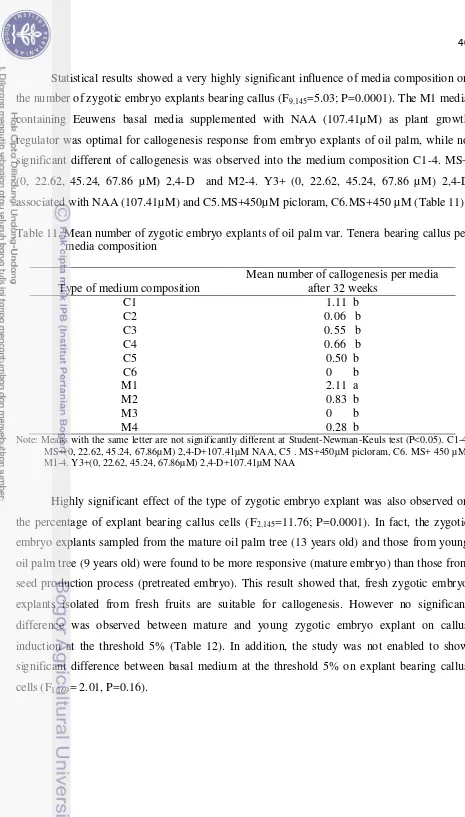
9. Percentage of contamination per medium and type of zygotic embryo explant of
oil palm after 28 weeks of culture (%)……….. 38 10. Effect of medium composition and type of zygotic embryo explants of oil palm
(E. guineensis) cv. Tenera on percentage of explant bearing callus after 32 weeks... 39
11. Mean number of zygotic embryo explants of oil palm var. Tenera bearing callus
per media composition ……… 40 12. Mean number of callogenesis per type of zygotic embryo explant of oil palm
var. Tenera ……… 41
13. Effect of medium composition and type of zygotic embryo explants of oil palm (E. guineensis) cv. Tenera on the percentage of explant producing
germinated shoot after 25 weeks in medium ………..……… 41 14. Mean number of shoot germination production by zygotic embryo explant per
LIST OF FIGURES
Number Pages
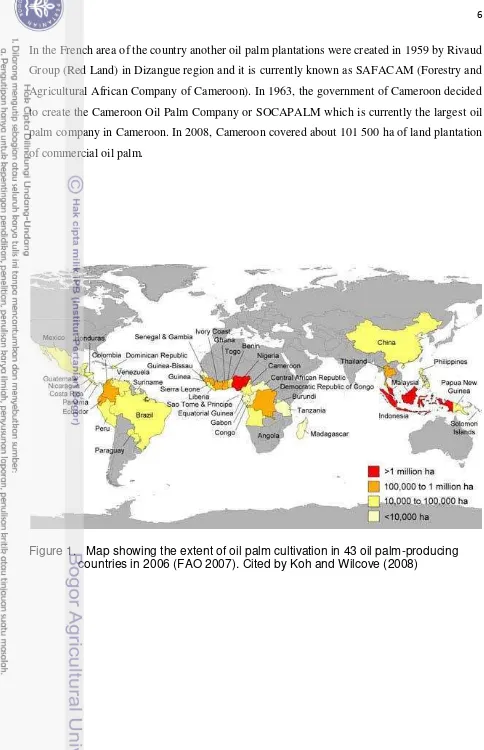
1. Map showing the Extent of Oil Palm Cultivation ……….. 6
2. Adult Oil Palm (Elaeis guineensisJacq.) Tree……… 8
3. Male and Female Inflorescence of Oil Palm……… 10
4. Mature and Immature Fresh Fruit Bunches of Oil Palm……….. 10
5. Cross section of the different varieties of oil palm (E. guineensisJacq.)………… 11
6. Different Type of Oil Palm Explants used……….. 22
7. Young Leaf Sample Started Material Preparation Process……….. 26
8. Mature Zygotic Embryo Explant Preparation Process……….. 29
9. Immature Female Flower Explant Preparation Process……… 30
10. Friable nodular callus (NC) proliferation from young leaves explant ………. 33
11. Root-like callus initiation from young leaf explant of oil palm ……….. 34
12. Embryogenic callus (EC) obtained from young leaf of oil palm……….. 36
13. Direct embryoid cells obtained from young leaf of oil palm……….. 37
14. Germinated shoots from mature zygotic embryo explant of oil palm…………... 38
15. Root-like callus (RC) initiation from zygotic embryo explant ……… 39
16. Embryogenic callus (EC) initiation of zygotic embryo………. 43
LIST OF APPENDICES
Number Pages
1. ANOVA of callus induction from young leaves explant of oil palm………. 53 2. Student-Newman-Keuls test of different medium concentrations for callus
induction of young leaves explant………. 53 3. Student-Newman-Keuls test of different leaf ranks of oil palm for callus induction of young leaves explant………..……….. 54 4. Student-Newman-Keuls test of different basal medium for callus induction of
young leaf explant………..……. 54
5. ANOVA of callus induction from zygotic embryo explant of oil palm………… 54
6. Student-Newman-Keuls test of different medium for callus induction of mature
zygotic embryo explant………..………… 55 7. Student-Newman-Keuls test of different type of zygotic embryo explant for
callus induction……….. 55 8. ANOVA of callus induction from zygotic embryo explant of oil palm for basal
medium and type of zygotic embryo……….……… 56
9. Student-Newman-Keuls test of different basal medium for callus induction of
mature zygotic embryo explant……….. 56
10. ANOVA of direct shoot formation from zygotic embryo explant of oil palm….. 56
11. Student-Newman-Keuls test of different medium for direct shoot formation of
mature zygotic embryo explant……….. 57 12. Student-Newman-Keuls test of different type of zygotic embryo explant for
shoots germination………..……… 57 13. ANOVA of shoot formation from zygotic embryo explant of oil palm for basal
medium and type of zygotic embryo………. 58 14. Student-Newman-Keuls test of different basal medium for shoots germination
INTRODUCTION
Background
Oil palm (Elaeis guineensis Jacq.) is an important commercial crop from all tropic producer countries. Cultivated for palm oil and palm kernel oil, it is the highest yielding oil-bearing crop, by producing more than five times the yield of oil per year per hectare of any annual oil crop. The best oil palm is met in Indonesia, regularly producing 8 tons of oil/hectare/year (Suprianto et al. 2008). The worldwide palm oil production since 2005 was 39.8 million metric tons, of which 4.3 million tons was in the form of palm kernel oil. As compare to the other oleaginous crops, Elaeis guineensis oil is the most widely-produced tropical oil, and constitutes 30 % of total edible oil production worldwide. Since 2007, Indonesia emerged the world's largest producer of palm oil, by producing approximately 50 % of world palm oil volume, giving a total of 23 million ton/year (htt://Google Wikipedia oil palm, U.S Department of Agriculture 2010). Palm oil production has contributed to economic benefits such as government revenues, profits for companies, employment, and raised incomes for
smallholders. In Indonesia, the world‟s largest producer, the industry generated US$12.4
billion in foreign exchange from palm oil exports in 2009, and supports millions of jobs and opportunities for rural farmers (Gingold 2011). Therefore, this economic sector employs about 1 544 641 people in Indonesia and the planted area is increasing every year by 372 000 hectares. Oil palm was planted on a total land area of 8 036 431 hectares in the year 2010 in Indonesia (D.T.E 2011).
