IN VITRO ANALYSIS OF BIPHASIC CALCIUM PHOSPHATE
AND HYDROXYAPATITE AS BONE IMPLANTS
RAHMI SOLIHAT
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
STATEMENT ON THESIS
I hereby declare that the thesis of “In vitro analysis of biphasic calcium phosphate and hydroxyapatite as bone implants” is my work under direction of the supervising committee and has not been submitted in any form to any college. The source of information is derived or quoted from the published or unpublished work by other authors mentioned in the text and listed in the reference at the end of this thesis.
Bogor, 2011
ABSTRACT
RAHMI SOLIHAT. In Vitro Analysis of Biphasic Calcium Phosphate and Hydroxyapatite as Bone Implants. Supervised by KIAGUS DAHLAN and BOY M. BACHTIAR.
Hydroxyapatite (HA) and Biphasic Calcium Phosphate (BCP) are widely used as bone implant materials because of its biocompatibility. The minimum requirement of biocompatible materials is nontoxic. The reduction of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to a purple formazan is used to measure toxicity of implant materials that exposed to the cell. The biocompatibility also can be observed from the attachment between cell and implant materials under electron microscope. This research reported that HA and BCP derived from eggshell using precipitation and hydrothermal method were nontoxic. MTT toxicity analysis was held during 1, 2 and 3 days immersion of osteocalcifications that secret protein collagen were appeared. This MTT analysis result was appeared in a good agreement with Scanning Electron Microscope (SEM) characterization, that showed the attachments of either HA or BCP to the osteoblast cells after 1 day immersion. The SEM photos after incubated 3 days showed that cells start to calcify and secret protein. The calcification and secretion of protein collagen performed much better after 14 days immersion. In conclusion, HA and BCP derived from eggshell were nontoxic and performed good adhesion interaction to the host cells in vitro.
ABSTRAK
RAHMI SOLIHAT. Pengujian kalsium fosfat dua fasa dan hidroksiapatit secara in vitro sebagai implan tulang. Dibimbing oleh KIAGUS DAHLAN dan BOY M. BACHTIAR.
Hidroksiapatit (HA) dan Kalsium Fosfat Dua Fasa (KFDF) digunakan secara luas sebagai bahan implan tulang karena sifat biokompatibilitasnya. Persyaratan minimum bahan biokompatibel adalah tidak toksik. Reduksi 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) menjadi formazan yang berwarna ungu digunakan untuk mengukur toksisitas bahan implan yang diberikan terhadap sel. Biokompatibilitas juga dapat diamati melalui adanya pelekatan antara sel dan bahan implan melalui mikroskop elektron. Penelitian ini melaporkan bahwa HA dan KFDF yang diperoleh dari cangkang telur melalui metode presipitasi dan hidrotermal bersifat tidak toksik. Pengujian toksisitas dilakukan dengan perlakuan perendaman HA dan KFDF di dalam sel osteoblas MG-63 selama 1, 2, dan 3 hari. Viabilitas sel yang direndam dengan HA dan KFDF lebih dari 100% untuk 1, 2, dan 3 hari perendaman, yakni 476.12%, 380.60%, 182.59% dan 307.21%, 128.36%, 155.47%. Hasil ini menunjukkan bahwa HA dan KFDF bersifat tidak toksik dan menginduksi sel-sel untuk tumbuh. Viabilitas sel cenderung menurun karena terjadinya proses osteokalsifikasi yang mensekresikan protein kolagen. Hasil pengujian MTT ini sesuai dengan hasil karakterisasi Scanning Electron Microscope (SEM) yang menunjukkan terjadinya pelekatan antara HA atau KFDF dengan sel osteoblas setelah 1 hari perendaman. Foto SEM sampel setelah inkubasi selama 3 hari menunjukkan bahwa sel mulai mengalami kalsifikasi dan mensekresikan protein. Kalsifikasi dan sekresi protein kolagen semakin terlihat setelah perendaman selama 14 hari. Jadi, HA dan KFDF yang diperoleh dari cangkang telur bersifat tidak toksik dan memiliki interaksi adesi yang baik dengan sel secara in vitro.
SUMMARY
RAHMI SOLIHAT. In Vitro Analysis of Biphasic Calcium Phosphate and Hydroxyapatite as Bone Implants. Supervised by KIAGUS DAHLAN and BOY M. BACHTIAR.
The minimum requirement of synthetic biomaterial is must be biocompatible. Biocompatibility means that material is nontoxic and able to interact with the host cells. The analysis of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to detect toxicity. Interaction between cells and bone implants were characterized by Scanning Electron Microscopy (SEM). Previously, hydroxyapatite is well known as biocompatible implantable materials. But, the dense hydroxyapatite is almost non-resorbable when used as bone implant. In the other hand, the porous β-tricalcium phosphate (TCP) displays affinity for high speed biological degradation. TCP was later identified as Biphasic Calcium Phosphate (BCP) consisting of hydroxyapatite (HA) and tricalcium phosphate (β-TCP).
Cytotoxicity analysis of bone implants were exposed to the osteoblast cells. The bone implants are powder of HA and BCP. HA synthesis was using precipitation method as Dewi’s work (2009). BCP synthesis was using hydrothermal method as Fajriyah’s work (2010). Both HA and BCP were using CaO and (NH4)2PO4 as the raw materials. CaO was derived from chicken
eggshells that rich of calcium carbonates (CaCO3). Chicken eggshells were
calcined at 1000°C for 5 hours based on Nurlaela’s work (2009). Calcinations of eggshell formed CaO and removed carbonate ion. Dewi (2009) was then optimizing the molarities of CaO and (NH4)2PO4, which were 0.1, 0.2, 0.3, 0.4,
and 0.5 with Ca/P 1.67 as the characteristic of HA. X-Ray Diffraction (XRD) characterizations showed that pure HA was produced from 0.3 M of CaO. Fajriyah (2010) was done an optimation of sintering temperature in synthesizing BCP, which were 2, 4, and 6 hours. XRD characterization showed that BCP phase was formed by 6 hours of sintering. MG63-cell line was using as the prototype of human osteoblast cells. The osteoblast cells are one type of human bone cells.
that proved by the increasing of cells viability after 2 and 3 days of immersion. All samples that exposed by bone implants have higher than 100% of cells viability which enable us to categorize either HA or BCP as nontoxic biomaterials.
SEM photos show that either HA and BCP were small cubes crystallites. In the other hand, SEM photos of cells only incubated for 1, 3, and 14 days show that cells structure presented as small flatten balls-like morphology. Interaction between cells and bone implants were showed by the adhesion that covered almost all structure of HA or BCP after 1 day of immersion. Crystallite HA and BCP structure were still be recognized for 1 day of immersion. SEM photos after 3 days of immersion show that cells start to calcify and secret sticky extracellular matrices protein which covered all of HA and BCP structures. This osteocalcifications were appeared in a good agreement as MTT result after 3 days of immersion. SEM photos after 14 days of immersion show perfectly greater interaction of secreted protein and bone implants.
In conclusion, HA and BCP derived from eggshell were nontoxic and performed good adhesion interaction to the host cells in vitro.
© Copyright of IPB, the year 2011 Copyright reserved by the law
IN VITRO ANALYSIS OF BIPHASIC CALCIUM PHOSPHATE
AND HYDROXYAPATITE AS BONE IMPLANTS
RAHMI SOLIHAT
A THESIS
in partial fulfillment of the requirements for the degree of Master of Science of
Biophysics Program
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
APPROVAL SHEET
Theses Title : In Vitro Analysis of Biphasic Calcium Phosphate and Hydroxyapatite as Bone Implants
Name : Rahmi Solihat
NRP : G751090011
Approved by:
The Commission of Supervisors
Date of Examination: 8 Februari 2010 Date of Graduation:
Dean of the IPB Graduate School
Prof. Dr. Ir. Khairil Anwar Notodiputro, MS Head of Biophysics
Postgraduate Program
Dr. Agus Kartono
Certified by:
Prof. drg. Boy M. Bachtiar, MS., Ph.D Co-Supervisor
ACKNOWLEDGEMENTS
The author would like to expresses sincere appreciation of Dr. Kiagus Dahlan, Prof. drg. Boy M. Bachtiar, MS., Ph.D, and Prof. Djarwani Soejoko for directing the research, Setia Utami Dewi, M.Si for the help in the laboratory sample preparation, Maysyaroh, S.Si for in vitro analysis, Ir. Basril Abbas for gamma radiation sterilization, and Wikanda for SEM characterization. The author is also grateful to the National Education Department for awarding her a scholarship under the programme Beasiswa Unggulan and Hibah Kompetensi for funding this research. To all of these people, I owe its whole-hearted gratitude that impossible to describe.
