THE FRESHWATER ATYID SHRIMPS OF THE GENUS
CARIDINA
(CRUSTACEA: DECAPODA: CARIDEA)
FROM LAKE LINDU, CENTRAL SULAWESI, INDONESIA
ANNAWATY
SEKOLAH PASCASARJANA INSTITUT PERTANIAN BOGOR
PERNYATAAN MENGENAI DISERTASI DAN
SUMBER INFORMASI SERTA PELIMPAHAN HAK CIPTA*
Dengan ini saya menyatakan bahwa disertasi berjudul The freshwater atyid shrimps of the Genus Caridina (Crustacea: Decapoda: Caridea) from Lake Lindu, Central Sulawesi, Indonesia adalah benar karya saya dengan arahan dari komisi pembimbing dan belum diajukan dalam bentuk apa pun kepada perguruan tinggi mana pun. Sumber informasi yang berasal atau dikutip dari karya yang diterbitkan maupun tidak diterbitkan dari penulis lain telah disebutkan dalam teks dan dicantumkan dalam Daftar Pustaka di bagian akhir disertasi ini.
Dengan ini saya melimpahkan hak cipta dari karya tulis saya kepada Institut Pertanian Bogor.
Bogor, Agustus 2014
Annawaty
RINGKASAN
ANNAWATY. Udang air tawar Genus Caridina (Crustacea: Decapoda: Caridea) dari Danau Lindu, Sulawesi Tengah, Indonesia. Dibimbing oleh BAMBANG SURYOBROTO, ACHMAD FARAJALLAH, DAISY WOWOR, dan DEDI SETIADI.
Udang air tawar endemik Danau Lindu, Caridina linduensis pertama kali dideskripsi lebih dari satu abad yang lalu oleh J. Roux berdasarkan koleksi milik naturalis dari Swiss, Paul dan Fritz Sarasin (Roux 1904). Dalam tulisan ini dideskripsi ulang C. linduensis berdasarkan specimen baru yang diperoleh dari Danau Lindu yang merupakan type locality dari spesies tersebut. Hasil penelitian ini juga mengungkapkan adanya dua spesies udang air tawar endemik lain yang belum pernah dideskripsi sebelumnya, yaitu Caridina sp A dan Caridina sp B, kedua spesies tersebut dideskripsi dan dibuat ilustrasinya dalam tulisan ini.
Ketiga spesies Caridina danau Lindu dapat dibedakan terutama berdasarkan bentuk rostrum dan struktur mandibulanya. Caridina linduensis memiliki rostrum yang panjang dengan ujung rostrum mencapai atau sedikit melewati ujung segmen ketiga antennular pedunclenya. Spesies ini memiliki rostrum yang paling panjang dibandingkan dengan kedua spesies Caridina yang lain. Rostrum Caridina sp B lebih pendek dengan ujung rostrum melewati ujung segmen basal tetapi tidak pernah mencapai ujung segmen kedua dari antennular peduncle. Caridina sp A dapat dibedakan dari kedua spesies lainnya berdasarkan bentuk rostrum yang sangat pendek, hanya mencapai ujung distal segmen basal dari antennular peduncle. Bentuk rostrum yang sangat pendek ini membuatnya sangat berbeda dari kedua caridina yang lain. Disamping itu Caridina sp A memiliki ukuran tubuh yang lebih besar dibanding kedua spesies yang lain. Panjang carapas individu betina bertelur Caridina sp A mencapai 6.8 mm sementara C. linduensis
hanya 4.9 mm dan 5.4 mm pada Caridina sp B. Struktur mandibula pada Caridina
sp A dan Caridina sp B memiliki kemiripan dengan adanya 6 gigi pada incisor process dengan 2 deret granula yang tidak beraturan di antara gigi, molar process lurus. Struktur ini sangat berbeda dengan C. linduensis, yang memiliki lebih banyak gigi pada incisor processnya yaitu 16-18 gigi dengan struktur molar process yang melengkung.
Ketiga spesies Caridina tidak hanya berbeda secara morfologi, tetapi juga berbeda dalam pemilihan habitat serta distribusinya. C. linduensis ditemukan menggantung di akar tanaman air di daerah littoral danau dan ditemukan juga di permukaan serasah dan ranting ranting kayu yang sudah lapuk di tepi outlet danau yang memiliki arus lambat cenderung stagnan. Caridina sp Bditemukan di bagian danau yang berada tidak jauh dari inlet, dan juga ditemukan di inlet dengan air keruh dengan arus yang lambat cenderung stagnan. Spesies ini menggantung di akar tumbuhan pakis atau tanaman air yang terdapat di tepi danau atau sungai..
Caridina sp A hanya dapat ditemukan di sungai ataupun selokan kecil berair jernih dengan substrate baru kerikil dan kerakal serta aliran air yang cukup deras. Spesies ini melimpah di sungai-sungai yang terletak di bagian barat danau, dimana kemiringan lahan berkisar antara 15 hingga 40 derajat.
yang lain, dan tampaknya distribusinya dipengaruhi oleh temperatur dan adanya ikan-ikan introduksi seperti mas (Cyprinus carpio) dan mujair (Oreochromis mossambicus), serta adanya udang introduksi (Macrobrachium lanchesteri). Fauna akuatik introduksi ini, baik yang disengaja maupun tidak, merupakan ancaman serius bagi kelangsungan hidup Caridina terutama spesies yang hidup di danau karena berpotensi sebagai predator maupun kompetitor bagi Caridina.
Semua spesies Caridina Danau Lindu memiliki jumlah telur yang relatif sedikit (26–47 telur) dengan ukuran telur yang cukup besar (diameter lebih dari 1 mm). Hal ini mengindikasikan sifat mereka sebagai spesies land-locked yang berarti tidak lagi membutuhkan air payau/asin untuk menyelesaikan siklus hidupnya karena mereka sepenuhnya menghabiskan seluruh hidupnya di air tawar.
SUMMARY
ANNAWATY. The freshwater atyid shrimps of the genus Caridina (Crustacea: Decapoda: Caridea) from Lake Lindu, Central Sulawesi, Indonesia. Supervised by BAMBANG SURYOBROTO, ACHMAD FARAJALLAH, DAISY WOWOR, and DEDI SETIADI.
The freshwater atyid shrimps from Lake Lindu, Caridina linduensis are described for the first time by J. Roux in 1904. He described the species based on the specimen collection of Paul and Fritz Sarasin. After neglected for more than a century, here, we redescribe and figure this poorly known species based on new material from its type locality, Lake Lindu. Two new species, Caridina sp B and
Caridina sp A are also found in this lake and they are described and illustrated. The three endemic species have different morphological characters mainly in rostrum shape and mandible structure. Rostrum on C. linduensis relatively long with the rostrum tip reaching near to or slightly beyond end of the third segment of antennular peduncle. This species have the longest rostrum compared with the other species. Rostrum of Caridina sp B is rather short which is tip of rostrum overreach the end of basal segment, but never reach end of second segment of antennular peduncle, while Caridina sp A have a very short rostrum with maximum reach of the end of basal segment of antennular peduncle. Caridina sp A have a shorter rostrum among the Lindu’s species. The shortest rostrum in
Caridina sp A make this species very different from the two Caridina species found in the Lake Lindu and its catchment area. Caridina sp A also have larger body size of ovigerous females with maximum carapace length 6.8 mm whileonly 4.9 mm in C. linduensis and 5.4 mm in Caridina sp B. The second morphological difference of three Caridina species is its mandible structure. The mandibles of
Caridina sp B and Caridina sp A are similar, i.e. the incisor process has 6 irregular teeth with 2 very fine granulated rows between the teeth, and the molar process is straight. On the other hand, the mandible of C. linduensis is quite different; the incisor process has 16–18 irregular teeth, and the molar process is truncated.
The Caridina from Lake Lindu not only different in their morphology but also in their habitat preferences. C. linduensis is a true lake inhabitant, Caridina
sp B can be found both in the lake itself and associated streams while Caridina sp A is an obligate stream species. There is no overlapping distribution among the species.
Caridina sp A is abundant in streams and ditches with moderate flow running water gravel–cobble substrate. It is mainly spread in the western part of the lake where slope varies between 15 and 40%. Both Caridina sp B and C. linduensis can be found in lake and streams with very slow current to almost stagnant water, muddy sand substrate and associated with roots of water plants and leaf litter. However, Caridina sp B is never occurred together with C. linduensis and they are less abundant compare to Caridina sp A. Distribution of
lanchesteri present in Lake Lindu.These exotic fish and the alien shrimp can have the potency become predators and or competitors for the Caridina spp.
The less egg number (26–47) and the large egg size (more than 1 mm in diameter) of the all Caridina species from Lake Lindu system indicated they are probably land-locked species which are complete their entire life cycle in freshwater. In combination with their land-locked species type and ecological and physical barrier, the Lindu’s Caridina distribution is restricted within the lake system. The environmental condition prevents Caridina to disperse into other area. It is also associated with the reduction of dispersal ability and gene-flow among the populations, which may explain the high degree of endemism in
Caridina of Lake Lindu. There is no overlap of Caridina species among Lake Lindu, Lake Poso and Malili lake system. Most of the species which belong to the lake system are endemic to lake system itself.