Cameroon is the 13th world producer of palm oil with 190 000 metric tons and the 3rd African country producer after Nigeria and Ivory Coast (U.S Department of Agriculture, 2010). Palm oil is mainly required for food purposes for about 80% as cooking oil. In Europe, 10% of consumption high quality products sold contains palm oil. In addition, palm oil is present into several industrial products and public restaurant food (www.econo-ecolo.org). Besides producing oils and fats, at present there is a continuous increasing interest
products such as methane gas, bio-plastic, organic acids, bio-compost, ply-wood, activated carbon, and animal feedstock. Even waste effluent; palm oil mill effluent (POME) has been
converted to produce energy. Oil palm has created many opportunities and social benefits for the locals. In the above perspective, the objective of the present work is to give a concise and up-to-date picture of the present status of oil palm industry enhancing sustainable and renewable energy (Sumathi et al. 2007). But the demand for palm oil has mainly increased in recent years due to its use as a biofuel,
Oil palm (Elaeis guineensis Jacq.) is a monocot plant, originating from West and Central Africa. Since the establishment of the first plantations in the 1920s and 1930s, five generations of selection and breeding have been completed in oil palm. The oil palm breeding program is limited by the long generation, selection cycles with typically 12 years and the tissues in plant culture was first recognized by Steward et al. (1958) and Reinert (1958, 1959) in culture of Daucus carota tissue. According to oil palm, propagation through tissue culture is also widely used. Initially tissue culture techniques were used to propagate elite oil palm clones (Jones, 1974). Otherwise several explants sources have been used to establish tissue culture. These include mature embryos (Rabechault et al. 1970), inflorescences (Smith and Thomas 1973), roots (Jones 1974), seedlings (Ong 1977), and young leaves (Staritsky 1970; Schwendiman et al. 1988). Unfortunately some early aberration were observed from plant produced (Corley et al. 1986). However, oil palm tissue culture techniques have undergone continuous improvement in these recent years. This resulted in the production of clonal palms with minimal abnormality less than 5% (Jones, 1995, Rival et al. 1998). Moreover, Texeira et
explants of oil palm. Plant propagation using explants from flower organs either anther or ovary through embryogenesis techniques have been successfully carried out although their
regeneration is more difficult than that using young leaves (Perera et al. 2007, 2008, Texeira et al., 1994; Verdeil et al. 1994). Although these skills are applied, the frequencies for complete plant regeneration from some explants are still inefficient (Rival et al. 1999). The low response of embryogenesis is influenced mainly by genotype and donor plant age (Ruslan, 1993), in addition to the influence of the appropriate media and explants source. Explants of young tissues still undergoing cell division and generally form callus more readily than those from older parts of plant, where the meristematic cell activities are reduced or lost. In general, the more juvenile the explant material, the greater the likelihood of success (Edwin and Sherrington, 1984; Lydiane and Kleyn, 1983). Embryogenesis rates of oil palm, averaging usually less than 5% per ortet (Soh et al., 2006). Embryoids are multiplied until the total number reaches the required 350. Of these 350 embryoids only 250 will produce shoots (shooting percentage of 75%). Nevertheless, about 18% of the ortets will provide the bulk cultures for mass propagation (Wong et al., 1997).
Thus some new protocols contributions are still needed for oil palm plant regeneration based on embryogenesis. It is in this light that the study was carried out.
Aim of Research
The long term objective is to formulate the protocol of in vitro oil palm regeneration using young leaves, mature zygotic embryo and immature female flower explants.
Research Activities
In short term, it was aimed to:
Find out the optimum medium for callus induction and somatic embryo formation from young leaf explant of oil palm of Tenera variety.
Find out the optimum medium for callus induction and somatic embryo formation from oil palm mature zygotic embryo explants of Tenera variety.
Find out the optimum medium for callus induction and somatic embryo formation from oil palm immature female flower explant source of Tenera variety.
Importance of Study
This work will contribute to obtain protocols on elite oil palm plants propagation for seedlings production centre. Protocols will be served as tool of clonal oil palm production to ensure the best use of oil palm resources in germplasm management. The skills can be used for the plant breeding program purpose and also during the oil palm in vitro selection study.
Hypothesis
1. There is an optimal type of medium composition for each explant source of Tenera variety oil palm.
2. There is at least one explant for embryogenesis of Tenera variety of oil palm.
LITERATURE REVIEW
Origin and History of oil Palm
It is generally agreed that the Oil Palm (Elaeis guineensis Jacq.) is originated from the equatorial tropical rain forest region of Africa, precisely along the gulf of guinea. It exists in the wild type and cultivated state. The American oil palm, Elaeis oleifera is native to tropical Central America and South America. The main belt runs through the southern latitudes of
Cameroon, Côte d‟Ivoire, Ghana, Liberia, Nigeria, Sierra Leone, Togo and into the equatorial
region of Angola and the Congo. Oil palm was first illustrated by Nicholaas Jacquin in 1763, hence its name, Elaeis guineensis Jacq (en.wikipedia.org/wiki/Oil_palm#mw-head).
During the 14th to 17th centuries some palm fruits were taken to the Americas and from there to the Far East. The plant appears to have thrived better in the Far East, thus providing the largest commercial production of an economic crop far removed from its centre of origin. Oil palms were introduced to Java (Indonesia) by the Dutch in 1848 (Lötschert and Beese 1983) and to Malaysia (then British colony of Malaya) in 1910 by Scotsman William Sime and English banker Henry Darby. The first plantations were mostly established and operated by British plantation owners, such as Sime Darby and Boustead. The large plantation companies remained listed in London until the Malaysian government engineered their
"Malaysianisation" throughout the 1960s and 1970s (Stevenson 2006).
