THE UTILIZATION OF EPIDIDYMAL SPERM AND
CAUSATIVE MUTATIONS RELATED TO SPOTTED
COAT COLOR PATTERN IN SWAMP BUFFALO
YULNAWATI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
STATEMENT OF COPYRIGHT FOR DISSERTATION AND
SOURCES OF INFORMATION *
I hereby declare that the dissertation entitled “The Utilization of Epididymal Sperm and the Causative Mutations Related to Spotted Coat Color Pattern in Swamp Buffalo” is true of my work under the guidance of the supervising committee and has not been submitted in any form to any college. Resources derived or quoted from works published and unpublished from other writers mentioned in the text and listed in the References at the end of this dissertation.
I hereby bestow the copyright of my papers to the Bogor Agricultural University (IPB).
Bogor, June 2014
Yulnawati
RINGKASAN
YULNAWATI. Pemanfaatan Sperma Epididimis dan Mutasi Penyebab Munculnya Warna Belang Pada Kerbau Lumpur. Dibimbing oleh CECE SUMANTRI, ARIEF BOEDIONO, RONNY RACHMAN NOOR dan GÖRAN ANDERSSON.
Kerbau belang merupakan salah satu keanekaragaman hayati Indonesia yang termasuk dalam kelompok kerbau lumpur atau kerbau rawa (Bubalus bubalis carabanensis). Populasi kerbau belang umumnya ditemui di sekitar wilayah Toraja, Sulawesi Selatan. Jenis ternak ini memiliki ikatan yang sangat erat dengan kebudayaan setempat. Kerbau belang jantan dikorbankan untuk dijadikan sebagai persembahan pada saat upacara kematian. Rendahnya tingkat kelahiran akibat tradisi yang tidak menginginkan terjadinya kawin alam pada kerbau belang jantan, serta tingkat pemotongan yang tinggi akibat upacara adat, menyebabkan populasi ternak ini menurun drastis, mendekati kepunahan.
Disertasi ini bertujuan untuk i) menginvestigasi metode reproduksi yang dapat diterapkan untuk meningkatkan angka kelahiran kerbau belang, dan ii) mengidentifikasi mutasi pada gen MITF sebagai penyebab munculnya variasi warna belang. Disertasi ini terdiri dari tiga kajian yang meliputi aspek reproduksi kerbau belang jantan dan variasi genetik penyebab munculnya warna belang pada kerbau rawa.
Pada kajian pertama, dilakukan investigasi mengenai pengaruh warna kulit terhadap kualitas sperma epididimis segar dan setelah thawing. Sampel sperma epididimis dikoleksi dari 12 ekor kerbau belang yang terdiri dari 5 ekor Saleko, 4 ekor Bonga dan 3 ekor Lotong Boko, dibandingkan dengan sperma epididimis dari 5 ekor kerbau hitam. Tidak ditemukan adanya perbedaan signifikan pada setiap parameter kualitas sperma yang diamati, baik pada kondisi sperma segar maupun setelah thawing. Motilitas progresif sperma epididimis setelah thawing dari kelompok Saleko, Bonga, Lotong Boko dan hitam secara berturut-turut adalah 44%, 42%, 40% dan 42%. Sementara itu, daya hidup dan keutuhan membran plasma sperma epididimis dari keempat kelompok tersebut secara berturut-turut adalah 64,9%; 65,2%; 62,6%; 62,7% and 64,6%; 67,1%; 64,5%; 64,1%. Disimpulkan bahwa variasi warna kulit belang ternyata tidak memberikan pengaruh terhadap kualitas sperma epididimis segar maupun beku.
CEY20, dapat digunakan untuk mempertahankan kualitas dan fertilitas sperma epididimis kerbau belang setelah thawing.