© Hak Cipta Milik IPB, Tahun 2014
Hak Cipta Dilindungi Undang-Undang
Dilarang mengutip sebagian atau seluruh karya tulis ini tanpa mencantumkan atau menyebutkan sumbernya. Pengutipan hanya untuk kepentingan pendidikan, penelitian, penulisan karya ilmiah, penyusunan laporan, penulisan kritik, atau tinjauan suatu masalah; dan pengutipan tersebut tidak merugikan kepentingan IPB
Disertasi
sebagai salah satu syarat untuk memperoleh gelar Doktor pada Program Studi Biosains Hewan
THE FRESHWATER ATYID SHRIMPS OF THE GENUS
CARIDINA
(CRUSTACEA: DECAPODA: CARIDEA)
FROM LAKE LINDU, CENTRAL SULAWESI, INDONESIA
SEKOLAH PASCASARJANA INSTITUT PERTANIAN BOGOR
Penguji pada Ujian Tertutup: 1. Prof Dr Mulyadi, MSc
Professor riset Pusat Penelitian Biologi, Lembaga Ilmu Pengetahuan Indonesia 2. Prof Dr Ir MF Rahardjo, DEA
Guru Besar Fakultas Perikanan dan Kelautan IPB
Penguji pada Ujian Terbuka:
1. Prof Dr Ramadhanil Pitopang, MSi
Guru Besar Fakultas MIPA Universitas Tadulako 2. Prof Dr Ir MF Rahardjo, DEA
Judul Disertasi : The freshwater atyid shrimps of the genus Caridina (Crustacea: Decapoda: Caridea) from Lake Lindu, Central Sulawesi, Indonesia
Nama : Annawaty
NIM : G362090011
Disetujui oleh Komisi Pembimbing
Dr Bambang Suryobroto Ketua
Dr Ir Achmad Farajallah, MSi Anggota
Dr Ir Daisy Wowor, MSc Anggota
Prof Dr Ir Dede Setiadi, MS Anggota
Diketahui oleh
Ketua Program Studi Biosains Hewan
Dr Ir Rd Roro Dyah Perwitasari, MSc
Dekan Sekolah Pascasarjana
Dr Ir Dahrul Syah, MScAgr
PRAKATA
Puji dan syukur penulis panjatkan kepada Allah SWT atas segala karunia-Nya sehingga karya ilmiah ini berhasil diselesaikan. Tulisan yang berjudul “The freshwater atyid shrimps of the genus Caridina (Crustacea: Decapoda: Caridea) from Lake Lindu, Central Sulawesi, Indonesia” ini mengungkapkan adanya penemuan dua spesies baru udang air tawar dari danau Lindu yaitu Caridina sp A dan Caridina sp B. Tulisan ini juga mendeskripsikan kembali Caridina linduensis,
udang air tawar endemik Danau Lindu yang merupakan satu-satunya udang air tawar yang pernah dilaporkan dari Danau Lindu lebih dari 100 tahun yang lalu. Tulisan ini mengungkapkan juga adanya pereferensi habitat dan distribusi yang berbeda pada ketiga spesies Caridina tersebut.
Terima kasih penulis ucapkan kepada Dr Bambang Suryobroto, Dr Ir Achmad Farajallah, MSi, Dr Ir Daisy Wowor, MSc dan Prof Dr Ir Dede Setiadi, MS selaku komisi pembimbing. Ucapan terima kasih juga saya sampaikan kepada seluruh staf pengajar di Program Studi Biosains Hewan serta teknisi di Laboratorium Molekuler Program Studi BSH. Kepada tenaga lokal di Danau Lindu serta mahasiswa Jurusan Biologi Universitas Tadulako yang telah membantu selama proses sampling di Danau Lindu, penulis juga mengucapkan terima kasih. Kepada rekan-rekan seprofesi di Jurusan Biologi Universitas Tadulako serta rekan-rekan di Himpunan Mahasiswa Pascasarjana Sulawesi Tengah, saya juga ucapkan terima kasih atas dukungannya.
Terima kasih penulis sampaikan kepada Dekan Fakultas MIPA Universitas Tadulako dan Rektor Universitas Tadulako yang telah memberi izin untuk melanjutkan pendidikan S3, Dirjen Dikti Kementerian Pendidikan dan Kebudayaan atas beasiswa BPPS dan beasiswa Sandwich-like. Penulis juga mengucapkan terima kasih kepada Dr Kristina von Rintelen yang telah mengirimkan deskripsi asli Caridina linduensis dan Prof Rudolf Meier yang telah membimbing penulis selama mengikuti program Sandwich-like di Evolutionary Biology Laboratory, National University of Singapore.
Kepada rekan seangkatan 2009 Dr. Irma Shita Arlyza dan Puji Rianti MSi. serta seluruh angkatan 2011 S3 BSH: Andy Darmawan, MSi, RR. Sri Catur Setyawaningsih MSi, FX Widadi Padmarsari Soetignya MSi dan Yuliadi Zamroni M.Si. penulis juga mengucapkan terima kasih atas diskusi dan dukungannya selama penulis menyelesaikan penulisan disertasi.
Ungkapan terima kasih juga disampaikan kepada kedua orangtua penulis, bapak dan ibu mertua, seluruh keluarga besar penulis, serta suami penulis, Zulfan Hasibuan MPd atas segala dukungan dan kesabarannya selama penulis mengikuti pendidikan doktor.
Bogor, Agustus 2014
DAFTAR ISI
DAFTAR TABEL xi
DAFTAR GAMBAR xi
1 INTRODUCTION 1
2 LITERATURE STUDY 2
3 MATERIAL AND METHODS 3
Study area 3
Collecting specimen 4
Environmental Data Analysis 4
Morphometric Measurements for Taxonomical Analysis 5
4 RESULTS AND DISCUSSION 6
Results 6
Discussion 25
5 CONCLUSION 29
REFERENCES 29
DAFTAR TABEL
Table 1 Distribution of Caridina species collected in Lake Lindu system …..21
Table 2 Distribution of Caridina species in Lake Lindu, Lake Poso and Malili lake system ……...………..24
DAFTAR GAMBAR
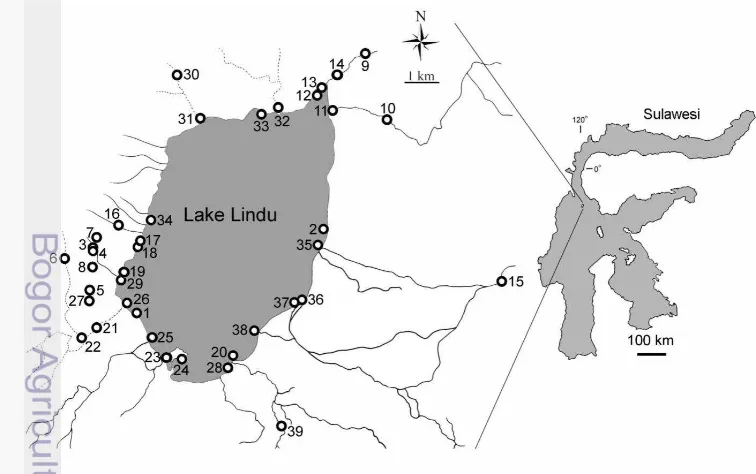
Figure 1 Morphology of Caridea shrimp ... 2Figure 2 Location of the Lake Lindu system in Sulawesi Island (right) and overview of the sampling sites in the lake (left). Circle refer to the sampling site ... 4
Figure 3 Line drawing of Caridina linduensis Roux, 1904. ... 7
Figure 4 Line drawing of Caridina linduensis Roux, 1904. ... 8
Figure 5 Line drawing of Caridina sp B. ... 13
Figure 6Line drawing of Caridina sp B. ... 14
Figure 7 Line drawing of Caridina sp A. ... 18
Figure 8 Line drawing of Caridina sp A. ... 19
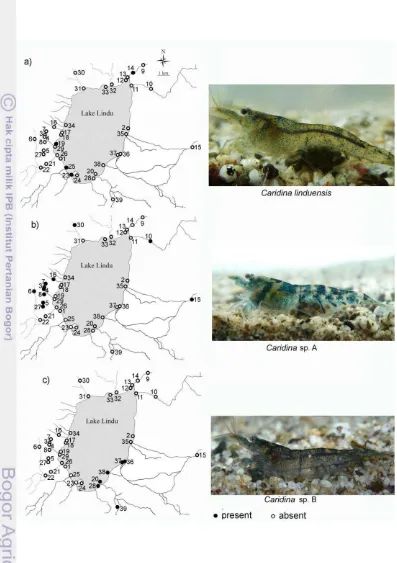
Figure 9 Map of the Lake Lindu system, showing distribution of a) Caridina linduensis b) Caridina sp A and c) Caridina sp B, presence and absence as indicated. ... 23
1
INTRODUCTION
Freshwater atyid shrimps are widely distributed in Indonesia. They can be found in various water bodies such as lake, pond, river, stream and ditch, both in surface and underground waters (Wowor et al. 2004). The shrimp habit as scavengers or detritus feeders make this family very important in ecosystem to recycle organic material. Leaf decay rate in the tropical stream was significantly greater in the presence of shrimps than in their absence (March et al. 2001).