The cameroon‟s wild oil palm material contains some interesting genotypes which are
In the French area of the country another oil palm plantations were created in 1959 by Rivaud Group (Red Land) in Dizangue region and it is currently known as SAFACAM (Forestry and
Agricultural African Company of Cameroon). In 1963, the government of Cameroon decided to create the Cameroon Oil Palm Company or SOCAPALM which is currently the largest oil palm company in Cameroon. In 2008, Cameroon covered about 101 500 ha of land plantation of commercial oil palm.
Taxonomy of Oil Palm
The scientific classification of oil palm is as follows:
Kingdom : Plantae
Phylum/Division : Phanerogam/Spermatophyta
Subdivision : Angiospermae
Class : Monocotyledonae
Order : Arecales
Family : Arecaceae
Subfamily : Arecoideae
Tribe : Cocoseae
Subtribe : Elaeidinae
Genus : Elaeis
Species : guineensis and oleifera
Botany of Oil Palm
plant in alternating cycle of variable duration depending on genetic factors, age and environmental conditions (Corley, 1976).
Figure 2. Adult oil palm (Elaeis guineensis Jacq.) tree
The Foliage System of Oil Palm
At the mature stage, E. guineensis Jacq. is constituted with a large crown of 30 to 45 palms measuring 5 to 9 meters long topping the single cylindrical pseudo-trunk. Every year, about 20 to 26 new pinnate leaves are produced and each mature leaf bears 250 to 300 leaflets. The leaf bases are persistent for years, and prominent leaf scars are arranged spirally on the trunk of mature palms where bases have fallen. The leaflets cover the distal 2/3 of the leaf, and the lower 1/3 is spined with spines increasing in length acropetally. The stem or
pseudo-trunk can extend a rate of 30 to 60 cm/year between the ages of 6 to 15 depend on hereditary and environmental factors. It functions as a supporting, vascular and storage organ. Generally, the height of trees determines the exploitation times of oil palm plantations. Thus, palm plantations are exploited up to 25 to 30 years when the trees height is between 12 to 15 meters. Above of this, the harvest of fresh fruit bunches becomes difficult and replanting should be required.
The Roots System of Oil Palm
uptake). The different morphological types of oil palm roots have been distinguished according to their development pattern and state of differentiation. As all the monocot, the
root system of E. guineensis is fasciculata. The oil palm has an adventitious root system; with primary roots generally about 6-10 mm in diameter, originating from the base of the trunk and either spreading horizontally or descending at varying angles into the soil. The primary roots bear secondary roots, of about 2-4 mm in diameter. Tertiary roots, about 0.7-1.2 mm in diameter, branch out from the secondary roots, which in turn bear the quaternary roots. Quaternary roots are unlignified, about 0.1-0.3 mm in diameter and 1-4 mm long and they are often assumed to be the main absorbing roots (Corley et al., 1976). The total length of tertiary and quaternary roots in the soil is the most important root characteristic as they are the absorbing roots that affect fertilizer use efficiency. Most of the root biomass is found within 1m of the soil surface, but tertiary and quaternary roots are found mostly in the upper 30 cm from the soil surface. Primary roots can grow up to 20 m away from the base of the palm and some primary roots could penetrate below the water table at 90 cm from the surface (Ng et al., 2003). The distribution of roots depends largely on the nature of the soil. Oil palm being a monocot needs a friable soil for root branching (Zuraidah et al. 2010).
The Reproductive System of Oil Palm
Figure 3. A. Male inflorescence, and B. Female inflorescence of oil palm.
In the nature, the pollination is mainly entomophily (by insects) but can also be done by the wind and the rain. The main pollinator of the oil palm is the insect from the genus
Elaeidobius and 14 species were found to be associated to the male and female inflorescences. The most common one is Elaeidobius kamerunicus, which was introduced in Asia, South and Central America. Its introduction led to an increase in yields of more than 35%. Upon pollination at the anthesis stage, the female inflorescence may develop and give rise to a fruit bunch 22 to 26 weeks later. In the field, fruit bunches (Figure 4) which are as compact and ovoid mass spiked bearing spines can be found on the oil palm 2 to 3 years after planting. The fruit bunches become heavier as the palms get older. On 10 years old palms, the bunch weights can be ranged from 10 to 50 kg for a total of 500 to 4 000 fruits per bunch. The fruit is a sessile drupe generally of ovoid shape measuring 2 to 5 cm length and weighing from 3 to 30g. The oil palm fruit consists of the pericarp which includes the outer and smooth exocarp, the fibrous and fleshy mesocarp or pulp rich in palm oil, the hard endocarp or shell protecting the seed and finally the kernel or seed. Inside the shell is the endosperm or kernel. The endosperm contains large amount of fats and carbohydrates on which the seedling will be entirely dependent after germination. The oil palm produces two main vegetable oils, namely palm oil extracted from the mesocarp of the fruit and palm kernel oil extracted from seed.
Figure 4. Mature and Immature Fresh fruit bunches of oil palm
A B
Commonly three fruit forms or “varieties” of oil palm can be identified based on the
thickness of the shell criterion (Figure 5). The Dura has a shell thickness between 2 to 8 mm, while the Pisifera has no shell. The shell thickness of the hybrid Tenera is between 0.2 to 2 mm, the fleshy mesocarp of Dura yields between 15 to 17% oil while in Tenera yields
between 21 to 23% oil and Pisifera more than 23% oil. According to commercial purpose, Pisifera is not cultivated on large scale because of its ability to fruits abortion and thereby virtual empty bunches production. Tenera which is a hybrid of Dura and Pisifera varieties remains the commercial variety with 60 to 96% of mesocarp. The hybrid produces more fruit
bunches than Dura.
Figure 5. Cross section of the different varieties of oil palm (E. guineensis Jacq.). A. Dura palm fruit with thick shell, B. Tenera palm fruit with less thick shell, C. Pisifera palm fruit with no shell
Climatic and Soil Requirements of Oil Palm
Oil palm culture is done well in low altitude (less than 500 m above sea level), 15º from the equator in the humid tropics. Evenly distributed rainfall of 1,800 to 2,000 mm/year, but will tolerate rainfall up to 5,000 mm/year, provided the soil is properly drained. Oil palm is sensitive to poor drainage and drought. Potential yield is reduced where there are more than three consecutive months with less than 100 mm rainfall per month. Irrigation may increase economic returns in areas with pronounced dry periods. More than 2,000 sunshine hours (i.e., low cloud cover during daytime) are required.