Dalam kajian ketiga, dilakukan penelusuran variasi genetik pada gen
microphthalmia-associated transcription factor (MITF) dalam kaitannya terhadap kemunculan warna belang. Studi ini berhasil mengidentifikasi dua jenis mutasi penting, yaitu mutasi nonsense yang menyebabkan terjadinya premature stop codon pada exon 3, dan mutasi donor splice-site yang menyebabkan perpanjangan exon 8. Akibat mutasi splice-site ini terjadinya penambahan 8 residu asam amino yang diperkirakan terjadi langsung sebelum leucine ketiga dari leucine zipper
bHLH-Zip domain, sehingga mempengaruhi dimerisasi dan kapasitas ikatan protein MITF dengan DNA.
Secara umum dapat disimpulkan bahwa IB menggunakan sperma epididimis kerbau belang yang dibekukan baik dalam bahan pengencer TEY20 maupun CEY20 dapat diaplikasikan guna meningkatkan angka kelahiran kerbau belang. Selanjutnya, dapat dipastikan bahwa mutasi nonsense dan splice-site yang diidentifikasi pada gen MITF merupakan faktor penyebab munculnya warna belang pada kerbau rawa.
SUMMARY
YULNAWATI. The Utilization of Epididymal Sperm and Causative Mutations Related to Spotted Coat Color Pattern in Swamp Buffalo. Supervised by CECE SUMANTRI, ARIEF BOEDIONO, RONNY RACHMAN NOOR and GÖRAN ANDERSSON.
The spotted buffalo is an Indonesian domestic buffalo, which is a variant of the swamp buffalo (Bubalus bubalis carabanensis) type. The spotted buffalo is mainly found in the Toraja region, South Sulawesi. Apart from its importance in agriculture, this species is also closely tied to local spiritual beliefs. The sacrificial offering of spotted buffalo is considered a fundamental part of funeral ceremonies. Due to the spotted buffalo’s low birth rate of natural reproduction, but yet high demand in funeral ceremonies, the species is close to extinction.
The objectives of this dissertation were i) to investigate the applicative reproductive methods that are useful to increase the birth rate of spotted buffalo, and ii) to identify the causative mutations and variations in MITF gene that are associated with spotted coat color pattern in swamp buffalo. This dissertation consists of three studies, two studies were related to the reproductive aspect of spotted buffalo bulls and the third study was related to the variation of a pigmentation gene in swamp buffalo.
In the first study we investigated if fresh and frozen-thawed epididymal sperm quality was influenced by the buffalo’s coat color pattern. Therefore, epididymal sperm from 12 buffaloes with spotted coat color pattern (5 Saleko, 4
Bonga and 3 Lotong Boko) were compared to sperm from 5 buffaloes with solid coat color pattern. Results showed that the coat color pattern did not influence the sperm quality. No significant difference was found in any of the analyzed parameters among groups of fresh and frozen-thawed sperm. The percentage of frozen-thawed progressive motility from Saleko, Bonga, Lotong Boko and Solid was 44%, 42%, 40% and 42%, respectively. Moreover, the percentage of viability and membrane integrity of frozen-thawed sperm were 64.9%; 65.2%; 62.6%; 62.7% and 64.6%; 67.1%; 64.5%; 64.1%, respectively. In conclusion, the buffalo coat color pattern variation did not affect the quality of fresh and frozen-thawed epididymal sperm.
The objective of the second study was to investigate the ability of chemically defined extenders in maintaining the quality and fertility of frozen-thawed epididymal sperm of spotted buffaloes. Two extenders, Tris-based egg yolk(TEY20) and Citrate-based egg yolk (CEY20), were used in this study. The results showed a similar progressive motility, viability and membrane integrity of frozen-thawed epididymal sperm in both extenders. With 30 buffalo cows, the resulting success rate of pregnancy was 47% (7/15) using frozen-thawed epididymal sperm in TEY20 and 40% (6/15) using the one in CEY20 extender. In conclusion, the two chemically defined extenders (TEY20 and CEY20) were equally suitable to maintain the quality and fertility of frozen-thawed epididymal sperm.
color pattern. We identified two important causative mutations: first, a nonsense mutation that caused premature stop codonin exon 3 and likely removal of the resulting mRNA via nonsense-mediated mRNA decay pathway; second, a donor splice-site mutation that led to aberrant mRNA splicing of exon 8 of MITF
resulting in an mRNA coding for protein with eight additional amino acid residues. Most likely the insertion of the eight amino acid residues negatively influences dimerization and DNA binding capacity because the insertion was located immediately before the third leucine in the leucine zipper bHLH-Zip domain.