The shrimps are characterized by having chelipeds with setae on the tip of the fingers which are used for filtering small aquatic organisms or scraping detritus during feeding (Fryer 1977). The atyid shrimp is consisted of 469 species dominated by the genus Caridina. The genus has 290 species members (De Grave and Fransen 2011). In Indonesia, there are 62 species of atyid shrimps and 52 are reported to be found in Sulawesi and nearby islands (De Grave and Fransen, 2011; Klotz and von Rintelen 2013). The majority of the species are belong to the genus
Caridina with more than half of them are lacustrine and endemic to the island. In the last ten years, the study of the lacustrine Caridina spp in Sulawesi has been focused in taxonomy and evolution of the species. The studied lakes are Poso and Malili lakes system which were considered by Schön and Martens (2004) as ancient lakes. Recently, the ancient status of the lakes was confirmed both in terms of their estimated age as well as their fauna (von Rintelen et al. 2012). It is agree with the definition of ancient lake which is usually perceived as more or less long‐lived lake that often associated with both high species numbers and endemicity in various systematic groups (Albrecht 2012).
Before 2006, there are only nine species known from the Lake Poso and Malili lakes system. Recent studies record 12 new Caridina species from the lakes, viz. C. caerulea, C. dennerli, C. glaubrechti, C. holthuisi, C. longidigita, C. Swiss naturalists Paul Benedict Sarasin and Karl Friedrich Sarasin during the 1901–1903 Sulawesi Expedition.
During year 2011 field work, Annawaty and Wowor (2012) came across two other undescribed Caridina species from the Lake Lindu, i.e. Caridina sp A and Caridina sp B. The three Caridina species can be distinguished by the shape of the rostrum and the structure of mandible (Annawaty and Wowor, submitted manuscript).
2
2
LITERATURE STUDY
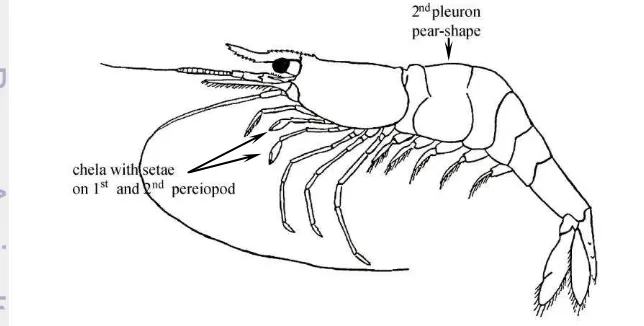
Freshwater shrimp belong to the order Decapoda, infraorder Caridea which is characterized by the second abdominal pleuron (lateral plate) greatly expanded, pear-shaped and overlapping posterior part of first pleuron and anterior part of third pleuron (Chan 1998). Caridea shrimp consists of approximately 3438 species in 35 families, one of the most speciose freshwater family is the Atyidae which is
consisted of 469 species and more than half of them belongs to the genus
Caridina (de Grave and Fransen 2011). The Family Atyidae are characterised by unique feeding chelipeds, with the complex brushes on the first and second pereiopods (Fig. 1) filtering out suspended matter or sweeping up microbial films (Fryer 1977).
The study of the freshwater atyid shrimp fauna of Sulawesi was began more than a century ago when the first atyid species, Atya wyckii described from the Lake Tondano, North Sulawesi. The species was described by Hickson on 1888 (Klotz et al. 2007).
According to de Grave et al. (2008) half of 28 species of atyid shrimp reported from Sulawesi are endemic to the island. The number of species increased rapidly in the last ten years mainly for the lacustrine species. Cai and Wowor (2007) described C. longidigita and re-described 3 other species i.e. C. sarasinorum, C. ensifera and C. acutirostris from Lake Poso. The next 2 year, von Rintelen and Cai (2009) described two new species from the lake, C. caerulea and
C. schenkeli and increase the number of Caridina species in the lake to become 6. The other lake which is received considerable attention is Malili lakes system. The lake system composed of three major lakes that directly connected, Matano, Mahalona, and Towuti and two smaller satellite lakes, Lake Lontoa (or Wawantoa) and Lake Masapi. There are several species described from 2006 i.e.
C. spongicola (Zitzler and Cai 2006), C. woltereckae and C. mahalona (Cai et al.
2009), C. dennerli, C. glaubrechti, C. holthuisi, C. parvula, C. profundicola and C.
3
striata (von Rintelen and Cai 2009).
Caridina linduensis is the only atyid shrimp reported from Lake Lindu so far. The description of the species based on the collection of Swiss naturalis, Paul and Fritz Sarasin during the 1901–1903 Sulawesi Expedition (Roux 1904). Lake Lindu located in the central highlands of Sulawesi (Lukman 2007) at an altitude of 982 m asl. This tectonic lake is found along a strike-slip fault zone, the Palu-Koro Fault, formed from convergence of three major tectonic plates, i.e. Pacific, Indo-Australia and Eurasia. This fault zone is a fast slipping area but with a relatively low level of seismicity (Bellier et al. 2001).
The age of Lake Lindu was estimated by Sarasin and Sarasin (1905) based on the lake mollusk fauna, and it is believed that geologically, it is a very recent structure that was formed through the sinking of a part of a mountain range during the Pleistocene. However, the exact age of Lake Lindu is still not known. Lake Lindu is drained by the Rawa River towards the north and in a point turns left to join the Sopu River through a long, deep gorge. The confluence of Rawa and the Sopu rivers marks the start of the Gumbasa River (Deschamps and Turland 2001) which runs into Palu River and empties to Palu Bay.
3
MATERIAL AND METHODS
Study area
4
Collecting specimen
Purposive sampling method was applied to 39 sampling sites (Fig. 1). Samples were collected with tray net and hand net in July, August and November 2011. All specimens obtained were fixed in 96% ethanol. The ethanol was changed after 24 hours with fresh 96% ethanol. The specimens are deposited in Division of Zoology, Research Center for Biology, Indonesian Institute of Sciences (LIPI) and Laboratory of Molecular, Division of Animal Function and Behavior, Department of Biology, Bogor Agricultural University, Indonesia.
During collecting, we recorded environmental parameters i.e. 1) the temperature of the water with a mercury thermometer, 2) the qualitative water clarity, 3) the qualitative water current (slow current water to almost stagnant or moderate flow), 4) substrate (sediment classification) based on Wentworth (1922), 5) presence or absence of water plant associated with the Caridina habitat, and 5) the coordinate and altitude of sampling location, measured using GPS.
Environmental Data Analysis
The coordinate and altitude of each sampling site were overlaid to the map of Lake Lindu system published by BAKOSURTANAL. The percentage of each
Caridina species found was measured by the amount of particularly species caught to the total of Caridina caught.
5
Morphometric Measurements for Taxonomical Analysis
Morphometric measurements were taken using a Nikon SMZ 800 stereo-microscope with an ocular micrometer. Drawings of chepalothorax were made with a camera lucida mounted on the microscope while other cephalic and body appendages were made with a microphotograph mounted on a Nikon Phase Contrast 0.90 DRY light microscope. Drawings were then digitized and processed with Adobe Photoshop CS3 extended.
Comparative data for Caridina linduensis is based on the original description of Roux (1904), and for C. sarasinorum and C. schenkeli is based on the published data of Cai and Wowor (2007) and von Rintelen and Cai (2009), respectively. That for C. parvidentata and C. sulawesi is from Cai and Ng (2009).
6
4
RESULTS AND DISCUSSION
Results
Taxonomy of the Genus Caridina from Lake Lindu
Systematic account
Family Atyidae De Haan, 1849
Caridina H. Milne Edwards, 1837
Caridina linduensis Roux, 1904
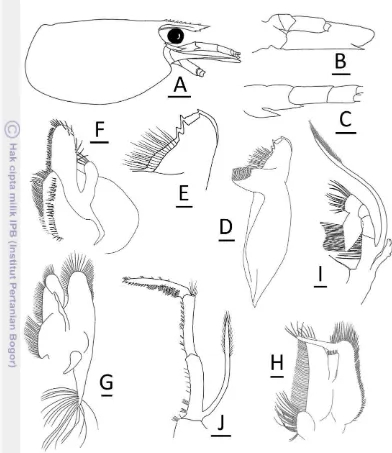
(Figs. 3–4)
Caridina linduensis J. Roux 1904: 541, pl. 9, figs. 1–4 [type locality: Lake Lindu, Sulawesi (Celebes), Indonesia].–Bouvier 1925: 224, figs. 497–503.–Chace 1997: 13.–von Rintelen et al. 2008: 2244.–De Grave and Fransen 2011: 276.