It is adapted to a range of soil types. Tolerates low pH, but does not thrive at very high pH (greater than 7.5). Soil must be free draining. The table below shows the suitable and extreme values of climate and soil that are required by oil palm.
A
B
Table 1. Agro ecological factors affecting growth and production of oil palm
Characteristic Suitability class
Good Moderate Severe Very severe
Rainfall (mm) 2500 1450-1700 1250-1450 <1250
Temperature (oC) 25-30 20-22 10-20 <16
Many authors have put forward various arguments on the origin of plant tissue culture. Rehwald (1927) is thought to have been the first person who has obtained undifferentiated callus tissue in sterile culture. But according to most scientists, the real scientific work on tissue culture was done in 1934 by P.R. White in the USA (United States) and in the same year, by Gautheret in France, using respectively tomato plant and the cambium of several tree species (Acer pseudoplatanus, Salix caprea, sambucus) as sources of explant. Before that period, in 1924, callus culture of carrots (Daucus carota) was reported by two physicians, R. Blumenthal and P. Meyer in the pathological implication studies.
In 1934, the plant hormone or auxin named indole-3-acetic acid (IAA) was identified the first time by F. Kögl, A.J. Haagen-Smit, and H. Erxleben. In 1955 C.O Miller has discovered kinetin, which is a plant growth regulator known as cytokinin.
Plant tissue culture is a technique or a process by which small organs or pieces of tissue of plants (explants) are grown in aseptic conditions within glass or clear plastic vessels. These are usually termed in vitro techniques or propagation. It is also called micropropagation
on a large scale by the orchid industry in the 1950s. Later, it became clear that any plant would respond to tissue culture as long as the right formula and the right processes were
developed for its culture. The process of tissue culturing plant the explant stage to the final stage of plantlet requires four basic stages namely: establishment or initiation of explant (Stage I), multiplication of cells (Stage II), rooting of shoots (Stage III), and acclimatization or hardening off (Stage IV).
Explants
The kind of explant chosen, its size, age and the manner in which it is cultured, can all affect whether tissue culture can be successfully initiated and whether morphogenesis can be induced. The juvenile form had pre-existing competent cells that were able to respond to auxin and become determined to form organs. However, the adult form appeared to lack cells with pre-existing competence to form organs, but competence was acquired by some callus cells once they had been initiated. Explants taken from mature shoots are frequently more liable than juvenile material to suffer necrosis, especially when surface disinfested and placed in culture (Hanus and Rohr, 1987). For tissue culture, juvenile explants are usually more readily established in vitro and grow and proliferate at a more rapid rate than adult material. This is particularly true with tree species where micropropagation of adult material is often difficult (Edwin et al., 2008).
Medium of Tissue Culture
gelling efficiency of agar. The initial pH can often be selected to ensure the integrity of the medium and the most rapid rate of culture growth (Edwin and Sherrington, 1984).
However a supply of reduced nitrogen is generally necessary for embryogenesis to occur in callus or suspension cultures.
Plant Growth Regulators
Endogenous compounds occur naturally within plant tissues. They have a regulatory role rather than nutritional activity in growth and development. These compounds are always active at very low concentrations and known as plant growth substances or plant hormones. Otherwise synthetic chemicals with similar physiological activities can be found and generally supplied in the medium for growth and morphogenesis in vitro. They are termed plant growth regulators (exogenous). Some recognized plant growth substances classes include such chemical compounds as auxins, cytokinins, gibberellins, ethylene and abscisins. The first two compound classes are by far the most important for regulating growth and morphogenesis in plant tissue and organ cultures.
Auxins are phytohormones very widely used in micropropagation medium to promote the growth of callus, cell suspensions or organs and to regulate morphogenesis, mainly when
combined with cytokinins. They influence cell enlargement, root initiation, and adventitious bud formation. The potential levels of endogenous auxin in the explant tissues are found to depend on the mother plant (donor plant growth state, growth conditions and season of sampling) from which they were collected. Therefore, the natural auxin will vary between cell strains of the same origin. Meristematic cells are referred to be particular active sites for the biosynthesis and/or the release of natural growth factors (Clare and Collin, 1974). The commonly auxins used in tissue culture consist of NAA (1-naphthaleneacetic acid); 2,4-D (2,4-dichlorophenoxyacetic acid); IAA (indole-3-acetic acid); IBA (indole-3-butyric acid); Dicamba (3,6-dichloro-0-anisic acid or 3,6-dichloro-2-methoxybenzoic acid); Picloram (4-Amino-3,5,6-trichloro-2-pyridinecarboxylic acid ).
Formerly called kinins, cytokinins are growth regulators that are required in tissue culture media for cell division, shoot multiplication, and axillary bud proliferation. In addition, they help delay senescence, and influence the transport of auxins. Cytokinins which are commonly used in tissue culture media include Kinetin (6-furfurylaminopurine); Zeatin (6-[4-hydroxy-3-methylbut-2-enlyamino]purine); BA or BAP (6-benzylaminopurine); 2iP
Zygotic Embryo Culture
Zygotic embryo culture is the aseptic isolation and growth of sexually produced embryo
in vitro with the objective of obtaining viable plants. Two principle types of embryo culture are concerned: a) culture of immature embryos, where isolated embryos are originated from unripe or hybrid seeds which failed to germinate are used to be grown; b) culture of mature embryos, where mature embryos are excised from ripe seeds and culture mainly to avoid inhibition in the for germination. An embryo callus possesses a high regenerative capacity compared to those derived from mature organs such as the leaf, stem and root. The age of embryo considerably influences the regenerative ability of plant calluses. The most useful and popular application of embryo culture, has been to raise rare hybrids by rescuing embryos of incompatible crosses. In fact, poor or abnormal development of the endosperm causes starvation and eventual abortion. Isolation of hybrid embryos before abortion and their in
vitro culture may circumvent these strong post-zygotic barrier. Plant embryo culture is an invaluable breeding technique as far as it is possible to synthesize hybrids from incompatible crosses. The regenerative potential is an essential prerequisite in non conventional method of plant genetic manipulation. Because of their juvenile nature, embryos have a high potential for regeneration and hence may be used for in vitro clonal propagation (Razdan, 2003).