In conclusion, AI using frozen-thawed epididymal sperm, which was diluted either in TEY20 or CEY20, could be applied to increase the birth rate of spotted buffaloes. Furthermore, the nonsense and splice-site mutations in MITF gene were strongly associated with spotted coat color pattern in swamp buffaloes.
© All Rights Reserved IPB, in 2014
Copyright Reserved
Prohibited from quoting part or all of this paper without including or citing sources. Citations only for educational purposes, research, writing papers, preparing reports, writing criticism, or review a matter, and citations are not detrimental to the interests of the University.
THE UTILIZATION OF EPIDIDYMAL SPERM AND
CAUSATIVE MUTATIONS RELATED TO SPOTTED
COAT COLOR PATTERN IN SWAMP BUFFALO
YULNAWATI
Dissertation
As a requirements for Doctoral degree at
Major of Livestock Production and Technology
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
Examiners on preliminary defense: 1. Dr agr Asep Gunawan, SPt, MSc
2. Dr Ni Wayan Kurniani Karja, DVM, MP
Examiners on final defense:
ACKNOWLEDGEMENT
Many thanks to supervising committee, Prof Dr Ir Cece Sumantri, MAgrSc, Prof Arief Boediono, DVM, PhD, PAVet (K), Prof Dr Ir Ronny R. Noor, MRurSc and Prof Dr Göran Andersson, who has provided guidance and assistance during my study at the Graduate School, Bogor Agricultural University (IPB), Bogor, Indonesia and Department of Animal Breeding and Genetics, Swedish University of Agricultural Sciences (SLU), Uppsala, Sweden.
Sincere appreciation presented to the main sponsor, Graduate Scholarship Program from Ministry for Research and Technology (2010-2014) and the Guest Scholarship Program from Swedish Institute (2011-2013), which has provided financial support for the implementation of study and research. My appreciation also presented to Research Centre of Biotechnology, Indonesian Institute of Sciences.
My high appreciation is also to Dr Ni Wayan Kurniani Karja, DVM, MP, Dr agr Asep Gunawan, SPt, MSc, Dr Ir Bess Tiesnamurti, M.Sc and Prof Dr Mohamad Agus Setiadi, DVM as examiners, which have provided feedback and comments that are very useful for improving this dissertation. To the Head of Animal Production and Technology study program at the Graduate School, IPB, the Head of Department of Animal Breeding and Genetic, Swedish University of Agricultural Science (SLU) and his staff, Prof Dr Leif Andersson, all my teachers, technical assistance and administrative staff, I presented infinite gratitude for all help and support that given to me during my study.
Special thanks and appreciation for help and cooperation to all collaborators and co-authors, Dr Muhammad Rizal, Dr Hera Maheshwari, Dr Maria Wilbe and Astri Olivia Herlino, MSc. Many thanks to Ir Isak Maraya Allosomba, MSi, Ir Andarias Tandung Sale, MSi, the Governmental Animal Division of North Toraja District, buffalo owners and farmers, who have given full support for collecting samples and performing this study. To Dr Ir Niken Ulupi, MSi and Dr Ir M. Amrullah Pagala, MSi, thank you very much for friendship, support and help. Special thanks to Dr Ir Takdir Saili, MSi, Prof Dr Herdis, DVM, MSi, Dr Ir Ristika Handarini, MP, Kemaz Aditya Dewangga, DVM, LL B, MPN and Agus Teguh Suryaman, BVM, LL B, LL M, who had come and given support during the dissertation defense.