Material examined: Four males, cl 3.0–3.3 mm, 9 females, cl 3.6–4.4 mm,
6 ovigerous females, cl 3.8–4.2 mm (MZB Cru 3793), Uwe Rawa at the outlet of Lake Lindu, about 50 m from the lake, 01°16’20.1”S 120°06’32.7”E, on leaf litter and dead wood, coll. Annawaty and D. Wowor, 13 Nov 2011; 1 male, 1 ovigerous female (ZRC), same data as MZB Cru 3793; 7 males, cl 2.7–3.4 mm, 4 females, cl Wowor, 17 Nov 2011; 1 female, cl 3.9 mm (MZB Cru 3796), at Tomado beach, 01°19’31.9”S 120°03’10.0”E, on root of macrophytes, coll. Annawaty and D. Wowor, 15 Nov 2011.
Description: Rostrum slender, reaching near or slightly beyond end of third
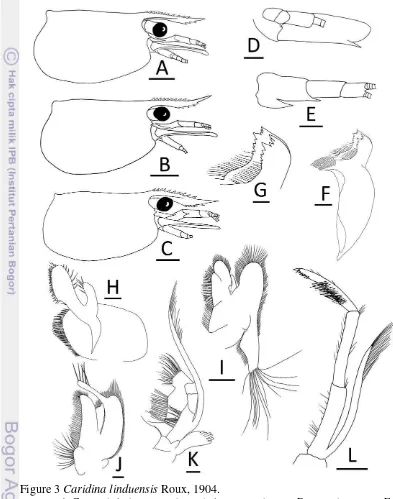
segment of antennular peduncle, does not overreach end of scaphocerite, tip of rostrum straight or slightly curved upwards, 0.5–0.7 times as long as carapace, dorsal margin horizontal or slightly sinuous, dorsal with 7–12 (mode 9) teeth, about one-third distal unarmed, no tooth behind orbital margin nor sub-apical tooth, ventral with 0–6 (mode 2 or 3) teeth. Antennal spine short, situated below inferior orbital angle. Pterygostomian margin rounded (Fig. 3A–C).
7 Scaphocerite 3.2 times as long as wide (Fig. 3D). Incisor process of mandible ending in 16–18 irregular teeth, molar process truncated (Fig. 3F–G). Lower lacinia of maxillula broadly rounded, upper lacinia elongated, with numerous distinct teeth on inner margin, palp slender (Fig. 3H). Upper endite of maxilla
Figure 3 Caridina linduensis Roux, 1904.
8
Figure 4 Caridina linduensis Roux, 1904.
9 subdivided, palp short, scaphognathite tapering posteriorly with numerous long, curved setae at posterior end (Fig. 3I). Palp of first maxiliped ending in triangular projection (Fig. 3G). Podobranch of second maxiliped reduced to lamina (Fig. 3K). Third maxiliped reaching end of scaphocerite, with ultimate segment slightly longer than penultimate segment (Fig. 3L).
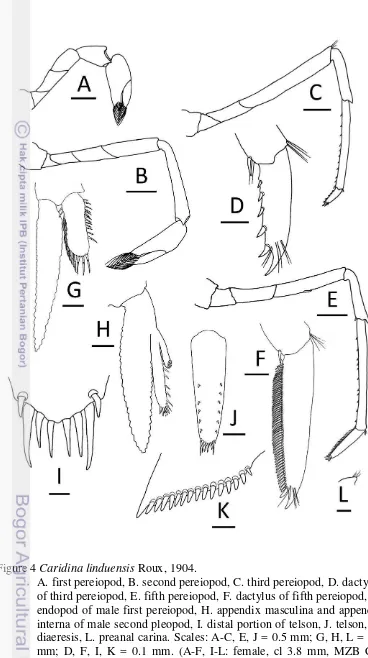
Epipod present on first 2 pereiopods, reduced in size posteriorly, absent on last 3 pereiopods. Chela and carpus of first pereiopod distinctly stouter, broader than chela and carpus of second pereiopod. First pereiopod stout, reaching to distal end of basal segment of antennular peduncle; merus 1.8–2.7 times as long as wide, slightly shorter than carpus; carpus excavated anteriorly, 1.6–2.4 times as long as wide, shorter than chela, chela 2.1–2.9 times as long as wide; movable finger as long as or slightly longer than palm, finger tip round (Fig. 4A).
Second pereiopod reaching to distal end of third segment of antennular peduncle; merus distinctly shorter than carpus, 4.4–5.8 times as long as wide; carpus slender, 5.1–6.9 times as long as wide, 1.2–1.3 times length of chela; chela 2.5–3.7 times as long as wide; movable finger 1.2–1.8 times as long as palm, finger tip round (Fig. 4B).
Third pereiopod slender, dactylus, half distal propodus overreach third segment of antennular peduncle; propodus 10.4–13.0 times as long as wide, 4.1– 4.5 times as long as dactylus; dactylus 2.8–4.0 times as long as wide (terminal spine included, without spines of flexor margin), terminating in 1 large claw with 5–9 (mode 5) accessory spines on flexor margin, reducing in size proximally (Fig. 4C–D). Sexual dimorphism present. Third, fourth pereiopods of male with numerous hooked spinules on inner, outer margins of propodus, carpus, respectively.
Fifth pereiopod slender, tip of dactylus reach end third segment of antennular peduncle; propodus 10.8–13.7 times as long as wide, 2.5–3.3 times as long as dactylus; dactylus 4.7–5.8 times as long as wide (terminal spine included, without spines of flexor margin), terminating in 1 large claw, with 53–56 (mode 54) accessory spinules on flexor margin (Fig. 4E–F).
Endopod of male first pleopod subrectangular, without appendix interna, 2.8 times as long as wide, 0.4 times length of exopod (Fig. 4G).
Appendix masculina of male second pleopod very slender, 0.5–0.6 times length of endopod, with appendix interna 0.3–0.5 times length of appendix masculina (Fig. 4H).
Sixth abdominal somite 0.5–0.6 times of carapace, 1.4–1.6 times as long as fifth somite, 0.9 times as long as telson. Telson 2.8 times as long as wide, distal margin rounded without posteromedian projection, with 4–6 (mode 5) pairs of dorsal spinules, 1 pair of dorsolateral spinules; distal end with 3 or 4 (mode 3) pairs of spine, lateral pair of spines distinctly longer than intermediate pairs (Fig. 4I–J). Preanal carina sub-rectangular, without spine (Fig. 4L).
Uropodal diaresis with 14–16 (mode 16) movable spinules (Fig. 4K).
Ovigerous females with 26–47 eggs (n = 3); Egg size 1.0–1.1 x 0.7 mm in diameter (n = 40, eggs with eyes).
10
the mouth of the only outlet stream with mud-sandy substrates with very slow currents or almost stagnant water. The temperature of the habitat varies between 28.0° and 29.0°C.
Distribution: Caridina linduensis is found only in Lake Lindu and the mouth
of outlet stream. It is distributed in the south and southwestern of the lake, and around the junction of the lake and Uwe Rawa at the north. A single specimen was also caught at the west coast of the lake at Tomado village.
Remarks: Although we did not compare our specimens with the type
material of C. linduensis, but we have seen the excellent drawings of the type material in the thesis of Cai (2004) which confirms our observations that our collected specimens from the type locality is indeed C. linduensis. However, our material has few differences from those examined by Roux (1904) in the higher number of accessory spines on the flexor margin of the fifth pereiopods (53-56, mode 54 vs. 50) and the ratio of the dactylus to the palm of the first pereiopod (dactylus as long as or slightly longer than palm vs. dactylus slightly shorter than palm). The differences might be an intraspecific variation within the population.
In this study, we observe that there are two characters which were not mentioned by Roux (1904), i.e. the mandible and the presence of sexual dimorphism in C. linduensis. The results show that the terminal of the incisor process of the mandible is ending in many irregular sharp teeth, and the inner margin of the propodus and the outer margin of the carpus of both third and fourth pereiopods of males are covered by numerous claw-shape spinules.