I am also thankful for Ibu, Bapa, Aa Diki & Teh Tina, De Rahmat, Aa Rudy, Mama, Papa, Teh Mira & Ka Haris, De Putri and whole families, 2009’s Biophysics for the pray and support in bringing about this thesis. I give all respect that impossible to describe, thank you so much. Nonetheless, I also welcome any critical feedback and advice from readers in order to maintain it as successful project. Hope this thesis could be useful.
CURRICULUM VITAE
TABLE OF CONTENTS
Biphasic Calcium Phosphate (BCP)……….. 5
BCP Properties………..………. 5
BCP Synthesis……..………6
In vitro Study... 7
MATERIALS AND METHODS... 9
Place and Time Schedule... 9
Materials and Equipments... 9
Experimental Method... 11
HA Synthesis... 11
BCP Synthesis... 12
Preparation for In vitro analysis... 13
Cells Culture...13
Cells Concentration Counting...13
Sample Preparation for SEM Characterization…………...15
Sample Characterization... 15
MTT analysis...15
Scanning Electron Microscopy (SEM) characterization... 15
TABLES LIST
Page 1. Family of calcium phosphate compounds... 4 2. Concentration and volume in culturing cells... 19 3. Absorbance of cells, HA, and BCP after 1, 2, and 3 days of
immersion……… 20
FIGURES LIST
Page 1. The crystal structure of hydroxyapatite……... 3 2. XRD pattern of HA from wet precipitation method………... 5 3. BCP X-Ray Diffraction Pattern from 9000C sintering... 6 4. SEM picture showing the morphology of osteoblast cells on BCP
implant materials after 2 days immersion in vitro... 7 5. Precipitation process in HA synthesis………. 12 6. Hydrothermal process in BCP synthesis………. 12 7. Scheme of grid on hemocytometer glass board……….. 14 8. Scheme of 96-well plates for MTT analysis………... 14 9. Visible spectrophotometer for absorbance analysis……….... 14 10. (a) JEOL JCM-35C scanning electron microscope and (b) Ion
Sputter JFC-1100 machine……….. 15 11. XRD Pattern of HA from precipitation method……….. 17 12. XRD Pattern of BCP from hydrothermal method………... 17 13. Color change effect of MTT solution dropped to the samples……… 19 14. Percentage of cells viability of cells, HA, and BCP samples 1, 2,
and 3 days of immersion... 21 15. SEM photos of bone implants (a) HA (b) BCP powder with 5000
times-magnification... 21 16. SEM photos of cells only after (a) 1, (b) 3, and (c) 7 days of
immersion with 5000 times-magnification... 22 17. SEM photos of cells exposed by BCP after (a) 1, (b) 3, and (c) 14
days of immersion with 5000 times-magnification... 23 18. SEM photos of cells exposed by HA after (a) 1, (b) 3, and (c) 14
APPENDICES LIST
Page 1. Flow Chart of BCP Synthesis……….………… 31 2. Flow Chart of Hydroxyapatite Synthesis………..……..… 32 3. Flow Chart of (a) cell culture (b) cytotoxicity analysis by MTT
analysis ………... 33
INTRODUCTION
Background
Recent developments on bone reconstruction concern mainly in implantable synthetic materials, although there are different kind materials in bone reconstruction as like as autograft, allograft and xenograft. Autograft are the materials from others body part of patient itself for bone reconstruction. However, the clinical use involves some difficulties such as septic complication, viral transmission and the unavailability of native bone (Daculsi 2004). On the other hand, allografts are more readily available than autograft since it is from the donor. In spite of that, allograft bone has revealed a risk of disease transmission (such as HIV and hepatitis), post-surgery pain, increased blood loss, secondary surgical wounds, risk of thrombosis and it is also difficult to shape (Daculsi 2004 & Ooi et al 2007). A significant additional limitation of allograft is the delayed remodeling by the host. In the case of very large defects, the allograft may remain in the implant site throughout the patient’s life, creating area more prone to fracture or infection (Ooi et al 2007). Likewise, xenograft also bears limitation since it is from others species as like as animals, which has different characteristic in mineral bone composition (Dewi 2009). In short, synthetic biomaterials were preferable in answering others method limitation in bone reconstruction.
The minimum requirements of synthetic biomaterial include the following: (1) the material must be biocompatible, such as nontoxic, blood- or tissue-compatible, noncarciogenic; (2) the material must not leach or release harmful components into the living system; (3) the mechanical and physical properties of the material, such as strength, elasticity, durability, and stability, must be appropriate for the intended application; and (4) the desired mechanical properties must last for the expected life of the implant; (5) the materials must be sterilizable (Shi et al 2004).
Gnanam 2002). While, the porous β-tricalcium phosphate (TCP) displays affinity for high speed biological degradation, they are bioactive and bioresorbable materials (Victoria & Gnanam 2002). TCP were later identified as Biphasic Calcium Phosphate (BCP) consisting of hydroxyapatite (HA) and tricalcium phosphate (β-TCP) (Li et al 2003). Recently, there is a growing interest in developing Biphasic Calcium Phosphate (BCP) ceramics as implant materials because they are more effective in bone repair or regeneration which proved in vitro and in vivo (Ramay & Zhang 2004).
Hypotheses
1. Both BCP and HA in vitro analysis show as nontoxic materials.
2. There appear morphological properties change of BCP and HA after in vitro indicating adhesion interaction between HA or BCP and cells.
Objective
In vitro analysis that has been held from 0 up to 14 days could explain the biocompatibility. In this study, the biocompatibility of BCP compared to HA was being performed through in vitro analysis by cytotoxicity screening by using MTT analysis. Furthermore, the change of morphological properties after in vitro was being characterized using Scanning Electron Microscopy (SEM). BCP was being synthesized through hydrothermal method while HA through precipitation method.
Benefit
LITERATURE REVIEW
Hydroxyapatite (HA)
HA Properties
There are many apatite compounds, including fluorapatite, chlorapatite, carbonate-apatite, and hydroxyapatite (Oliveira et al 2006). Hydroxyapatite is chemically similar to the mineral component of bones and hard tissues in mammals; its chemical formula is Ca10(PO4)6(OH)2 (Fernandes & Laranjeira
1999). The chemical nature of hydroxyapatite lends itcellsf to substitution, meaning that it is common for non-stoichiometric hydroxyapatites to exist. The most common substitutions involve carbonate, fluoride and chloride substitutions for hydroxyl groups, while defects can also exist resulting in deficient hydroxyapatites.
Hydroxyapatite is bioactive material; the ability to integrate in bone structures and support bone ingrowths, without breaking down or dissolving. Hydroxyapatite is a thermally unstable compound, decomposing at temperature from about 800-1200°C depending on its stoichiometry. Hydroxyapatite is a calcium phosphate including hydroxide, and its Ca/P ratio is represented as 1.67. The structure of hydroxyapatite is hexagonal, which has unit cell size, a = 9.418 Å and c = 6.883 Å (Shi et al 2004). This structure can be assumed as ideal hexagonal crystal structure (closed-packed) from PO4-3 ion, which is inserted by
Ca+2 ion and OH- ion among the empty space of PO
4-3 ions (Figure 1) (Shi et al
2004).