Ovary Culture
The culture of unfertilized ovaries to obtain haploid plants from egg cell or other haploid cells of the embryo sac (synergid, antipodal) is called ovary culture, and the process is termed as gynogenesis (in vitro parthenogenesis). The rate of success varies considerably with species and is markedly influenced by explant genotype so that same cultivars do not respond at all. As in anther culture, gynogenesis may occur either via embryogenesis or through plantlet regeneration from callus. The frequency of responding ovaries (1-5%) and the number of plantlets per ovary (1-2) is quite low (Yunbi, 2010).
Tissue Culture Problems and Preventions
and disease-free material from genetically uniform plant. Basically in mother plant, vegetative parts are composed of cells that all have the same genetic potential (genotype), however
sometimes, intact plants may be composed of cells with different genotypes in separate layers or segments, or occurring in a random mosaic fashion. This occasionally genetic variable of mother plant tissues can result to genetic changes in culture. Thus, care must be taken during explant sampling to be sure that origin explants are not taken from plant tissue likely to be endopolyploid. Subsequent exposure to high levels of growth hormones such as 2,4-D should be also avoided as far as possible. Besides of this, polyploid or aneuploid cells, or cells which contain structurally altered chromosomes may arise directly in culture from callus or suspension cultures (atypical or abnormal plants). But the extent and occurrence of genetic disturbance depend on the genetic constitution of the cells which are cultured (Edwin and Sherrington, 1984).
Blackening or Browning. Plants naturally contain tannin substances or hydroxyphenol which affect growth. Explants from those plants frequently turn brown or black shortly after isolation and growth is inhibited and the tissue usually dies. Young tissues are often less liable to browning on excision than older one. Some pretreatments of plant material can be used to remove phenolic compounds such as: (a) leaching or soaking in running water during 24
hours or in sterile water for 2-3 hours; (b) absorption by activated charcoal in the medium; (c) absorption by polyvinylpyro-lidone (PVP); (d) modifying redox potential by using reducing agents (anti-oxidant); (e) inhibiting the action of phenol oxidase enzymes (darkness, pH) (Edwin F.G and Sherrington P.D, 1984).
Embryogenesis
development and need only to be released. The second is indirect embryogenesis where cell proliferation or callus is required. This occurs in differentiated non-embryogenic cells or
induced embryogenic determined cells. Therefore, under certain cultural and environmental conditions, it is possible to regulate initiation and development of embryogenic cells. In addition, the frequency of response will vary considerably with the genotypes of the species used. However the plants from monocotyledonae class have proven difficult to grow in culture and regenerate somatic embryos.
Tissue Culture Field in Plant Breeding
Tissue culture is a field of many facets. It is the ever-ready tool for breeders who hybridize plants by either sexual or asexual means. Cell and tissue culture are the innovative breeding techniques applied to meet the increasing need for improved crop varieties. Tissue culture techniques can shorten the time and can lessen the labor and space requirements needed to produce a new variety. It is a clean and rapid mean for genetic engineers to grow material for identifying and manipulating genes or to transfer interesting individual characteristics from one plant to another. Cell and tissue culture and recombinant DNA
technology constitutes an important aspect of plant biotechnology (Chawla, 2002). Generally, tissue culture plays an important role in wide array of fields, such as molecular biology, hybrid development, genetic engineering, pesticide testing, botany, chemistry, physics, and food science (Lydiane Kyte and John Kleyn, 1983).
Pathogen-free plants from tissue culture have opened the door to a freer exchange of
plants between states and countries. They have gained acceptance in world trade because of the virtual elimination of the disease introduction dangers which can occur. Most tissue cultured plants are true to type, more vigorous, more disease resistant, and disease free. The free exchange of tissue cultures will have a significant impact on global food problems by allowing for more improved cultivars to be obtained more rapidly to growers worldwide.
or ovary culture and chromosome elimination after inter specific hybridization. Double haploids or homozygous plants can then be obtained by colchicines treatment, fusion of
pollen nuclei and endomitosis. The interest in haploids by plant breeder stems largely from their considerable potential in plant breeding, especially for the quick production of homozygous plants and isogenic lines that would result in the early release of crop varieties. This would also facilitate work in inducing and detecting desirable mutations, transformations, and biochemical genetics. In other ways, somatic cell fusion (protoplast) under in vitro condition leads to the formation of cell hybrids which are very important for somatic cell genetics and crop improvement in the fields of plant breeding program. In addition, as the genetic variability is an essential component of any breeding program designed to improve the characteristics of crop plants, somaclonal variation through plant cell culture has been promoted as one of the potential sources of useful genetic variation. In another words, the genetic differences between plants regenerated from callus are sufficient to have attracted the interest of plant breeders as a new source of selectable variability. Thus in vitro culture of higher plants can be used for selection of mutants (in vitro selection) (Evans et
al.,1983 ; Chawla, 2002).
Oil Palm Genetic Improvement
The high world demand for oils and fats due to their uses as renewable bio-energy supplies and crucial source of food and without forgetting the environmental effect reduction need of oil palm have boosted the oil palm improvement sector. It was estimated about 155 million tons in 2007 (Oil world annual report 2008). Based on current demand for oil palm seeds in the world, Zamzuri et al., (1999) estimated that there is a ready market for more than 100 million tissue culture plantlets annually. The programme of crop improvement through the utilization of traditional breeding and selection methods, the development and benefits of tissue culture techniques and ongoing efforts to apply molecular and genetic engineering techniques to improve and modify oil composition, are continuously applied.
The four African Elaeis guineensis palms brought over by the Dutch in 1848, and planted in Buitenzorg Botanical Garden (now Bogor) Indonesia laid the foundation for the oil palm industry in Indonesia and Malaysia. From these, the Deli dura palms with unique and favourable fruit qualities were developed. In the conventional breeding methods, the Deli
Tenera palms (group B) which consists of large number of small bunches, are widely utilized for seed production and in genetic improvement programmes. Oil palm improvement is
carried out mainly in South East Asia by Indonesia and Malaysia. In Africa some research centre of oil palm include CEREPAH of La-Dibamba in Cameroon, CNRA of La-Me in Ivory Coast, NIFOR in Nigeria and Benin.