With regard to the shape of the rostrum, the position of the antennal spine to the inferior orbital angle, and the absence of the posteromedian projection of the telson, C. linduensis resembles C. sarasinorum and C. schenkeli, two endemic
Caridina species from Lake Poso in Sulawesi. However, it can be separated from both species by the absence of tooth behind the orbital margin (vs. 3–7 teeth in C. sarasinorum, 2–5 teeth in C. schenkeli), the lower number of the ventral rostral teeth (0–6 teeth vs. 8–17 teeth in C. sarasinorum, 9–13 teeth in C. schenkeli), the smaller ratio of the second segment to the third segment of the antennular peduncle (1.0–1.3 vs. 2.0 in C. sarasinorum, 1.6–2.0 in C. schenkeli), the smaller ratio of the antennular peduncle length to the carapace length (0.6–0.8 times vs. as long as in C. sarasinorum, 0.8–1.0 times in C. schenkeli), the relatively broader scaphocerite (3.2 times as long as wide vs. 4.4 times in C. sarasinorum, 3.3–4.7 times in C. schenkeli), the more slender dactylus of the fifth pereiopod (4.7–5.8 as long as wide vs. 3.0–3.9 in C. sarasinorum, 3.7–4.8 in C. schenkeli), the broader telson (2.8 times as long as wide vs. 4.1 times in C. sarasinorum, 3.0–3.6 times in
11
Caridina linduensis also shares several characters with C. schenkeli which can be used to distinguished both species from C. sarasinorum, i.e., by the presence of epipod on the first two pereiopods (vs. present on the first pereiopod and greatly reduced or absent on the second pereiopod in C. sarasinorum), and the larger egg size (1.0–1.1 x 0.7 mm vs. 0.9–1.0 x 0.5–0.6 mm in C. sarasinorum). Wowor, 21 Nov 2011. Paratypes: 11 females, cl 3.9–4.8 mm, 2 ovigerous females 4.2–4.9 mm (MZB Cru 3807), same data as holotype; 1 male, cl 3.5 mm, 1 ovigerous female, cl 4.4 mm (MZB Cru 3808), Uwe Poweroa, Lake Lindu, 01°20’05.6”S 120°05’55.2”E, on roots of macrophytes, coll. Annawaty and D. Wowor, 21 Nov 2011; 12 females, cl 3.8–4.5 mm (MZB Cru 3809), at the mouth of Air Dingin stream, Lake Lindu, 01°20’07.2’’S 120°05’52.2’’E, on roots of macrophytes, coll. Annawaty and D. Wowor, 21 Nov 2011; 2 males, cl 3.2 mm, 1 female, cl 4.8 mm (MZB Cru 3810), west side of mouth of Uwe Kati, Lake Lindu, 01°20’54.4”S 120°04’51.0”E, on roots of macrophytes, coll. Annawaty and D. Wowor, 18 Nov 2011; 2 females, cl 3.8 mm (MZB Cru 3811), at east side of mouth of Uwe Kati, Lake Lindu, 01°20’54.4”S 120°04’51.0”E, on roots of macrophytes, coll. Annawaty and D. Wowor, 16 Nov 2011; 26 males, cl 3.7–4.8 mm, 13 females, cl 4.0–5.3 mm, 5 ovigerous females, cl 4.8–5.4 mm, 1 male, cl 4.1 mm, dissected (MZB Cru 3812), Uwe Kati, Lake Lindu catchment, 01°21’54.5”S 120°05’35.2”E, on roots of macrophytes, coll. Annawaty and D. Wowor, 21 Nov 2011; 1 male, 1 female, 1 ovigerous female (ZRC), same data as MZB Cru 3812. Others: 29 juveniles, 8 males, 9 females, 1 ovigerous female (MZB Cru 3974), Uwe Kati, Lake Lindu catchment, coll. Annawaty and D. Wowor, 21 Nov 2011.
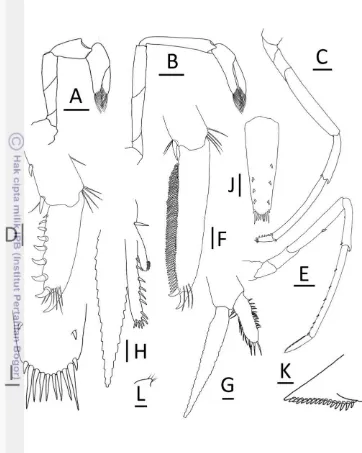
Description: Rostrum short, overreach end of basal segment to half
proximal of second segment of antennular peduncle, 0.4 times as long as carapace, dorsal margin bent downwards above orbit with tip directed anteriorly, dorsal with 1–9 (mode 6) teeth, without tooth behind orbital margin, no sub-apical tooth, ventral with 0–3 (mode 1) teeth. Antennal spine short, situated below inferior orbital angle. Pterygostomian margin rounded (Fig. 5A).
12
0.7 basal segment of antennular peduncle (Fig. 5C). Scaphocerite 3.2 times as long as wide (Fig. 5B).
Incisor process of mandible ending in 6 irregular teeth and 2 very fine granulated rows between teeth, molar process straight (Fig. 5D–E). Lower lacinia of maxillula broadly rounded, upper lacinia elongated, with numerous distinct teeth on inner margin, palp slender (Fig. 5F). Upper endite of maxilla subdivided, palp short, scaphognathite tapering posteriorly with numerous long, curved setae at posterior end (Fig. 5G). Palp of first maxiliped ending in triangular projection (Fig. 5H). Podobranch of second maxiliped reduced to a lamina (Fig. 5I). Third maxiliped reaching end of scaphocerite with ultimate segment slightly longer than penultimate segment (Fig. 5J).
Epipod present on first 2 pereiopods, reduced in size posteriorly, absent on last 3 pereiopods. Chela and carpus of first pereiopod distinctly stouter, broader than chela and carpus of second pereiopod.
First pereiopod stout, tip of chela not reaching basal segment of antennular peduncle; merus 2.2–2.5 times as long as wide, slightly shorter than carpus; carpus excavated anteriorly, distinctly shorter than chela, 1.7–2.5 times as long as wide, chela 2.0–2.4 times as long as wide; movable finger slightly longer than palm, finger tip round (Fig. 6A).
Second pereiopod reach end of third segment of antennular peduncle; merus distinctly shorter than carpus, 4.1–4.8 times as long as wide; carpus slender, 1.3– 1.4 times as long as chela, 6.1–6.7 times as long as wide; chela 3.2–3.5 times as long as wide; movable finger 1.4–1.6 times as long as palm, finger tip round (Fig. 6B).
Third pereiopod slender, dactylus, half distal propodus overreach third segment of antennular peduncle; propodus 10.0–10.6 times as long as wide, 3.6– 4.2 times as long as dactylus; dactylus 3.3–4.2 times as long as wide (terminal spine included, without spines of flexor margin), terminating in 1 large claw with 7–10 (mode 7) accessory spines on flexor margin, reducing in size proximally (Fig. 6C–D). Sexual dimorphism present. Third and fourth pereiopods of male with numerous hooked spinules on inner, outer margins of propodus, carpus respectively.
Fifth pereiopod slender, one-third distal of dactylus overreach third segment of antennular peduncle; propodus 11.5–13.7 times as long as wide, 2.7–3.2 times as long as dactylus; dactylus 5.5–6.0 times as long as wide (terminal spine included, without spines of flexor margin), terminating in 1 large claw with 63–80 accessory spinules on flexor margin (Fig. 6E–F).
Endopod of male first pleopod subrectangular, without appendix interna, 3.2 times as long as wide, 0.4–0.5 times length of exopod (Fig. 6G).
Appendix masculina of male second pleopod very slender, 0.5–0.6 times length of endopod, with appendix interna 0.4–0.5 times length of appendix masculina (Fig. 6H).
13
Figure 5 Caridina sp B.
14
Figure 6Line drawing of Caridina sp B.
15 Uropodal diaresis with 13–15 (mode 15) movable spinules (Fig. 6K).
Ovigerous females with with 39–40 eggs (n = 2); Egg size 1.1–1.2 x 0.7–0.8 mm in diameter (n= 40, eggs with eyes).
Habitat: Caridina sp B is a soft substrate dweller on roots of macrophytes
(water grass and ferns). This species mainly occurs at the edge of inlet streams and the edge of the lake that have muddy-sandy substrates. It was found in streams of 0.5 to 3 m depth, with murky and slow running water that may be almost stagnant. The temperature of the habitat varies from 22.0° to 28.5°C.
Distribution: Caridina sp B is endemic to Lake Lindu and the catchment area. So far, it was found only at the relatively flat southeastern shore of the lake and Uwe Kati. This species has not been encountered in the north and the west coasts of the lake.
Etymology: The species is named after its main habitat, a local name for water grass, where this species mainly occur clinging around. The name is used as a noun in apposition.
Remarks: With regard to the shape of the rostrum, the absence of a tooth behind the orbital margin, the position of the antennal spine to the inferior orbital angle, the absence of a median projection at the terminal margin of the telson, the absence of a preanal carina spine, and the absence of an appendix interna at the terminal margin of the sub-rectangular endopod of the first pleopod of male,
Caridina sp B is similar to C. linduensis and C. parvidentata Roux, 1904. However, the new species can be differentiated from C. linduensis by the shorter rostrum (ratio of rostrum length to carapace length 0.4 vs. 0.5–0.7 in C. position of the antennal spine (below inferior orbital angle vs. fused with inferior orbital angle in C. parvidentata), the brouder scaphocerite (3.2 times as long as wide vs. 3.8 times in C. parvidentata), the presence of epipod on the first two pereiopods (vs. on the first three pereiopods in C. parvidentata), the stouter chela of the second pereiopod (3.2–3.5 times as long as wide vs. 3.8 times in C. parvidentata), the more slender dactylus of the fifth pereiopod (5.5–6.0 times as long as wide vs. 4.1 times in C. parvidentata), the lower ratio of appendix masculina to endopod of male second pleopod (0.5–0.6 times vs. 0.8 times in C. parvidentata), the proportionately broader telson (2.7 times as long as wide vs. 3.0 times in C. parvidentata), the smaller ratio of sixth abdominal somite to telson length (0.9 vs. as long as in C. parvidentata), and the relatively larger egg size (1.1–1.2 x 0.7–0.8 mm vs. 0.9 x 0.6 mm in C. parvidentata). In addition, C. sp B can be distinguished from both C. linduensis and C. parvidentata by having more accessory spinules on the flexor margin of the fifth pereiopod (63–80 vs. 53–56 in
17 females, 12 ovigerous females (MZB Cru 3980), Uwe Laga, Lake Lindu catchment, coll. Annawaty, 10 Aug 2011.