31
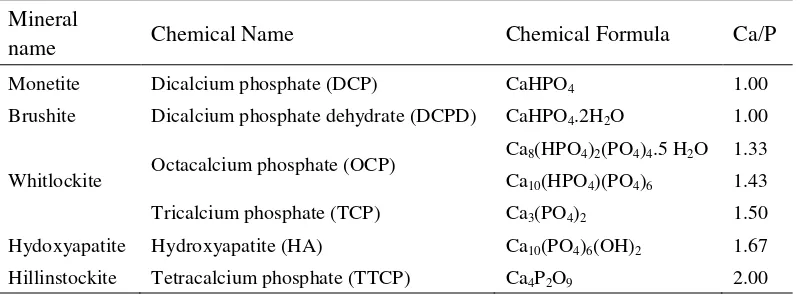
Table 1 Family of calcium phosphate compounds (Shi et al 2004).
Mineral
name Chemical Name Chemical Formula Ca/P
Monetite Dicalcium phosphate (DCP) CaHPO4 1.00
Brushite Dicalcium phosphate dehydrate (DCPD) CaHPO4.2H2O 1.00
Whitlockite Octacalcium phosphate (OCP)
Ca8(HPO4)2(PO4)4.5 H2O 1.33
Ca10(HPO4)(PO4)6 1.43
Tricalcium phosphate (TCP) Ca3(PO4)2 1.50
Hydoxyapatite Hydroxyapatite (HA) Ca10(PO4)6(OH)2 1.67
Hillinstockite Tetracalcium phosphate (TTCP) Ca4P2O9 2.00
There are different phases of calcium phosphate ceramics that can be used in medicine, depending on whether a bioactive or a resorbable material is desired (Table 1) (Shi et al 2004). Generally, dense hydroxyapatite does not have the mechanical strength to enable it to succeed in long term load bearing applications. But, hydroxyapatite may be employed as bone fillers in forms such as powders, porous blocks or beads to fill bone defects or voids. These may arise when large sections of bone have had to be removed (such as bone cancers) or when bone augmentations are required (such as dental applications). The bone filler would provide a scaffold and encourage the rapid filling of the void by naturally forming bone and provides an alternative to bone grafts. It would also become part of the bone structure and would reduce healing times compared to previous bone prostheses.
HA Synthesis
Hydroxyapatite in particulate form can be produced by using a variety of methods, such as wet method, dry method and hydrothermal method (Shi et al 2004). In this study, hydroxyapatite synthesis was being performed through wet method that is precipitation. Santos et al (2004) have been succeeding in synthesizing hydroxyapatite through wet precipitation method based on the chemical reaction below:
32
Figure 2 XRD pattern of HA from wet precipitation method ( HA) (Santos et al 2004).
The 0.5 M Ca(OH)2 suspension was prepared using Ca(OH)2 powder. The
suspension was degassed, vigorously stirred and heated for one hour at 40°C temperatures. The 0.3 M H3PO4 solution was dropped into the Ca(OH)2
suspension at same temperature for approximately one hour at the rate 6 mL/min.
The pH was adjusted become pH = 7 by addition of 1 M NH4OH solution at the
end of the precipitation process. The XRD result showed below match to the hydroxyapatite pattern (Santos et al 2004).
Biphasic Calcium Phosphate (BCP)
BCP Properties
Development of biphasic calcium phosphate (BCP), especially with hydroxyapatite (HA: Ca10(PO4)6(OH)2) and tricalcium phosphate (TCP:
Ca3(PO4)2) has drawn considerable attention. HA and TCP, although have similar
33
bioactivity relies on physical and chemical properties of biphasic calcium phosphate ceramics (Victoria & Gnanam 2002).
It is also noted that the presence of small amount of β-TCP 1100°C and 1200°C may be associated with the partial decomposition of HA phase. If HA is annealed in air at 1200°C, it decomposed into the β-TCP phase according to chemical reaction below (Ooi et al 2007).
Ca10(PO4)6(OH)2 → 3β-Ca3(PO4)2 + CaO + H2Ogas
BCP Synthesis
Kumar et al (2005) was succeed in synthesizing BCP from sintering process. Firstly, the BCP granules were synthesized by the microwave. Calcium hydroxide and diammonium hydrogen ortho phosphate (DAP) were used as raw materials. The amounts of reactants used for the reaction were calculated based on the Ca/P molar ratio of 1.58. Weighed amounts of the starting granules were dissolved in water and the DAP solution was added to the calcium hydroxide solution. The solution is then exposed to 900°C microwave irradiation in a microwave oven during 20 minutes. The product was then dried in an oven. The result of XRD has a major peak indicating TCP that is (0 2 0) peak as shown below.
34
In vitro study
Biocompatibility testing in vitro often involves the detection of cell damage and death as like as cytotoxicity. Coelho et al (2000) was used the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to a purple formazan product in the MTT assay to estimate cell viability. The screening test is useful to detect over toxic effects of a test material in showing incompatibility. For instance, the rate of growth, proliferation and differentiation of cells on a material may be dependent on successful initial attachment and spreading of the cells on the surface of the implant materials. In this respect, the initial and short-term responses of cells to an implant material in vitro may provide valuable indicators of the long-term biocompatibility in vivo (Lin et al 1997).
Riberio et al (2009) have been succeed in showing cell attachment to the BCP implant materials within 2 days of immersion in osteoblast cells as SEM pictures show below.
MATERIALS AND METHODS
Place and Time Schedule
This research was being conducted from February through September 2010 which took place in IPB-Biophysics Laboratory in sample preparation, while in vitro sample analysis was done in Oral Biology Laboratory of Dental Faculty, University of Indonesia. Sample sterilization was done in National Nuclear Energy Agency (BATAN) Pasar Jumat. Sample characterization was done in National Nuclear Energy Agency (BATAN) Serpong for SEM characterization.
Materials and Equipments Materials
1. BCP synthesis materials :
a. Calcium oxide from chicken eggshell
b. Pro-analyze ammonium hydro phosphate; (NH4)2HPO4
c. Aquabides
2. MG-63 cell line as osteoblast cell 3. Cell culture medium materials :
a. Dulbecco’s Modified Eagle’s Medium (DMEM) b. Fetal Bovine Serum (FBS)
c. Penicillin Streptomycin d. Fungizone
4. Trypsin EDTA 5. Trypan blue
6. Washing medium materials : Phosphate Buffered Saline (PBS) 7. Cytotoxicity test materials :
a. MTT (3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide)
37
8. SEM preparation materials :
a. Phosphate Buffered Saline (PBS) b. 8% glutaraldehyde
c. Ethanol
Equipments
1. BCP and HA synthesis equipments (Appendix 4) : a. Analytical balance
2. Gamma radiation sterilization with cobalt 60-radiation source (Appendix 6)
3. In vitro analysis equipments (Appendix 5) :
a. 0.2 m-sterile syiringe filter (Corning, Germany) b. 50 mL-syringe (Terumo, Japan)
c. 15 mL- and 50 mL-tube (Falcon, USA) d. Scrapper
e. Micropipette (Eppendorf, Germany) f. Tips micropipette
38
i. Incubator (Memert)
j. Cell culture dish (35 mm×10 mm) k. 96-well plates (NUNC, Denmark ) l. Microscope (Nikon Elipse 80i)
m. Biohazard safety cabinet (ESCO Micro PTE Ltd.) n. Water bath
o. Centrifuge (Sorvall)
p. Vortexer (Bio-rad BR 2000) q. Shaker (Certomat)
4. Characterization equipments (Appendix 6) :
a. Scanning Electron Microscope (SEM) (JEOL JCM-35C) b. Ion Sputter JFC-1100 machine
c. Bio-Rad Microplate Reader Benchmark Visible Spectrophotometer
Experimental Method
Experimental method is shown on flow chart on Appendices 1, 2, and 3.