Apart from raising the total yield of fresh fruit bunches (FFB), breeding and selection
also focus on achieving high FFB oil and kernel content. The quality of the oil in terms of a high level of unsaturation [high iodine value (I.V)] and minor but important constituents such as vitamin E and carotenoids, is also being selected for. Vegetative characters are also taken into account where reduced rates of trunk extension and long bunch stalks are desirable attributes to facilitate harvesting while compact palms may allow higher planting densities of up to 180 palms/hectare (Basri et al., 2003). Elaeis oleifera, an oil palm species endemic to South and Central America readily hybridizes with Elaeis guineensis. This American species offers several desirable traits including slow height increment, high unsaturation and resistance to disease such as Fusarium wilt, which can be introgressed into the economically important Elaeis guineensis. Generally the objective of plant breeding in oil palm is to create genotypes with the maximum potential for oil and kernel production per unit of area, and
some morphological characters, the tolerance to abiotic and biotic stress.
Breure & Bos (1992) and Breure & Verdooren (1995) described the strategy for selecting superior materials, which involves three steps: 1) phenotypic selection of superior
200,000 plantlets per year (Zamzuri et al., 1999). As compared to seed production, tissue culture of oil palm offers several advantages (Sogeke, 1998). It allows rapid multiplication of
uniform planting materials with desired characteristics. This enables improvement of planting materials using existing individuals which have all or most of the desired qualities such as good oil yield and composition, slow vertical growth and disease resistance. Additionally, it also opens new avenues for producing novel planting materials via genetic engineering, because tissue culture is the means for regeneration of tissues transformed with genes for traits of interest.
The selection of the donor palm (ortet) is based on a range of characteristics such as yield, extraction rates, height and disease or drought tolerance. These palms are graded and a list submitted to the tissue culture laboratory for propagation (clonal propagation). Plant tissue aims to clone a minimum of 50 grade 1 palms, 40 grade 2 and 30 grade 3 suitable for planting in the field. The most responsive parts of the oil palm in regard to tissue culture are those areas which are meristematic (rapidly dividing) and young. Ideally the explant should provide large numbers of starting tissues and have little or no contamination. Oil palm tissue culture is employed both as a means for producing good tenera palms for commercial planting and to multiply good parents (both dura and pisifera) for seed production. It is also practised to expedite the exploitation of progenies from interspecific E. oleifera X E. guineensis crosses.
MATERIALS AND METHODS
Location and Time
This work was carried out in the Laboratory of Plant Biotechnology and Tissue Culture, Faculty of Agriculture, Bogor Agricultural University. It was started from August 2010 until September 2011.
Plant Materials and Equipments
Three kinds of plant materials have been collected to follow this study. The starting materials for callus induction include immature female flower, immature leaves and mature zygotic embryos isolated from Tenera variety donor palm. The selected trees were about 9 and 13 years old. The donor palm was selected according to some phenotypical characters such as fresh bunches borne, height, vigor, and health conditions. The vegetative material samples have been collected from the IPB Experimental Station of Cikabayan oil palm plantation. The three types of explants used in this study are shown in Figure 6.
Figure 6. The type of oil palm explants used in these experiments. A. Leaf explant,
B. Zygotic embryos explant, and C. Female flower explant
Media Preparation
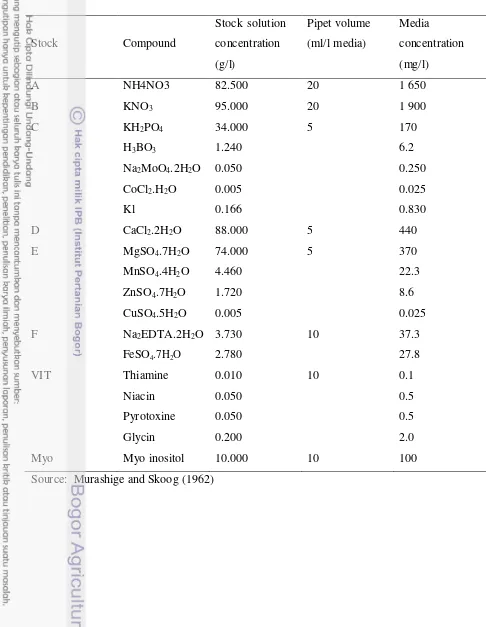
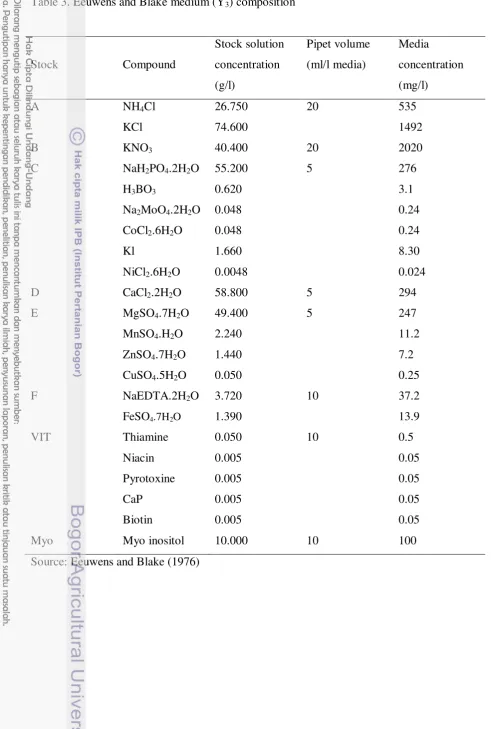
Two modified basal nutrient medium, Murashige and Skoog (1962) medium and Eeuwens and Blake (1976) medium, containing macro and micro salts (Table 2 & 3), were used for callus induction and embryo proliferation. These medium were supplemented with NAA, glutamine, asparagine, 2,4-D and Picloram.