My parents, brother, sister and families, thank you for love and supports that given to me. Mama, Papa and Claudi, thank you for support and love. Last but not least, special thanks to Tobias Wellnitz, for love, support, help and great comments in my dissertation.
Bogor, June 2014
i
2 SPOTTED COAT COLOR PATERN AND ITS EFFECT ON THE QUALITY OF EPIDIDYMAL SPERM
Introduction 7
Materials and Methods 10
Results and Discussion 12
Conclusion 14
3 THE QUALITY AND FERTILITY OF FROZEN-THAWED EPIDIDYMAL SPERM IN TWO DIFFERENT EXTENDERS
Introduction 15
Materials and Methods 16
Results and Discussion 17
Conclusion 20
4 MITF MUTATIONS ARE CAUSING SPOTTED COAT COLOR IN SWAMP BUFFALOES
6 CONCLUSION AND RECOMMENDATION 39
REFERENCES 41
SUPPLEMENTS 49
Sequence Listing of MITF Gene 55
ii
TABLE LISTS
2.1 Classification of swamp buffalo based on coat pattern and iris color
8
2.2 The quality of fresh epididymal sperm 13
2.3 The quality of frozen-thawed epididymal sperm 14
3.1 The composition of sperm extenders 16
3.2 The quality of fresh epididymal sperm of spotted buffalo 18 3.3 The quality of epididymal sperm during cryopreservation and
after thawing
19
4.1 Identified SNPs in swamp buffalo MITF gene 27 4.2 The genotype frequencies of the nonsense mutation (chr22:
32,322,242a) and the splice site mutation (chr22: 32,297,683a) in MITF gene of swamp buffaloes with different coat color phenotypes
29
iii
FIGURE LISTS
1.1 The estimated spotted buffalo population over the last five years in Northern Toraja District
1
1.2 The framework of studies 5
2.1 A compilation data of buffalo classification and domestication time period
8
2.2 Different type of coat color pattern in swamp buffalo 9 2.3 Testicle and epididymis of buffalo bull 10 4.1 The schema of MITF gene that uses cattle genome as reference 23 4.2 The roles of action of MITF in pigmentation 24 4.3 The results of Sanger sequencing of MITF DNA products
generated from mutant spotted and wild-type swamp buffaloes
28
4.4 Variations of coat and eye color phenotype in swamp buffaloes 29 4.5 The mRNA sequences of wild-type versus splice-site mutation 30 4.6 Amino acids alignment of splice-site mutation (SS) of spotted
buffaloes versus wild-type of solid buffaloes (Wt) and 9 other species
30
4.7 The binding affinity of mutant versus wild-type MITF protein on electrophoretic mobility shift assay (EMSA)
31
4.8 The estimated position of splice-site mutation in MITF gene and the position of helix-loop-helix DNA binding (HLH DNA-bd)
S.1 Offspring generated from AI using frozen-thawed epididymal sperm of a Saleko buffalo bull
S.3 A Saleko spotted buffalo bull as the source of epididymal sperm that were used for AI in our study
52
S.4 The mating pattern of swamp buffalo with different coat color phenotype and the coat color of the offspring
1
INTRODUCTION
Background
The swamp buffalo (lat. Bubalus bubalis carabanensis) is an important domestic animal in Indonesia. Particularly in the Toraja region, South Sulawesi, the role of swamp buffalo goes beyond its general significance in agriculture. For the indigenous ethnic group Toraja, swamp buffalo bulls with a spotted coat color pattern and white iris color are considered as holy animals. A fundamental part of Toraja’s animalistic beliefs is the sacrificial offering of these holy animals in large funeral ceremonies, called Rambu Solo’. During those ceremonies, dozens of animals, mainly spotted buffalo bulls but also cattle, pigs, horses, deer, and chickens, are sacrificed as an expression of sorrow and condolence. According to their spiritual beliefs, spotted buffalo bulls will give the deceased person a safe ride to heaven. Despite that most of Toraja’s population has converted to Christianity (>90%) or Islam (<10%), the polytheistic animalistic death ritual is practiced until today (BPS 2010). The amount of sacrificed animals and the difficulty of natural reproduction have brought this phenotype close to extinction.