Hydroxypatite synthesis
The same raw materials of CaO as synthesizing BCP that gained from chicken eggshells were used to prepare 100 mL-CaO suspension which was seen as white thick fluid. Chicken eggshells that mostly contain CaCO3 were calcined
at 1000°C for 5 hours as chemical reaction below:
CaCO3 CaO + CO2
0.3 M CaO suspension of eggshell product was dropped by 0.18 M of clear (NH4)2HPO4-solution in 100 mL aquabides in 37°C while stirring at 300 rpm at
the rate of 7 mL/min. The final suspension was then be filtered under vacuum. The filtered cake was then being dried in the furnace at 110°C during 5 hours. The dried powder was then be sintered at 900°C during 5 hours by using furnace. The chemical reaction of CaO and (NH4)2HPO4 was as below:
39
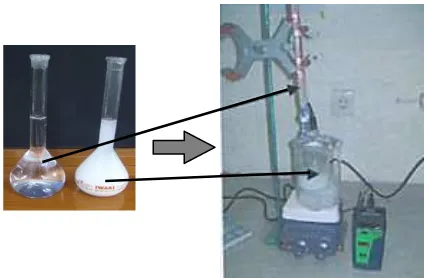
Figure 5 Precipitation process in HA synthesis.
BCP Synthesis
The solution of BCP in this research was prepared by a precipitation method. A hundred milliliters of 0.67 M (NH4)2HPO4 solution was then be added
dropwise to the 100 mL of 1 M CaO solution at the rate of 7 ml/min. Previously, CaO materials was prepared from chicken eggshell that calcined at 1000°C for 5 hours. The reaction was carried out at 300 rpm stirring. A white precipitate was obtained at the end of the reaction. The precipitate was heated hydrothermally at 300°C for 8 hours while stirring at 300 rpm. Then, the precipitate was be aged for 12 hours without stirring until it cooled down to room temperature. The solution was then be filtered under vacuum. The filtered cake was then being dried in the furnace at 110°C during 5 hours. The dried powder was then be sintered at 1000°C during 6 hours.
40
Preparation for In Vitro Analysis
BCP and HA need to be sterilized beforehand. Two milligram of BCP and HA powder was being put in each glass bottle that was being sterilized by gamma radiation with 25 kGy dozes.
Cells Culture
Culture medium was prepared in basic medium: DMEM supplemented with 10% FBS, penicillin streptomycin and fungizones. All basic medium was then melted beforehand inside 37°C-water bath within 15 minute. Osteoblast cell was be taken from liquid nitrogen storage (-198°C). The cells was then melted inside 37°C-water bath before being incubated for 24 hours at 37°C. The osteoblast cell wells washed with PBS before added by 1 mL-trypsin EDTA in order to release the attachment of cells from the bottom of the well. It was then be incubated again for 10 min (37°C) before replaced to the 15 mL-tube and added by basic medium. The 15 mL-tube was then centrifuge at 2000 rpm for 10 min (24°C) in order to concentrate the cell become a small pellet. Its supernatant needs to be removed and added by 5 mL of basic medium before homogenizing the pellet cell by several times pipetting in order to get cell solution.
Cells Concentration Counting
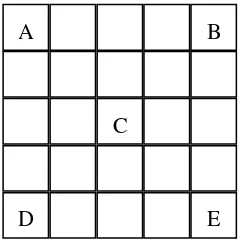
80 L-cells solution, 10 L-FBS and 10 L-trypan blue was then mixed in the 1.5 mL-eppendorf tube. Ten micro liters of solution in eppendorf tube was then dropped to the hemocytometer glass board. The cells counting were done by counting the cells on hemocytometer glass under optical microscope with 40 times-magnification. It has the separation grid to counting the cells as shown Figure 7. A, B, C, D, and E is the result of cells counting manually under optical microscope. The cells concentration was being calculated using Equation 1.
Cell suspension was prepared with a concentration of 2×105 cells ml-1 and seeded into 96 well-plates. HA and BCP powder was being poured to well then be incubated at 37°C in an atmosphere containing 5% of CO2 for 1, 2, and 3 days on
each sample in triplicate as scheme on Figure 8.
41
12
Figure 7 Scheme of grid on hemocytometer glass board
A is HA sample poured to the cells, B is BCP sample poured to the cells, C is the cells only and D is a blank that only contains basic medium (Figure 8). MTT test was performed to determine the cytotoxicity of BCP and HA. It was be calculated using the Equation 2.
(2)
If the percentage of cell viability above 100 %, the materials exposed to the cell would be categorized as nontoxic.
Figure 8 Scheme of 96-well plates for MTT analysis.
42
Sample preparation for SEM characterization
The surfaces and biocompatibility were examined by SEM. For this purpose, after each culturing period, samples was being removed from culture, washed in PBS, fixed in 2.5% glutaraldehyde, rinsed two times with PBS and dehydrated in series of ethanol concentrations. The samples were then are dried at room temperature and sputter coated with gold before observation under the SEM.
Sample characterization MTT analysis
The absorbance of cells was being analysis by visible spectrophotometer at wavelength 655 nm as shown on Figure 9. The output of absorbance measurement is performed in optical density (OD).
Scanning Electron Microscopy (SEM) characterization
Sample surfaces were being examined using a Scanning Electron Microscope after immersion in vitro (Figure 10a). Sample need to be coated by gold-palladium (80% of Au and 20% of Pd) beforehand. Coating process is using Ion Sputter JFC-1100 machine (Figure 10b). The magnification was being performed in 5000, 10000, 20000, and 40000 times- magnification.
Figure 9 Visible spectrophotometer for absorbance analysis.
(a) (b)
RESULT AND DISCUSSION
HA characterization
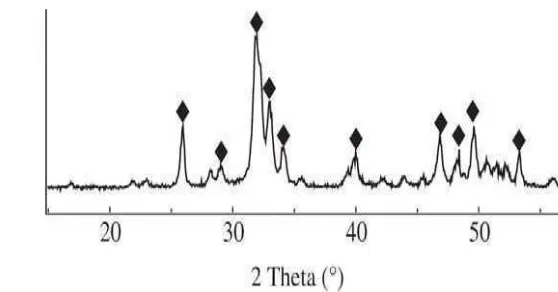
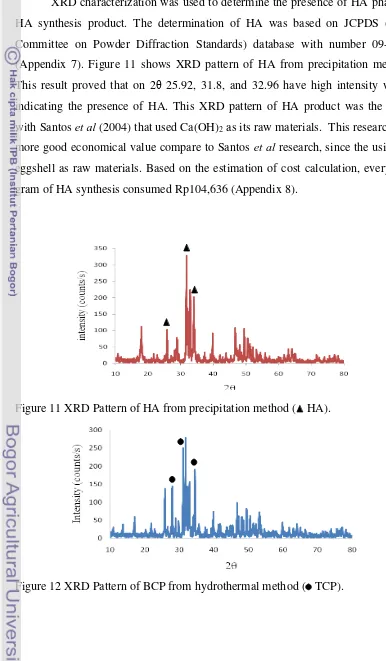
XRD characterization was used to determine the presence of HA phase in HA synthesis product. The determination of HA was based on JCPDS (Joint Committee on Powder Diffraction Standards) database with number 09-0432 (Appendix 7). Figure 11 shows XRD pattern of HA from precipitation method. This result proved that on 2θ 25.92, 31.8, and 32.96 have high intensity which indicating the presence of HA. This XRD pattern of HA product was the same with Santos et al (2004) that used Ca(OH)2 as its raw materials. This research has
more good economical value compare to Santos et al research, since the using of eggshell as raw materials. Based on the estimation of cost calculation, every one gram of HA synthesis consumed Rp104,636 (Appendix 8).
Figure 11 XRD Pattern of HA from precipitation method ( HA).
45
BCP characterization
The determination of BCP was based on JCPDS (Joint Committee on Powder Diffraction Standards) of TCP database with number 09-0169 (Appendix 7). Figure 12 shows XRD pattern of BCP from precipitation method. This result proved that on 2θ at 27.9, 31.14, and 34.56 have high intensity which indicating the presence of TCP. This XRD pattern of HA product was the same Kumar et al (2005) that used Ca(OH)2 as its raw materials. This research has more good
economical value compare to Kumar et al research, since the using of eggshell as raw materials. Based on the estimation of cost calculation, every one gram of BCP synthesis consumed Rp53,586 (Appendix 8).