Table 2. Murashige and Skoog (MS) medium composition used in these experiments
Stock Compound
Stock solution concentration (g/l)
Pipet volume (ml/l media)
Media
concentration (mg/l)
A NH4NO3 82.500 20 1 650
B KNO3 95.000 20 1 900
C KH2PO4 34.000 5 170
H3BO3 1.240 6.2
Na2MoO4.2H2O 0.050 0.250
CoCl2.H2O 0.005 0.025
Kl 0.166 0.830
D CaCl2.2H2O 88.000 5 440
E MgSO4.7H2O 74.000 5 370
MnSO4.4H2O 4.460 22.3
ZnSO4.7H2O 1.720 8.6
CuSO4.5H2O 0.005 0.025
F Na2EDTA.2H2O 3.730 10 37.3
FeSO4.7H2O 2.780 27.8
VIT Thiamine 0.010 10 0.1
Niacin 0.050 0.5
Pyrotoxine 0.050 0.5
Glycin 0.200 2.0
Myo Myo inositol 10.000 10 100
Sterilization Compounds
Standard sterilizing agents such as alcohol (5%; 10% and 70%), Sodium hypochlorite
(bleach) (5.25% NaClO), detergent,fungicide (Dithane M-45 2g/l, Agrept 2g/l) and antibiotic (Amoxicillin 1g/l), aquades steril were used in these experiments.
Laboratory Equipments and Tools
The equipments used in these experiments are pipet filler 1 ml and 10 ml, autoclave 2 l, wrapping plastic, glass bottles (baby food jar) 250 ml and 270 ml, magnetic stirrer, magnetic shaker, digital analytical balance scale, bunsen burners, erlenmeyer volumetric flask 250 ml; 500 ml and 1000 ml, graduate cylinder 100 and 500ml, hot plate, laminar air flow cabinet, spatula, pincet, sterilized blades, scalpel (knife), forceps, pH meter, petri dish, thermometer, pot holder, refrigerator, gas ring, labeler, cup holder 1000 and 2000 ml, towel, paper, sprayer, disposable mask, disposable hand gloves
Methods
Three independent experiments were applied in this study related to the three kinds of explant sources.
Experiment 1. Callus and Somatic Embryos Induction from Young Leaf Explant
Oil Palm Tree Sampling and Explant Processing
The donor palm was a commercial Tenera variety with 9 years old. After the donor palm was identified, the spear was carefully removed by using a curved knife and with the help of a specially designed protocol. The procedure should ensure that the donor palm and the candidate spear
are not damaged during the isolation process. As soon as the spear was isolated from the palm, it was sprayed with 96% alcohol, wrapped and placed inside a sampling bag for protection. The protected
spear was then taken immediately to the laboratory for processing (Figure 7A.).
The spear is a cluster of unopened young leaves or fronds which are different in terms of their
age (Figure7B). Regarding their age, the leaves were carefully removed from the spear in laminar air flow cabinet of the dissecting or plant preparation room and grouped per cluster (Figure 7D). The leaves were then soaked in a 10 % NaOCl (household bleach) solution for 10 minutes of sterilization and rinsed three times in sterile distilled water. Each cluster of leaves was then soaked in 10% sterile
Figure 7. Young leaves sample started material (spear) preparation process from the field to laboratory. A. Isolated spear, B. Young leaves clusters in the spear, C. Isolated cluster of young leaves, and D. Soaked Young leaves into sucrose solution after sterilization.
Callogenesis Mixing Medium (Combination)
Ten treatments have been applied C1, C2, C3, C4, C5, C6 for Murashige and Skoog (1962) medium and four others M1, M2, M3, M4, for Eeuwens and Blake (1976) medium (Y3). The tested medium compositions were as follow:
C1 = MS + 0 µM 2,4-D + 107.41µM NAA C2 = MS + 22.62 µM 2,4-D + 107.41µM NAA C3 = MS + 45.24 µM 2,4-D + 107.41µM NAA
C4 = MS + 67.86 µM 2,4- D + 107.41µM NAA C5 = MS + 450 µM picloram
C6 = MS + 450 µM2,4-D
M1 = Y3 + 0 µM2,4-D + 107.41µM NAA M2 = Y3 + 22.62 µM 2,4-D + 107.41µM NAA
M3 = Y3 + 45.24 µM2,4-D + 107.41µM NAA M4 = Y3 + 67.86 µM 2,4 D + 107.41µM NAA
The pH of nutrient media (C1, C2, C3, C4, M1, M2, M3, and M4) was adjusted to 5.7 with 1N NaOH or HCl before the purified agar was added and autoclaved. For each media, 5% of commercial sugar and 0.9 % of purified agar to make solid medium were used.
A B C
According to C5 and C6 media, 3% of commercial sugar was used while the pH was adjusted to 5.8 before adding 0.3g/l charcoal and 0.9% of purified agar. The Media were dispensed into
culture jar (250 ml) and covered by using heat resistant plastic. Then media were autoclaved for 20 minutes at 121 oC, 0.1 bar (17.5 psi).
The experiment was designed into factorial randomized complete block design with 3 replications. Each treatment was applied into 30 jars so that a total of 960 explants have been used per replication. The first factor is the rank of leaf with 5 levels (L-5, L-6, L-7, L-8, L-9) and the second factor is the concentration of the tested growth hormone with 10 levels (C1, C2, C3, C4, C5, C6, M1, M2, M3, M4). However another factor as the type of basal media solution with two levels (MS media and Eeuwens media) was also taken in account.
Environmental Culture Conditions
Cultures were incubated under darkness to induce callus in a temperature-controlled room at 28 ± 1 oC. Observations were done every week for the percentage of contamination and every four weeks for the percentage of explant bearing callus, the percentage of embryogenic callus, and the percentage of embryoid. Qualitative variables observing explants color (browning), the color of callus, and the callus structures (nodular, root-like, and embryogenic) were recorded. As soon as calluses were initiated, they were transferred in the
new same media composition. The subculture of the initial explant for callus initiation was done every 12 week intervals, while the initiated callus was subcultured every 8 week intervals.
Culture Conditions of Embryos
The embryoid stage marks the beginning of the light phase of the oil palm tissue culture process. The embryogenic cells and embryoid cultures were transferred from continuous dark culture to continuous light culture room for incubation with more light intensity of 1000 lux at 29 ± 1 oC. The number of embryo cells was recorded on the input sheet.
Embryogenesis Mixing Medium
gradually reduced concentration of 2,4-D and NAA from the 3rd to 4th subcultures. Each subculture was done after 6 week intervals.