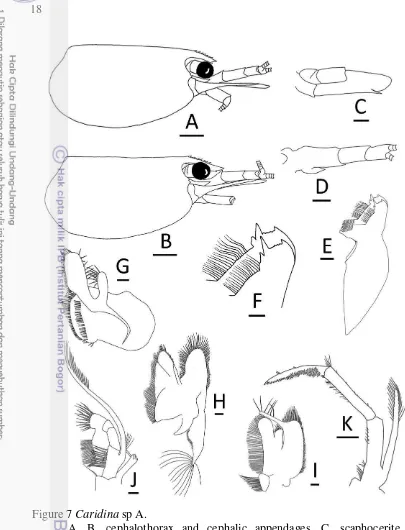
Description: Rostrum very short, reaching near to or reach end of basal segment of antennular peduncle, 0.3 times as long as carapace, dorsal margin bent downwards above orbit with tip directed anteriorly, armed dorsally with 2–10 (mode 6) teeth, without tooth behind orbital margin, no sub-apical teeth, armed ventrally with 0–2 (mode 1) teeth. Antennal spine short, situated below inferior orbital angle. Pterygostomian margin rounded (Fig. 7A–B).
Eyes well developed, anterior end 0.7 times length of basal segment of antennular peduncle. Antennular peduncle 0.6–0.7 times as long as carapace, basal segment of antennular peduncle longer than second, third segment lengths, second segment distinctly longer than third segment, anterolateral angle of basal segment of antennular peduncle reaching 0.3 proximal second segment of antennular peduncle. Stylocerite reaching between 0.6 and 0.8 proximal basal segment of antennular peduncle (Fig. 7D). Scaphocerite 3.6 times as long as wide (Fig. 7C).
Incisor process of mandible ending in 6 irregular teeth and 2 very fine granulated rows between teeth, molar process straight (Fig. 7E–F). Lower lacinia of maxillula broadly rounded, upper lacinia elongated, with numerous distinct teeth on inner margin, palp slender (Fig. 7G). Upper endite of maxilla subdivided, palp short, scaphognathite tapering posteriorly with numerous long, curved setae at posterior end (Fig. 7H). Palp of first maxiliped ending in a triangular projection (Fig. 7I). Podobranch of second maxiliped reduced to a lamina (Fig. 7J). Third maxilliped reaching end of scaphocerite, with ultimate segment slightly shorter than penultimate segment (Fig. 7K).
Epipod present on first 2 pereiopods, reduced in size posteriorly, absent on last 3 pereiopods. Chela and carpus of first pereiopod distinctly stouter, broader than chela and carpus of second pereiopod.
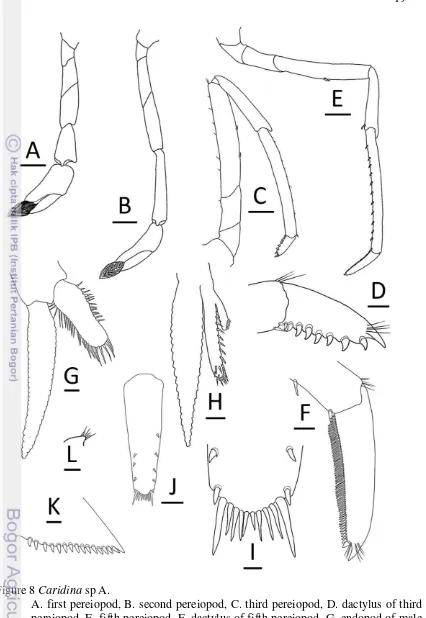
First pereiopod stout, tip of chela reaching middle of second segment of antennular peduncle; merus 2.1–2.7 times as long as wide, as long as or slightly shorter than carpus; carpus excavated anteriorly, distinctly shorter than chela, 1.7– 2.5 times as long as wide; chela 2.2–2.4 times as long as wide; movable finger longer than palm, finger tip round (Fig. 8A).
Second pereiopod slightly overreach end of third segment of antennular peduncle; merus distinctly shorter than carpus, 3.5–4.6 times as long as wide; carpus slender, 1.1–1.3 times as long as chela, 4.4–5.4 times as long as wide; chela 2.9–3.2 times as long as wide; movable finger 1.3–1.5 times as long as palm, finger tip round (Fig. 8B).
18
Figure 7 Caridina sp A.
19
Figure 8 Caridina sp A.
20
Fifth pereiopod slender, dactylus, half distal propodus overreach third segment of antennular peduncle; propodus 10.8–13.4 times as long as wide, 2.7– 3.2 times as long as dactylus; dactylus 4.4–5.3 times as long as wide (terminal spine included, without spines of flexor margin), terminating in 1 large claw with 61–66 (mode 66) accessory spinules on flexor margin (Fig. 8E–F).
Endopod of male first pleopod subrectangular, without appendix interna, 2.6 times as long as wide, 0.4–0.5 times length of exopod (Fig. 8G).
Appendix masculina of male second pleopod very slender, 0.5–0.6 times length of endopod, with appendix interna 0.4–0.5 times length of appendix masculina (Fig. 8H).
Sixth abdominal somite 0.4–0.5 times length of carapace, 1.8–1.9 times as long as fifth somite, 0.9 times as long as telson. Telson 2.8 times as long as wide, distal margin rounded without posteromedian projection, with 5 or 6 (mode 5) pairs of dorsal spinules, 1 pair of dorsolateral spinules; distal end with 3–5 (mode 3) pairs of spine, lateral pair of spines distinctly longer than intermediate pairs (Fig. 8I–J). Preanal carina sub rectangular, without spine (Fig. 8L).
Uropodal diaresis with 15–16 (mode 15) movable spinules (Fig. 8K).
Ovigerous females with with 29–46 eggs (n = 3); egg size 1.2–1.5 x 0.8–1.0 mm in diameter (n = 60, eggs with eyes).
Habitat: Caridina sp A is an exclusively riverine species which dwells in clear water streams and tributaries with sandy gravel substrate and moderate flow running water. This species clings on hard substrate such as gravel up to boulders and hangs on the roots of different macrophytes. The temperature of the habitat varies from 18.0° to 23.0°C.
Distribution: Caridina sp A is endemic to the catchment area of Lake Lindu, but does not occur in the lake itself. This species is mainly distributed in streams and tributaries at the west coast of the lake at the foot of hills, but it can also be found in two streams at the east coast, i.e. in Uwe Tokaroru and in Uwe Lembosa.
Etymology: The specific name refers to Kaili Tribe, resident of the plains and the hills around Lake Lindu where this new species was encountered. The name is used as a noun in apposition.
Remark: Although C. sp A shares several characters with the other two
Caridina species in Lake Lindu, such as the absence of a tooth behind the orbital margin, the antennal spine is below the inferior orbital angle, there is no median projection at the terminal margin of the telson, no preanal carina spine, and no appendix interna at the terminal margin of the endopod of the first pleopod of male; it can easily be distinguished by having an extremely short rostrum which is almost reaching near to or reach end of basal segment of antennular peduncle (vs. distinctly overreach end of basal segment of antennular peduncle in C. sp B, reaching near to or slightly beyond end of antennular peduncle in C. linduensis), and the larger body size of ovigerous females (maximum carapace length 6.8 mm vs. 4.9 mm in C. linduensis and 5.4 mm in C. sp B). The size of the eggs is also the largest among the three Caridina species endemic to the lake, i.e. 1.2–1.5 x 0.8–1.0 mm (vs. 1.0–1.1 x 0.7 mm for C. linduensis, 1.1–1.2 x 0.7–0.8 mm for C.
21
Caridina sp A also resembles C. sulawesi Cai and Ng, 2009, with regard to the shape of the rostrum, the lack of median projection at the terminal margin of the telson, the lack of a preanal carina spine, and the lack of appendix interna at the terminal margin of the endopod of the first pleopod of male. However, it can easily differentiated by the number of the dorsal rostral teeth (2–10, mode 6 vs. mostly unarmed or rarely with 4–6 in C. sulawesi), the position of the antennal spine (below inferior orbital angle vs. fused with inferior orbital angle in C. sulawesi), the more slender scaphocerite (3.6 times as long as wide vs. 3.0 times in C. sulawesi), the longer finger of the first pereiopod (finger longer than palm vs. finger shorter than palm in C. sulawesi), the stouter carpus of the second pereiopod (4.4–5.4 times as long as wide vs. 5.7 times in C. sulawesi), the more slender dactylus of the fifth pereiopod (4.4–5.3 times as long as wide vs. 3.2 in C. sulawesi), and the larger number of spinules on the flexor margin of the fifth pereiopod (61–66, mode 66 vs. 53–55 in C. sulawesi). Caridina sp A can also be distinguished from C. sulawesi by the shorter endopod of the first pleopod of male (ratio of the endopod to the exopod 0.4–0.5 vs. 0.7 in C. sulawesi), the longer appendix interna of the second pleopod of male (ratio of the appendix interna to the appendix masculina 0.4–0.5 vs. 0.25 in C. sulawesi), the higher number of the uropodal diaresis (15–16, mode 15 vs. 16–21 in C. sulawesi), and the larger egg size (1.2–1.5 x 0.8–1.0 mm vs. 0.9–1.0 x 0.55–0.70 in C. sulawesi).