MTT Analysis
Cytotoxicity analysis of bone implants exposed to the osteoblast cells. The bone implants are powder of HA and BCP. HA synthesis was using precipitation method as Dewi’s work (Dewi 2009). BCP synthesis was using hydrothermal method as Fajriyah’s work (Fajriyah 2010). MG63-cell line was using as the prototype of human osteoblast cells. The osteoblast cells are one type of human bone cells (Kim et al 2004).
Both HA and BCP were using CaO and (NH4)2PO4 as the raw materials.
CaO was derived from chicken eggshells that rich calcium carbonates (CaCO3).
Chicken eggshells were calcined at 1000°C for 5 hours based on Nurlaela (2009). Calcinations of eggshell formed CaO and removed carbonate ion. Dewi (2009) optimizing the molarities of CaO and (NH4)2PO4, which were 0.1, 0.2, 0.3, 0.4,
46
Table 2 Concentration and volume in culturing cells
C2 (cells/mL) C1 (cells /mL) V2 (mL) V1 (mL) medium (mL)
2×105 1.52×107 10 0.13 9.87
Figure 13 Color change effect of MTT solution dropped to the samples.
Cells concentration derived from culturing cells after 1 day is 1.52×107 cells/mL. The concentration is enough over 3 days of immersion for MTT analysis because this concentration is higher than 2×105 cells/mL (Table 2). The concentration for immersion is based on Oliveira et al. (Oliveira et al 2006). Cells volume was taken 0.13 mL from cells solution then 9.87 mL of basic medium was added based on Equation 2. The function of basic medium was life medium and nutrition for cells (Lin et al 1997).
Citotoxicity analysis was using MTT solution which is toxic and yellow. MTT solution reacted to the cells indicated by color exchange from red to the black. The MTT solution added to the blank which contained only basic medium was not resulted color exchange (Figure 13). Color exchange become black is because of the reduction of MTT to the formazan product (Ribeiro et al 2009). Degree of black color was measured by the absorbance devices. Light source was using red light so that it was absorbed when pointed to the black sample (contains cells) and transmitted when pointed to the blank sample. Table 3 showed absorbance measurement from spectrophotometer.
blank
47
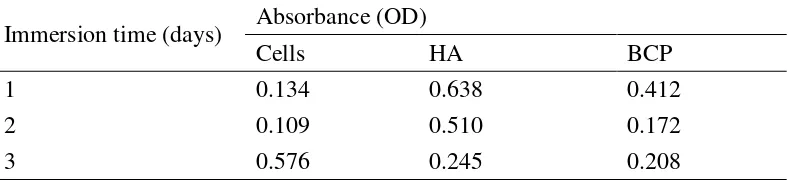
Table 3 Absorbance of cells, HA, and BCP after 1, 2, and 3 days of immersion
Immersion time (days) Absorbance (OD)
Cells HA BCP
1 0.134 0.638 0.412
2 0.109 0.510 0.172
3 0.576 0.245 0.208
Table 4 Cells viability of cells, HA, and BCP samples
Immersion time (days) Sample Cells viability (%)
1
Based on the calculation of absorbance into percentage of cells viability by using Equation 3 (Table 4), so that the chart shows on Figure 14 representing the change of viability cells on cells, HA, and BCP samples treated by 1, 2, and 3 days of immersion. One day immersion of cells either exposed by HA or BCP sample were higher than samples which contained cells only. This result indicated that HA and BCP implant acted as extra cellular matrices which inducing the cells to grow (Lin et al 1997).
48
Figure 14 Percentage of cells viability of cells, HA, and BCP samples 1, 2, and 3 days of immersion.
SEM Characterization
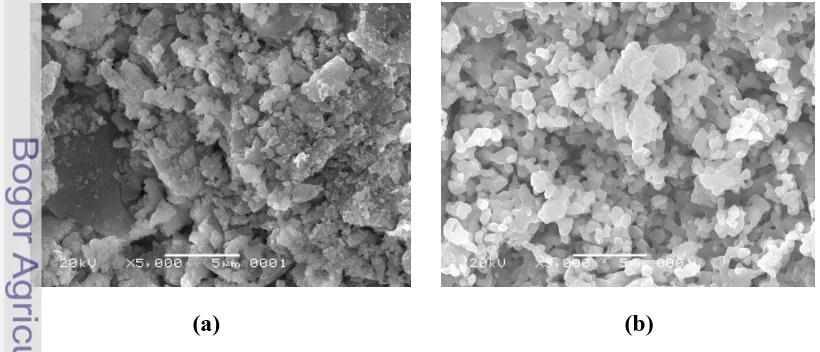
SEM photos show that either HA and BCP were small cubes crystallites (Figure 15). In the other hand, SEM photos of cells only incubated for 1, 3, and 14 days show that cells structure presented as small flatten balls-like morphology (Figure 16a, b, and c).
Figure 15 SEM photos of bone implants (a) HA (b) BCP powder with 5000 times-magnification.
49
Figure 16 SEM photos of cells only after (a) 1, (b) 3, and (c) 7 days of immersion with 5000 times-magnification.
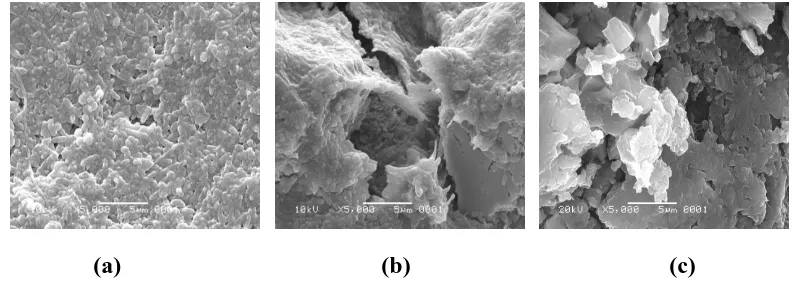
Interaction between cells and bone implants were showed by the adhesion that covered almost all structure of HA or BCP after 1 day of immersion as shown on Figure 17a and 18a. Crystallite HA and BCP structure was still be recognized for 1 day of immersion as shown on Figure 17a and 18a. SEM photos on Figure 17b and 18b after 3 days of immersion show that cells start to calcify and secret sticky extra cellular matrices protein which covered all of HA and BCP structures as Kim et al research (2004).
This osteocalcification was appeared in a good agreement as MTT result after 3 days of immersion. SEM photos after 14 days of immersion show perfectly greater interaction of secreted protein and bone implants as shown on Figure 17c and 18c. Based on Figure 17 (c), BCP was appeared more degraded than HA as shown on Figure 18 (c). This attachments between protein or cells and implants are same as Riberio et al research. Riberio et al showed SEM photos BCP implant materials within 2 days of immersion in osteoblast cells as shown on Figure 4. BCP implants that immersed on osteoblast cells as Riberio et al research was in densed shape of BCP scaffold while in this research in powder shape of HA and BCP.
(c) (b)
50
Figure 17 SEM photos of cells exposed by BCP after (a) 1, (b) 3, and (c) 14 days of immersion with 5000 times-magnification.
Figure 18 SEM photos of cells exposed by HA after (a) 1, (b) 3, and (c) 14 days of immersion with 5000 times-magnification.
(c) (b)
(a)
(c) (b)
CONCLUSION AND SUGGESTIONS
Conclusion
Based on in vitro analysis, it proves that HA and BCP powder which was synthesized in IPB Biophysics Laboratory was nontoxic through MTT analysis. So, it is possible to examine for further analysis through in vivo. Cells viability exposed by bone implants was higher than those which were not exposed by any bone implants. This result means that bone implants could be act as extra cellular matrices which induced the cells growth. The interaction between cells and implants were further proved by SEM photos. That interaction was the attachments cells on the implants which cover almost all implant structure.
Suggestions
54
REFERENCES
Coelho MJ, Cabral AT, Fernandes MH. 2000. Human bone cell cultures in biocompatibility testing. Part I: osteoblastic differentiation of serially passaged human bone marrow cells cultured in a-MEM and in DMEM. Biomaterials 21: 1087-1094.