Subculture’s Flow Chart of all the three Experiments
Experiment 2. Induction of Somatic Embryo from Mature Zygotic Embryo Explant
Explant Sampling and Sterilization Process
Oil palm fresh mature fruit bunches were cut from 9 and 13 years old palms of Tenera variety in the farm. A total of 3600 fruits were isolated from bunches (Figure 8A) in the tissue culture
laboratory and all their mesocarp was also removed (Figure 8B). 1800 seeds were treated for seed production process. The endocarp and endosperm of the obtained seeds from seed production process
were then cracked and zygotic embryos were isolated for sterilization. The endocarp of the other 1800 fresh seeds was also cracked so that the kernel of seeds was isolated (Figure 8C) and stored in closed
container to be sterilized. For both seeds, the sterilization have been done by mixing 4 g/l of fungicide (2g/l dithane and 2g/l benlate) for 1 hour; then soaked into 1 g/l of antibiotic for 1 hour, and 10 % commercial bleach for 20 minutes. In the laminar, isolated endosperm was then cracked to expose the fresh zygotic embryo (Figure 8D), so that these embryos were
carefully isolated and placed into petridishes for sterilization by using 5 % commercial bleach for 5 minutes. The sterilized zygotic embryos have been cultured into treatment media for 10 explants per vessel (Figure. 8E).
Cultured explants Subculture every 3 months in dark
Induced callus
Subculture every 2 months in dark Embryogenic callus
or embryoids
Figure 8. Mature zygotic embryo explants preparation process obtained from the laboratory. A. Isolated oil palm fresh fruits, B. Isolated seeds, C. Isolated kernels, D. Cracked endosperm seeds with zygotic embryo, and E. Isolated and cultured zygotic embryos
Callogenesis Mixing Medium (Combination)
Ten medium composition have been formulated as in the first experiment C1, C2, C3, C4, C5, C6 for Murashige and Skoog (1962) medium and four others M1, M2, M3, M4, for Eeuwens and Blake (1976) medium (Y3).
The experiment was designed into factorial randomized complete block design with 3 replications. Each treatment was applied into 12 jars so that a total of 1200 explants were used per replication. The first factor is the type of zygotic embryo explants with 3 levels (young embryo, mature embryo and embryo from seed production process) and the second factor is the concentration of the tested growth hormone with 10 levels (C1, C2, C3, C4, C5, C6, M1,
M2, M3, M4). However another factor as the type of basal media solution with two levels (MS media and Eeuwens media) was also taken in account.
Environmental Culture Conditions
As in the first experiment, the same environmental culture conditions were also applied for callus induction and embryogenic callus obtained from mature zygotic embryo explant in this experiment.
A B C
Embyogenesis Mixing Medium
Embryogenic calluses were transferred to embryogenic callus media for their
proliferation and maturation. This media was consisted of the same basal medium composition with 25% of gradually reduced concentration of 2,4-D and NAA from the 3rd to 4th subcultures. Each subculture was done after 6 week intervals.
Experiment 3. Induction of Somatic Embryo from Immature Female Flower Explant
Explant Sampling and Sterilization Process
Isolated immature female inflorescences from 9 and 13 years old oil palm trees of commercial Tenera variety were used as explant source. As soon as these inflorescences were cut, they were sprayed by using 96% alcohol and carefully plasticized (Figure 9A) in the field then brought to the laboratory. In the plant preparation room, the inflorescence spathes were carefully removed aseptically. Spikelets were isolated from inflorescence (Figure 9B) and
sterilized by 10% of commercial bleach for 30 minutes. Immature flowers were isolated from spikelets and sterilized using 70 % alcohol for 10 minutes then soak into 10 % of commercial bleach for 20 minutes and excised. From these inflorescences 3 600 pieces of flower explants (Figure 9C) were extracted and sterilized using 5 % of commercial bleach for 5 minutes then cultured onto callus initiation medium. The entire, half and quarters sliced length flowers
were used as explants. Firstly eight explants were inoculated into medium per vessel then after one month, the number of four explants was applied per bottles media.
Figure 9. Immature female flower explant preparation process in the laboratory. A. Isolated female inflorescence from selected donor palm, B. Isolated spikelets, and C. Isolated female flower explants
Callogenesis Mixing Medium (Combination)
As in the experiments one and two, the same ten medium composition were
formulated for this experiment. There are C1, C2, C3, C4, C5, C6 for Murashige and Skoog (1962) medium and four others M1, M2, M3, M4, for Eeuwens and Blake (1976) medium.
The treatments have been arranged into factorial randomized completely block design with 3 replications. A total of 1200 explants have been used per replication. The first factor is the age of the oil palm explant (9 and 13 years old), and the second factor is the concentration of the tested growth hormone with 10 levels (C1, C2, C3, C4, C5, C6, M1, M2, M3, M4). However another factor as the type of basal media solution with two levels (MS media and Eeuwens media) was also taken in account.
Environmental Culture Conditions
The same environmental culture conditions and observations were also used for this experiment as in the first and second experiment, to induce callus and embryogenic callus.
Data Analysis
The obtained data was analyzed using Excel software and ANOVA. The means were
RESULTS AND DISCUSSIONS
Experiment 1
Contamination and Oxidation of Young Leaf Explant
The level of contamination of leaves explant after 36 weeks varied from 1.11 to 100 % and was no longer depend on the type of medium and the rank of leave (Table 4). The level was quite high because of the autoclave mechanical problem in the laboratory. In fact, the autoclave could not perform the sterilization of medium. In addition, the spear was sampled from the ortet (donor palm) during the raining season.
Table 4. Percentage of contamination of oil palm leaves explant per medium composition and rank of leaf after 36 weeks of culture (%) Note: C1-4. MS+(0, 22.62, 45.24, 67.86µM) 2,4-D+107.41µM NAA, C5 . MS+450µM picloram, C6. MS+
450 µM, M1-4. Y3+(0, 22.62, 45.24, 67.86µM) 2,4-D+107.41µM NAA. L-x. leaf number in the spear.
Y3 medium as compare to MS media, and the increase of activated charcoal from 0.3% to 05% was considerably reduced the level of oxidation on oil palm explants used.
Tale 5. Influence of various medium composition (NAA and 2,4-D) and young leaves rank of oil palm (E. guineensis) cv. Tenera spear on percentage of oxidized calli after 4 subcultures
The leaf explants swollen after 2-3 weeks in callus induction culture medium. Tisserat (1987) reported that callus production is always extremely slow for palms. This assertion was in harmony with our result. In fact callus induction was first observed from young leaf number nine (L-9) after 8-12 weeks of culture on medium C1 containing only NAA (107.41µM) as plant growth regulator. These calli appeared at the top of explant surface on the vascular region as shown in Figure 10.
Figure 10. Friable nodular callus (NC) proliferation from young leaves explant of oil palm (E. guineensis) cv. Tenera 24 weeks after inoculation