Habitat Preferences and Distribution of Caridina in Lake Lindu
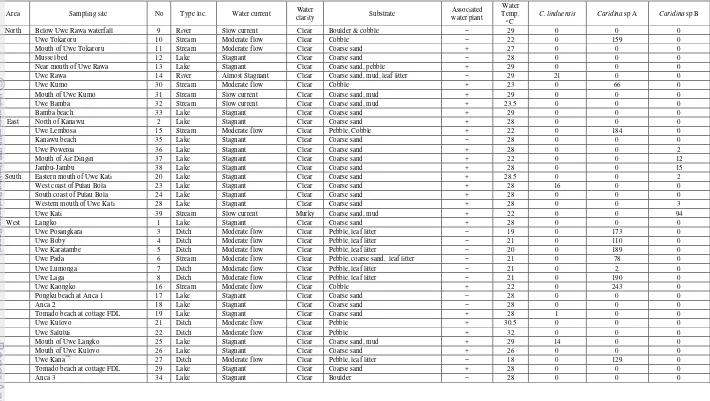
A total of 1703 Caridina spp were obtained. They were consisted of 3.05% C. linduensis, 89.43 % Caridina sp A, and 7.52% Caridina sp B. Distribution of each species collected in the lake system is shown in Table 1. From 39 sites sampled, only 21 sites were inhabit by Caridina shrimps. Caridina linduensis was only found on 4 sites. While Caridina sp A was the most common and it was present at 11 sampling sites; Caridina sp B was obtained from 6 sites. Water temperatures of the lake and its catchment area ranged from 18 to 29°C, and the altitude of the 39 sampling sites were ranged between 982 and 1020 m.
Distribution of the three Caridina species in Lake Lindu and the catchment area showed there is no overlap among the species. Caridina linduensis
is never found in sympatry with Caridina sp A nor Caridina sp B.
Caridina linduensis was found both in lake and the outlet of the lake with small amount of specimen. There are 3 sites in the lake where C. linduensis
present (Fig. 9), its habitat is always associated with the water plant such as fern or water grass. The species are clinging around the roots of the plant. Caridina linduensis is also found in the outlet with very slow current and almost stagnant. They dwell in leaf litter and dead wood in the river bank (Fig. 11).
Even though this species was never found in sympatry with the other
22
Tabel 1 Distribution of Caridina in Lake Lindu and catchment area.
Area Sampling site No Type loc. Water current Water
C. linduensis Caridina sp A Caridina sp B
23
24
Figure 10 Habitat of Caridina in Lake Lindu system
25
Caridina sp A is the true riverine species, inhabit streams and ditches with moderate flow running water and boulder and cobble substrate (Fig. 10). Most of the species are disperse in the streams and the ditches in western part of the lake and only found in two streams in the eastern part of the lake i.e Uwe Tokaroru and Uwe Lembosa.
Caridina sp Bis only distribute in the eastern part of the lake, particularly near the mouth of the inlet stream (Fig. 9). The species is not only found in the lake but also in a stream with slow current in eastern part (Kati stream). More of the specimens of this species were collected in Kati stream compare to the catch from the lake (Table 1). Caridina sp B was also found in sympatry with M. lanchesteri in the western mouth of Uwe Kati.
Discussion
Morphological differences among the Caridina species
According to Klotz and von Rintelen (2013), there are 35 Caridina species endemic to Sulawesi and nearby islands. With the discovery of the two new
Caridina species from Lake Lindu system, the total number of Caridina endemic to Sulawesi and its nearby islands, i.e. Peleng and Buton, increases to 37 species. There are now a total of 54 atyid species found in Sulawesi and few surrounding islands.
The three endemic Caridina species in Lake Lindu system have different morphological characters mainly in rostrum shape and mandible structure (see Fig. 3, 5 and 7). Caridina linduensis have the longest rostrum compared to the other species. Rostrumof Caridina sp B is rather short which tip overreaches the end of basal segment of antennular peduncle but never reaches end of the second segment of antennular peduncle, while Caridina sp A has a very short rostrum which tip has maximum reach at the end of the basal segment of the antennular peduncle. Caridina sp A has the shortest rostrum among Caridina species of Lake Lindu and its catchment area. This make Caridina sp A is very different from the other two Caridina species. Caridina sp A has also larger body size with ovigerous females maximum carapace length 6.8 mm, while C. linduensis and
Caridina sp B in have only 4.9 mm and 5.4 mm carapace length, respectively. Von Rintelen (2009) mentioned the morphology of the rostrum as a good character to delimit the different species from Lake Poso and Malili lakes system. The recent examination of the specimens from the Lake Lindu and its catchment area confirmed that the shape and the denticulation of the rostrum are good characters for recognizing species.
26
Habitat Preferences and Distribution of Caridina species.
The Lindu’s Caridina not only differ in their morphology but also in their habitat preferences. Caridina sp A was found in large amount both in western and eastern parts of the lake system. The abundance of this species in the streams and the ditches may be linked to the accumulation of large mass of detritus in the streams which provides food source for them. However, there was no Caridina sp A found in the lake. The limiting factor restricting Caridina sp A to disperse into the lake probably associated with the stream physical condition which is not present in the lake. Most of the streams in the western side have moderate running water with boulder and pebble substrate and slope varies between 15 and 40% (Lukman 2007). The water debit of some streams decrease at the lower part. It is caused by the porous surface of the bottom of the stream where some of the water infiltrates into the ground. Some streams even disappear before they reach the lake, such as Pada and Lumonga streams.
The streams in the eastern side are running on flat slope where the slope varies between 0 and 3% (Lukman 2007). The distribution of Caridina sp Ain the eastern side of the lake is restricted to Tokaroru and Lembosa streams which is around 3 and 7 km away from the lake shore, respectively. Apparently the shrimps were also prevented to disperse into the lake due to temperature differences between the streams and the lake. The maximum temperature of the habitat of
Caridina sp A is 23°C, while the water–temperature in the lake ranged between 28 and 29 °C. The high water–temperatures might be played a role as a natural barrier for Caridina sp A.
The high water–temperature might be performed as a limiting factor for
Caridina sp A to disperse into the lake, and on the other hand, the low water-temperature of the streams possibly prevents C. linduensis to distribute into the streams (Table 1). The only river where C. linduensis can inhabit is Uwe Rawa, the only outlet of the lake. The part of the river where it was found is very similar to the lake.
Contrary to the large amount of Caridina sp A gained from streams, C. linduensis and Caridina sp B were found in small numbers in the lake and near the inlets (Table 1). The fewer specimens caught probably affected by the occurrence of invasive fish species.
The Caridina in Lake Lindu System as Land-lock Species
The three endemic Caridina spp of Lake Lindu system have large-sized eggs with larval development of highly abbreviated type as shown in C. brevirostris
(average egg size 1.2 x 0.8 mm) (Shokita 1973) and C. pseudodenticulata
(average egg size 0.77 x 0.50 mm diameter) (Lai and Shy 2009). The early life stage of C. brevirostris from Iriomote Island, Japan, has only one zoea and one megalopa stage (Shokita 1973), while C. pseudodenticulata from western Taiwan goes through five larval stages before they become juveniles (Lai and Shy 2009). Based on the egg size, it is possible that the three Caridina species of the Lake Lindu system have very short larval developments as that of C. brevirostris.
27 We do not agree with von Rintelen et al.’s (2012) statement that Caridina with large-size eggs are direct developers. According to Gore (1985) and Rabalais and Gore (1985), “the young of direct development hatch more or less in the form of the adult, possessing distinct juvenile morphology and no free-swimming larval staged exist”, as shown in true freshwater crabs (see Cumberlidge and Ng 2009).
The large egg size of the all Caridina species from lake system indicate they are probably land-locked species which complete their entire life cycle in fresh water. Besides that, Uwe Rawa becomes several (at least three) waterfalls before it joint Gumbasa River. The authors had sampled shrimps in Gumbasa River but caught only Macrobrachium lepidactyloides (De Man, 1892), a palaemonid species typically associated with strong-current systems. Gumbasa is a relatively large river with very strong current and boulders substrate, not a habitat suitable for the lake Caridina species. In combination with their land-locked species type, ecological and physical barrier, the Lindu’s Caridina spp only distribute in the Lake Lindu system. The environmental factors limit the Caridina’s dispersal ability into other area. It is also caused the reduction of gene-flow among populations, which may explain the high degree endemism in Caridina spp in Lake Lindu.