Daculsi G. 2004. Micro macroporous calcium phosphate bioceramics. Business Briefing Global Surgery.
Dewi SU. 2009. Pembuatan komposit kalsium fosfat-kitosan dengan metode sonikasi [tesis]. Bogor: Program Pascasarjana, Insitut Pertanian Bogor. Fajriyah HI. 2010. Hydrothermal synthesis of biphasic calcium phosphate [tesis].
Bogor: Program Pascasarjana, Insitut Pertanian Bogor.
Fernandes GF, Laranjeira MCM. Calcium Phosphate Biomaterials from Marine Algae, Hydrothermal Synthesis and Characterization. Journal of Univeridade Federal de Sta Catarina, Florianopolis. 1999; 23 [4]
Kim HW, Knowles JC, Kim HE. 2005. Hydroxyapatite and gelatin composite foams processed via novel freeze-drying and crosslinking for use as temporary hard tissue scaffolds. J Biomed Mater Res 72A: 136–145. Kumar KR, Kumar TSS, Sunder M, Babu NR, Victor SP. 2005. Biphasic calcium
phosphates for antibiotic release. Trends Biomater. Artif. Organ 18 [2] : 213-218.
Li S, Wijn JD, Li J, Layrolle P, Groot KD. 2003. Macroporous biphasic calcium phosphate scaffold with high permeability/porosity ratio. Tissue Engineering 9 [3] : 535-548.
Lin FH, Liao CJ, Liu HC, Chen KS, Sun JS. 1997. Behavior of fetal rat osteoblasts cultured in vitro on the DP-bioactive glass substratum. Materials Chemist and Physics 49: 270-276.
Nurlaela A, Dewi SU, Dahlan K, Soejoko DS. 2009. The use of eggshells as calcium sources for synthesis of bone mineral. Proceeding of the 1st International Seminar on Science and Technology; 24-25 Jan 2009.
55
2006. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials 27: 6123–6137. Ooi CY, Hamdi M, Ramesh S. Properties of hydroxyapatite produced by
annealing of bovine bone. Ceramics International Elsevier. 2007 : 33 : 1171-1177
Ramay HRR, Zhang M. 2004. Biphasic calcium phosphate nanocomposite porous scaffolds for load-bearing bone tissue engineering. Biomaterials 25 : 5171–5180.
Ribeiro C, Rojas-Cabrera WI, Marques M, Bressiani JC, Bressiani AHA. 2009. In vitro characterization of porous ceramic based calcium phosphate processing with albumin. Key Engineering Materials Vols 396-398 : 27-30.
Santos MH, Oliveira M, Souza LPF, Mansur HS, Vasconcelos WL. 2004. Synthesis control and characterization of hydroxyapatite prepared by wet precipitation process. Materials Research 7[4] : 625-630.
Shi D. 2004. Biocompatibility of materials. Di dalam: Wang M, Zhang M, Clare A, Kasuga T, Lin Q. Biomaterials and Tissue Engineering. Springer-Verlag Berlin Heidelberg. Page 83-143.
Victoria EC, Gnanam FD. 2002. Synthesis and characterization of biphasic calcium phosphate. Trends Biomater. Artif. Organs 16 [1] : 12-14.
56
58
Appendix 1 Flow Chart of BCP Synthesis
Ready?
CaO dissolving
Materials and equipment preparation
Report Arrangement Data analysis Hydrothermal synthesis
while stirring
In vitro Analysis Solution aging and filtering
Sample drying Precipitation of (NH4)2HPO4
and Ca(OH)2 while stirring
Hydroxyapatite compound
Sample sintering
BCP compound
59
Appendix 2 Flow Chart of Hydroxyapatite Synthesis
Ready?
CaO dissolving
Materials and equipment preparation
Report Arrangement Data analysis In vitro Analysis Solution aging and filtering
Sample drying Precipitation of (NH4)2HPO4
and Ca(OH)2 while stirring
Sample sintering
HA compound
60
Cell washing with PBS Cell homogenization
Seeding cell into 96 well plates
Adding cell by MTT solution Cell incubation
Cells incubation (3 hours)
Replacing cells by isopropanol
1 hours-shaking Materials and equipment preparation
Adding cell by basic medium
61
Appendix 4 Experimental Equipments of HA and BCP synthesis
(b) Analytical balance (h) Digital pHmeter
62
Appendix 5 Experimental Equipments of cell Culture and cytotoxity analysis
63
Appendix 6 Experimental Equipments of sterilization
(a) Gamma radiation room (b) Gamma radiation room (c) Cobalt 60 radiation source
(c) (b)
64
65
Appendix 8 Cost Estimation of HA and BCP product
Kinds of Cost
Detailed cost
Estimation cost of HA synthesis Cost Power
(W) Duration (hour) Amount of power (kWh) Electrical cost (Rp.1245/kWh) Electrical
Materials ammonium hydrogen phosphate
Price/g Amount of materials (g) Chemical cost
Rp379,5 2 Rp759
Operator Fee
Duration
(days) Operational cost
Rp100,000 3 Rp300,000
Cost of HA product/3 g Rp313,909
Cost of HA product/g Rp104,636
Kinds of
Cost Detailed cost
Estimation cost of BCP synthesis Cost Power
(W) Duration (hour) Amount of power (kWh) Electrical cost (Rp.1245/kWh) Electrical
Price/g Amount of materials (g) Chemical cost
Rp379,5 5 Rp1,897
Operator Fee
Duration
(days) Operational cost
Rp100,000 3 Rp300,000
Cost of HA product/6 g Rp321,521
66
Appendix 9 BCP and HA SEM photos of various magnifications
Magnification HA BCP
5000
10000
20000
40000
67
Appendix 10 Cells SEM photos of various magnifications
Magnification Immersion time (days)
1 3 14
5000
10000
20000
68
Appendix 11 Cells exposed by HA-SEM photos of various magnifications
Magnification Immersion time (days)
1 3 14
5000
10000
20000
69
Appendix 12 Cells exposed by BCP-SEM photos of various magnifications
Magnification Immersion time (days)
1 3 14
5000
10000
20000
ABSTRACT
RAHMI SOLIHAT. In Vitro Analysis of Biphasic Calcium Phosphate and Hydroxyapatite as Bone Implants. Supervised by KIAGUS DAHLAN and BOY M. BACHTIAR.
Hydroxyapatite (HA) and Biphasic Calcium Phosphate (BCP) are widely used as bone implant materials because of its biocompatibility. The minimum requirement of biocompatible materials is nontoxic. The reduction of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to a purple formazan is used to measure toxicity of implant materials that exposed to the cell. The biocompatibility also can be observed from the attachment between cell and implant materials under electron microscope. This research reported that HA and BCP derived from eggshell using precipitation and hydrothermal method were nontoxic. MTT toxicity analysis was held during 1, 2 and 3 days immersion of osteocalcifications that secret protein collagen were appeared. This MTT analysis result was appeared in a good agreement with Scanning Electron Microscope (SEM) characterization, that showed the attachments of either HA or BCP to the osteoblast cells after 1 day immersion. The SEM photos after incubated 3 days showed that cells start to calcify and secret protein. The calcification and secretion of protein collagen performed much better after 14 days immersion. In conclusion, HA and BCP derived from eggshell were nontoxic and performed good adhesion interaction to the host cells in vitro.
INTRODUCTION
Background
Recent developments on bone reconstruction concern mainly in implantable synthetic materials, although there are different kind materials in bone reconstruction as like as autograft, allograft and xenograft. Autograft are the materials from others body part of patient itself for bone reconstruction. However, the clinical use involves some difficulties such as septic complication, viral transmission and the unavailability of native bone (Daculsi 2004). On the other hand, allografts are more readily available than autograft since it is from the donor. In spite of that, allograft bone has revealed a risk of disease transmission (such as HIV and hepatitis), post-surgery pain, increased blood loss, secondary surgical wounds, risk of thrombosis and it is also difficult to shape (Daculsi 2004 & Ooi et al 2007). A significant additional limitation of allograft is the delayed remodeling by the host. In the case of very large defects, the allograft may remain in the implant site throughout the patient’s life, creating area more prone to fracture or infection (Ooi et al 2007). Likewise, xenograft also bears limitation since it is from others species as like as animals, which has different characteristic in mineral bone composition (Dewi 2009). In short, synthetic biomaterials were preferable in answering others method limitation in bone reconstruction.
The minimum requirements of synthetic biomaterial include the following: (1) the material must be biocompatible, such as nontoxic, blood- or tissue-compatible, noncarciogenic; (2) the material must not leach or release harmful components into the living system; (3) the mechanical and physical properties of the material, such as strength, elasticity, durability, and stability, must be appropriate for the intended application; and (4) the desired mechanical properties must last for the expected life of the implant; (5) the materials must be sterilizable (Shi et al 2004).
Gnanam 2002). While, the porous β-tricalcium phosphate (TCP) displays affinity for high speed biological degradation, they are bioactive and bioresorbable materials (Victoria & Gnanam 2002). TCP were later identified as Biphasic Calcium Phosphate (BCP) consisting of hydroxyapatite (HA) and tricalcium phosphate (β-TCP) (Li et al 2003). Recently, there is a growing interest in developing Biphasic Calcium Phosphate (BCP) ceramics as implant materials because they are more effective in bone repair or regeneration which proved in vitro and in vivo (Ramay & Zhang 2004).
Hypotheses
1. Both BCP and HA in vitro analysis show as nontoxic materials.
2. There appear morphological properties change of BCP and HA after in vitro indicating adhesion interaction between HA or BCP and cells.
Objective
In vitro analysis that has been held from 0 up to 14 days could explain the biocompatibility. In this study, the biocompatibility of BCP compared to HA was being performed through in vitro analysis by cytotoxicity screening by using MTT analysis. Furthermore, the change of morphological properties after in vitro was being characterized using Scanning Electron Microscopy (SEM). BCP was being synthesized through hydrothermal method while HA through precipitation method.
Benefit
LITERATURE REVIEW
Hydroxyapatite (HA)
HA Properties
There are many apatite compounds, including fluorapatite, chlorapatite, carbonate-apatite, and hydroxyapatite (Oliveira et al 2006). Hydroxyapatite is chemically similar to the mineral component of bones and hard tissues in mammals; its chemical formula is Ca10(PO4)6(OH)2 (Fernandes & Laranjeira
1999). The chemical nature of hydroxyapatite lends itcellsf to substitution, meaning that it is common for non-stoichiometric hydroxyapatites to exist. The most common substitutions involve carbonate, fluoride and chloride substitutions for hydroxyl groups, while defects can also exist resulting in deficient hydroxyapatites.
Hydroxyapatite is bioactive material; the ability to integrate in bone structures and support bone ingrowths, without breaking down or dissolving. Hydroxyapatite is a thermally unstable compound, decomposing at temperature from about 800-1200°C depending on its stoichiometry. Hydroxyapatite is a calcium phosphate including hydroxide, and its Ca/P ratio is represented as 1.67. The structure of hydroxyapatite is hexagonal, which has unit cell size, a = 9.418 Å and c = 6.883 Å (Shi et al 2004). This structure can be assumed as ideal hexagonal crystal structure (closed-packed) from PO4-3 ion, which is inserted by
Ca+2 ion and OH- ion among the empty space of PO
4-3 ions (Figure 1) (Shi et al
2004).
31
Table 1 Family of calcium phosphate compounds (Shi et al 2004).
Mineral
name Chemical Name Chemical Formula Ca/P
Monetite Dicalcium phosphate (DCP) CaHPO4 1.00
Brushite Dicalcium phosphate dehydrate (DCPD) CaHPO4.2H2O 1.00
Whitlockite Octacalcium phosphate (OCP)
Ca8(HPO4)2(PO4)4.5 H2O 1.33
Ca10(HPO4)(PO4)6 1.43
Tricalcium phosphate (TCP) Ca3(PO4)2 1.50
Hydoxyapatite Hydroxyapatite (HA) Ca10(PO4)6(OH)2 1.67
Hillinstockite Tetracalcium phosphate (TTCP) Ca4P2O9 2.00
There are different phases of calcium phosphate ceramics that can be used in medicine, depending on whether a bioactive or a resorbable material is desired (Table 1) (Shi et al 2004). Generally, dense hydroxyapatite does not have the mechanical strength to enable it to succeed in long term load bearing applications. But, hydroxyapatite may be employed as bone fillers in forms such as powders, porous blocks or beads to fill bone defects or voids. These may arise when large sections of bone have had to be removed (such as bone cancers) or when bone augmentations are required (such as dental applications). The bone filler would provide a scaffold and encourage the rapid filling of the void by naturally forming bone and provides an alternative to bone grafts. It would also become part of the bone structure and would reduce healing times compared to previous bone prostheses.
HA Synthesis
Hydroxyapatite in particulate form can be produced by using a variety of methods, such as wet method, dry method and hydrothermal method (Shi et al 2004). In this study, hydroxyapatite synthesis was being performed through wet method that is precipitation. Santos et al (2004) have been succeeding in synthesizing hydroxyapatite through wet precipitation method based on the chemical reaction below:
32
Figure 2 XRD pattern of HA from wet precipitation method ( HA) (Santos et al 2004).
The 0.5 M Ca(OH)2 suspension was prepared using Ca(OH)2 powder. The
suspension was degassed, vigorously stirred and heated for one hour at 40°C temperatures. The 0.3 M H3PO4 solution was dropped into the Ca(OH)2
suspension at same temperature for approximately one hour at the rate 6 mL/min.
The pH was adjusted become pH = 7 by addition of 1 M NH4OH solution at the
end of the precipitation process. The XRD result showed below match to the hydroxyapatite pattern (Santos et al 2004).
Biphasic Calcium Phosphate (BCP)
BCP Properties
Development of biphasic calcium phosphate (BCP), especially with hydroxyapatite (HA: Ca10(PO4)6(OH)2) and tricalcium phosphate (TCP:
Ca3(PO4)2) has drawn considerable attention. HA and TCP, although have similar
33
bioactivity relies on physical and chemical properties of biphasic calcium phosphate ceramics (Victoria & Gnanam 2002).
It is also noted that the presence of small amount of β-TCP 1100°C and 1200°C may be associated with the partial decomposition of HA phase. If HA is annealed in air at 1200°C, it decomposed into the β-TCP phase according to chemical reaction below (Ooi et al 2007).
Ca10(PO4)6(OH)2 → 3β-Ca3(PO4)2 + CaO + H2Ogas
BCP Synthesis
Kumar et al (2005) was succeed in synthesizing BCP from sintering process. Firstly, the BCP granules were synthesized by the microwave. Calcium hydroxide and diammonium hydrogen ortho phosphate (DAP) were used as raw materials. The amounts of reactants used for the reaction were calculated based on the Ca/P molar ratio of 1.58. Weighed amounts of the starting granules were dissolved in water and the DAP solution was added to the calcium hydroxide solution. The solution is then exposed to 900°C microwave irradiation in a microwave oven during 20 minutes. The product was then dried in an oven. The result of XRD has a major peak indicating TCP that is (0 2 0) peak as shown below.
34
In vitro study
Biocompatibility testing in vitro often involves the detection of cell damage and death as like as cytotoxicity. Coelho et al (2000) was used the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to a purple formazan product in the MTT assay to estimate cell viability. The screening test is useful to detect over toxic effects of a test material in showing incompatibility. For instance, the rate of growth, proliferation and differentiation of cells on a material may be dependent on successful initial attachment and spreading of the cells on the surface of the implant materials. In this respect, the initial and short-term responses of cells to an implant material in vitro may provide valuable indicators of the long-term biocompatibility in vivo (Lin et al 1997).
Riberio et al (2009) have been succeed in showing cell attachment to the BCP implant materials within 2 days of immersion in osteoblast cells as SEM pictures show below.