The endemism of Lake Lindu’s fauna is not only present in the shrimp but also in the other species such freshwater crab (Parathelphusa linduensis), ricefishes (Oryzias sarasinorum and O. bonneorum), a bivalve (Corbicula linduensis) and one semi terrestrial gastropod (Oncomelania hupensis lindoensis) (Wowor and Annawaty, 2013).
There is also no overlap of Caridina species among Lake Lindu, Lake Poso and Malili lake system. Most of the species belong to the lake system are endemic to the lake itself (Table 2).
Threaten in the life of Caridina spesies
Some exotic fishes such as common carp (Cyprinus carpio) and Mozambique tilapia (Oreochromis mossambicus) have been introduced since 1950 for increasing fisheries productivity (Lukman 2006), and since 1970 tilapia has become the most common fish in the lake (Carney 1991). It is successfully compete with climbing perch (Anabas testudinus), a native fish of the lake (Graczyk and Fried 1998). The introduction of the tilapia is also correspond with the decline of freshwater mollusk in the lake particularly the genus Corbicula. The decline and near extinction of the mollusk probably due to the competition with the tilapia for phytoplankton and/or predation by the tilapia, known to be zooplankton feeders on the veliger stage of the mollusk (Carney 1991).
Observation during the sampling period indicated the high abundance of tilapia both adults and juveniles, even in the outlet and the mouth of the inlets. There are no Caridina found where the juveniles of tilapia present in large amount in the lake as well as in the mouth of the inlets such as mouth of Uwe Tokaroru and Uwe Kumo in the north, northern Kanawu in the east, and Langko in the west. It is suggested that the small population of C. linduensis and Caridina sp B in the lake is probably caused by the predation of adult tilapia. Herder et al.
28
al. (1987) mention that introduction of fish tilapia has led to the near extinction of indigenous fish species and mussels in Lake Lindu. On the contrary, we found large amount of Caridina sp B in Kati stream. It is probably related to the absence of introduced carp and tilapia in this site.
Macrobrachium lanchesteri is another alien species which can give negative impact to the Caridina community in the water bodies (Wowor et al. lanchesteri in the lake seem most probably by inadvertent introduction along with other introduced commercial fish. The occurrence of this alien shrimp can also affect the Caridina community in the lake. Macrobrachium lanchesteri is a competitor and predator for the Caridina spp in the lake.
Conservation of the endemic Caridina in Lake Lindu
The Lake Lindu as a putative ancient lake is very important to be conserved. It harbours many endemic aquatic fauna indicating its long term isolation. It is a good natural laboratory for studying evolution particularly speciation.
The small population of C. linduensis and Caridina sp B in the lake is quite worrying, because not only the occurrence of introduced aquatic species in the lake appear threatened the shrimps but also the progressive encroachment of
Table 2 Distribution of Caridina species in Lake Lindu, Lake Poso and Malili lake system
Lake Lindu Lake Poso Malili lake system
C. linduensis Roux, 1904 C. acutirostris Schenkel, 1902 C. dennerli von Rintelen & Cai, 2009 Caridina sp A C. caerulea von Rintelen & Cai, 2009 C. glaubrechti von Rintelen & Cai, 2009
Caridina sp B C. ensifera Schenkel, 1902 C. holthuisi von Rintelen & Cai, 2009 C. longidigita Cai & Wowor, 2007 C. lanceolata Woltereck, 1937
C. sarasinorum Schenkel, 1902 C. lingkonae Woltereck, 1937 C. schenkeli von Rintelen & Cai, 2009 C. loehae Woltereck, 1937
C. mahalona*) Cai et al, 2009
C. masapi Woltereck, 1937
C. parvula von Rintelen & Cai, 2009 C. profundicula von Rintelen & Cai, 2009
29 agriculture around the lake, human disturbance and buffalo grazing activity at the bank of the lake are likely to modify the lake ecosystem. In addition, the small population of these endemic shrimps in this restricted and isolated locality can cause their seriously endangered and might be led to their extinction.
It is recommended to have collaboration among university, government and non-government organization to increase knowledge-transfer program to raising local awareness about 1) the importance of sustainability of biodiversity and endemic fauna in the lake, and 2) the impact of presence of alien species and how to manage them in the lake system.
5
CONCLUSION
There are three Caridina species found in Lake Lindu, namely Caridina linduensis, Caridina sp A and Caridina sp B which are morphologically different in the rostrum shape and the mandible structure. All Caridina species from the lake have different habitat preferences. Caridina sp A is an obligate riverine species; while C. linduensis and Caridina sp B can be found both in lake and river/stream. All Caridina species have relatively large egg size that indicate them as land-locked species. Collaboration among the university, government, non-government organization and local people around the lake is needed to protect the sustainability of the endemic fauna in the lake and manage the alien species in the lake system.
REFERENCES
Albrecht C. 2012. Do Lake Characteristics Explain Biodiversity and Endemism? In: von Rintelen T, Stelbrink B, Wowor D, Heryanto, editors. Book of Abstract Speciation in Ancient Lakes 6, Classic concepts and new approaches. International Symposium: Speciation in Ancient Lakes 6; 2012 Aug 26–30; Bogor, Indonesia. Division of Zoology, Research Center for Biology, Indonesian Institute of Sciences. p 4.
Annawaty, Wowor D. 2012. Preliminary Study of Atyid Shrimps of Lake Lindu, Sulawesi, Indonesia In: von Rintelen T, Stelbrink B, Wowor D, Heryanto, editors. Book of Abstract Speciation in Ancient Lakes 6, Classic concepts and new approaches. International Symposium: Speciation in Ancient Lakes 6; 2012 Aug 26–30; Bogor, Indonesia. Division of Zoology, Research Center for Biology, Indonesian Institute of Sciences. p 5.
30
Bellier O, Sébrier M, BeaudouinT, Villeneuve M, Braucher R, Bourlès D, Siame L, Putranto E, Pratomo I. 2001. High slip rate for a low seismicity along the Palu-Koro active fault in central Sulawesi (Indonesia). Terra Nova, 13:463– 470.
Bouvier E-L. 1925. Recherches sur la morphologie, les variations, la distribution geographique des crevettes d’eau douce de la famille des Atyidés.
Encyclopédie Entomologique, series A, 4:1-370.
Cai Y. 2004. Systematics of the freshwater shrimps of the family Atyidae De Haan, 1849 (Crustacea: Decapoda: Caridea) of East and Southeast Asia. Unpublished PhD Thesis. National University of Singapore. 926 pp.
Cai Y, Ng PKL. 2005. Marosina, a new genus of troglobitic shrimps (Decapoda, Atyidae) from Sulawesi, Indonesia, with descriptions of two new species.
Crustaceana, 78:129–139.
Cai Y, Wowor D. 2007. Atyid shrimps from Lake Poso, Central Sulawesi, Indonesia, with description of a new species (Crustacea: Decapoda: Caridea).
The Raffles Bulletin of Zoology, 55:311–320.
Cai Y, Ng PKL. 2009. The freshwater shrimps of the genera Caridina and Parisia
from karst caves of Sulawesi Selatan, Indonesia, with descriptions of three new species (Crustacea: Decapoda: Caridea: Atyidae). Journal of Natural History,43:1093–1114.
Cai Y, Wowor D, Choy S. 2009. Partial revision of freshwater shrimps from Central Sulawesi, Indonesia, with descriptions of two new species (Crustacea: Decapoda: Atyidae). Zootaxa,2045:15–32.
Carney WP, 1991. Echinostomiasis – a snail–borne intestinal trematode zoonosis.
Southeast Asian J Trop Med Public Health 22:206–211.
Chace FA Jr. 1997. The Caridean shrimps (Crustacea: Decapoda) of the Albatross Philippine expedition 1907-1910. Part 7: Families Atyidae, Eugonatonotidae, Rhynchocinetidae, Bathypalaemonellidae, Processidae, and Hippolytidae.
Smithsonian Contribution to Zoology,587:1–106.
Chan TY. 1998. Shrimps and Prawns. In: Kent E. Carpenter and Volker H. Niem, editor. The Living Marine Resources of The Western Central Pasific. Food and Agriculture Organization of The United Nations, Rome p 852-906. Chia OKS, Ng PKL. 2006. The freshwater crabs of Sulawesi, with descriptions of
two new genera and four new species (Crustacea: Decapoda: Brachyura: Parathelphusidae). Raffles Bulletin of Zoology, 54:381–428.
Cumberlidge N, Ng PKL. 2009. Systematics, evolution, and biogeography of freshwater crabs. In: Martin JW, Crandall KA, Felder DL (Eds.), Decapod crustacean phylogenetics. Crustacean Issues 18. CRC Press, Boca Raton, Florida, pp. 491–508.
De Grave S, Fransen CHJM. 2011. Carideorum Catalogus: The Recent Species of the Dendrobranchiate, Stenopodidean, Procarididean and Caridean Shrimps (Crustacea: Decapoda). Zoologische Mededelingen, 85:195–589.
De Man JG. 1892. Decapoden des indischen archipels. In: weber, m. (ed.),