MATERIALS AND METHODS
Place and Time Schedule
This research was being conducted from February through September 2010 which took place in IPB-Biophysics Laboratory in sample preparation, while in vitro sample analysis was done in Oral Biology Laboratory of Dental Faculty, University of Indonesia. Sample sterilization was done in National Nuclear Energy Agency (BATAN) Pasar Jumat. Sample characterization was done in National Nuclear Energy Agency (BATAN) Serpong for SEM characterization.
Materials and Equipments Materials
1. BCP synthesis materials :
a. Calcium oxide from chicken eggshell
b. Pro-analyze ammonium hydro phosphate; (NH4)2HPO4
c. Aquabides
2. MG-63 cell line as osteoblast cell 3. Cell culture medium materials :
a. Dulbecco’s Modified Eagle’s Medium (DMEM) b. Fetal Bovine Serum (FBS)
c. Penicillin Streptomycin d. Fungizone
4. Trypsin EDTA 5. Trypan blue
6. Washing medium materials : Phosphate Buffered Saline (PBS) 7. Cytotoxicity test materials :
a. MTT (3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide)
37
8. SEM preparation materials :
a. Phosphate Buffered Saline (PBS) b. 8% glutaraldehyde
c. Ethanol
Equipments
1. BCP and HA synthesis equipments (Appendix 4) : a. Analytical balance
2. Gamma radiation sterilization with cobalt 60-radiation source (Appendix 6)
3. In vitro analysis equipments (Appendix 5) :
a. 0.2 m-sterile syiringe filter (Corning, Germany) b. 50 mL-syringe (Terumo, Japan)
c. 15 mL- and 50 mL-tube (Falcon, USA) d. Scrapper
e. Micropipette (Eppendorf, Germany) f. Tips micropipette
38
i. Incubator (Memert)
j. Cell culture dish (35 mm×10 mm) k. 96-well plates (NUNC, Denmark ) l. Microscope (Nikon Elipse 80i)
m. Biohazard safety cabinet (ESCO Micro PTE Ltd.) n. Water bath
o. Centrifuge (Sorvall)
p. Vortexer (Bio-rad BR 2000) q. Shaker (Certomat)
4. Characterization equipments (Appendix 6) :
a. Scanning Electron Microscope (SEM) (JEOL JCM-35C) b. Ion Sputter JFC-1100 machine
c. Bio-Rad Microplate Reader Benchmark Visible Spectrophotometer
Experimental Method
Experimental method is shown on flow chart on Appendices 1, 2, and 3.
Hydroxypatite synthesis
The same raw materials of CaO as synthesizing BCP that gained from chicken eggshells were used to prepare 100 mL-CaO suspension which was seen as white thick fluid. Chicken eggshells that mostly contain CaCO3 were calcined
at 1000°C for 5 hours as chemical reaction below:
CaCO3 CaO + CO2
0.3 M CaO suspension of eggshell product was dropped by 0.18 M of clear (NH4)2HPO4-solution in 100 mL aquabides in 37°C while stirring at 300 rpm at
the rate of 7 mL/min. The final suspension was then be filtered under vacuum. The filtered cake was then being dried in the furnace at 110°C during 5 hours. The dried powder was then be sintered at 900°C during 5 hours by using furnace. The chemical reaction of CaO and (NH4)2HPO4 was as below:
39
Figure 5 Precipitation process in HA synthesis.
BCP Synthesis
The solution of BCP in this research was prepared by a precipitation method. A hundred milliliters of 0.67 M (NH4)2HPO4 solution was then be added
dropwise to the 100 mL of 1 M CaO solution at the rate of 7 ml/min. Previously, CaO materials was prepared from chicken eggshell that calcined at 1000°C for 5 hours. The reaction was carried out at 300 rpm stirring. A white precipitate was obtained at the end of the reaction. The precipitate was heated hydrothermally at 300°C for 8 hours while stirring at 300 rpm. Then, the precipitate was be aged for 12 hours without stirring until it cooled down to room temperature. The solution was then be filtered under vacuum. The filtered cake was then being dried in the furnace at 110°C during 5 hours. The dried powder was then be sintered at 1000°C during 6 hours.
40
Preparation for In Vitro Analysis
BCP and HA need to be sterilized beforehand. Two milligram of BCP and HA powder was being put in each glass bottle that was being sterilized by gamma radiation with 25 kGy dozes.
Cells Culture
Culture medium was prepared in basic medium: DMEM supplemented with 10% FBS, penicillin streptomycin and fungizones. All basic medium was then melted beforehand inside 37°C-water bath within 15 minute. Osteoblast cell was be taken from liquid nitrogen storage (-198°C). The cells was then melted inside 37°C-water bath before being incubated for 24 hours at 37°C. The osteoblast cell wells washed with PBS before added by 1 mL-trypsin EDTA in order to release the attachment of cells from the bottom of the well. It was then be incubated again for 10 min (37°C) before replaced to the 15 mL-tube and added by basic medium. The 15 mL-tube was then centrifuge at 2000 rpm for 10 min (24°C) in order to concentrate the cell become a small pellet. Its supernatant needs to be removed and added by 5 mL of basic medium before homogenizing the pellet cell by several times pipetting in order to get cell solution.
Cells Concentration Counting
80 L-cells solution, 10 L-FBS and 10 L-trypan blue was then mixed in the 1.5 mL-eppendorf tube. Ten micro liters of solution in eppendorf tube was then dropped to the hemocytometer glass board. The cells counting were done by counting the cells on hemocytometer glass under optical microscope with 40 times-magnification. It has the separation grid to counting the cells as shown Figure 7. A, B, C, D, and E is the result of cells counting manually under optical microscope. The cells concentration was being calculated using Equation 1.
Cell suspension was prepared with a concentration of 2×105 cells ml-1 and seeded into 96 well-plates. HA and BCP powder was being poured to well then be incubated at 37°C in an atmosphere containing 5% of CO2 for 1, 2, and 3 days on
each sample in triplicate as scheme on Figure 8.
41
12
Figure 7 Scheme of grid on hemocytometer glass board
A is HA sample poured to the cells, B is BCP sample poured to the cells, C is the cells only and D is a blank that only contains basic medium (Figure 8). MTT test was performed to determine the cytotoxicity of BCP and HA. It was be calculated using the Equation 2.
(2)
If the percentage of cell viability above 100 %, the materials exposed to the cell would be categorized as nontoxic.
Figure 8 Scheme of 96-well plates for MTT analysis.
42
Sample preparation for SEM characterization
The surfaces and biocompatibility were examined by SEM. For this purpose, after each culturing period, samples was being removed from culture, washed in PBS, fixed in 2.5% glutaraldehyde, rinsed two times with PBS and dehydrated in series of ethanol concentrations. The samples were then are dried at room temperature and sputter coated with gold before observation under the SEM.
Sample characterization MTT analysis
The absorbance of cells was being analysis by visible spectrophotometer at wavelength 655 nm as shown on Figure 9. The output of absorbance measurement is performed in optical density (OD).
Scanning Electron Microscopy (SEM) characterization
Sample surfaces were being examined using a Scanning Electron Microscope after immersion in vitro (Figure 10a). Sample need to be coated by gold-palladium (80% of Au and 20% of Pd) beforehand. Coating process is using Ion Sputter JFC-1100 machine (Figure 10b). The magnification was being performed in 5000, 10000, 20000, and 40000 times- magnification.
Figure 9 Visible spectrophotometer for absorbance analysis.
(a) (b)
RESULT AND DISCUSSION
HA characterization
XRD characterization was used to determine the presence of HA phase in HA synthesis product. The determination of HA was based on JCPDS (Joint Committee on Powder Diffraction Standards) database with number 09-0432 (Appendix 7). Figure 11 shows XRD pattern of HA from precipitation method. This result proved that on 2θ 25.92, 31.8, and 32.96 have high intensity which indicating the presence of HA. This XRD pattern of HA product was the same with Santos et al (2004) that used Ca(OH)2 as its raw materials. This research has
more good economical value compare to Santos et al research, since the using of eggshell as raw materials. Based on the estimation of cost calculation, every one gram of HA synthesis consumed Rp104,636 (Appendix 8).
Figure 11 XRD Pattern of HA from precipitation method ( HA).