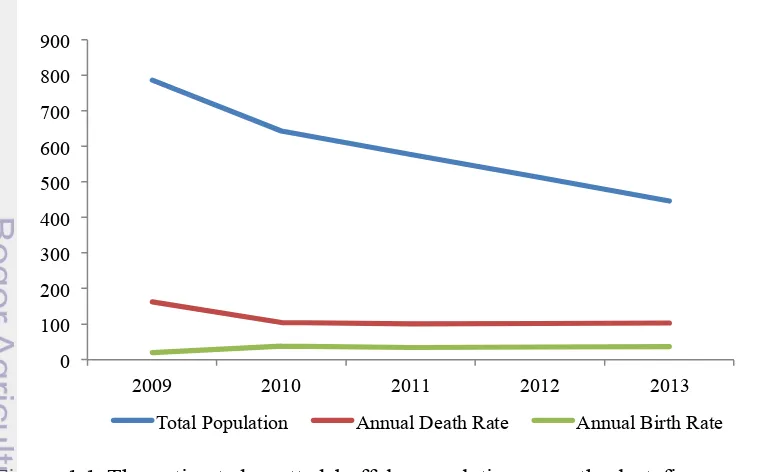
Unfortunately, the slaughter rate of spotted buffalo bulls is much higher than their birth rate, which has led over the last few years to a significant decrease of spotted buffalo population in Toraja region. The estimated number of population, including the annual death and the birth rate of spotted buffaloes during the last few years, is shown on Figure 1.1.
Figure 1.1 The estimated spotted buffalo population over the last five years in Northern Toraja District (source: Governmental Animal Husbandry Division of Northern Toraja District, unpublished data).
0
2
According to the data, spotted buffalo population has decreased from 787 animals in 2009, to just about 446 animals in 2013. An increase of the birth rate in 2010 occurred as a positive effect of the government's program in the salvation of pregnant buffalo cows and technical support for buffalo farmer community by the local Animal Husbandry Division.
Due to the tradition and culture of Toraja ethnic group, a holy animal is not allowed to perform the natural mating. Moreover, sperm collection by using an artificial vagina is also prohibited. As a consequence, the birth rate of spotted buffalo is very low.
Information concerning the reproductive potency including the pedigree of spotted buffalo is not available. Therefore, there is no explanation about the inheritance pattern of spotted coat color to the offspring. The birth of offspring with spotted coat color pattern regarded as a miracle to the owner. In opposite to that, the birth of offspring with white coat color, but blind, or even without eyes, also occurs and it is considered as a curse to the owner.
This study was performed as a concern to the threat of extinction of spotted buffaloes. The potential extinction of spotted buffaloes not only causes the loss of biodiversity, but also unknown changes of cultural tradition, which has been maintained over centuries. Since the funerals are also a major driver for tourism, they have become also important for the region’s economic stability.
This dissertation includes three studies. The two first studies were focused on reproductive aspects of spotted buffalo bulls, and the third study was focused on the identification of the genetic variantion in the MITF gene underlying the spotted coat color phenotype.
Due to the unavailability of sperm stock, cauda epididymis tissue has been identified as the alternative source of sperm. Our preliminary study showed that epididymal sperm of spotted buffalo had an equal quality to the ejaculated sperm (Yulnawati et al. 2010). Epididymal sperm could be used to fertilize the eggs and resulted in normal births in other species (Lone et al. 2011). However, scientific publications reported that the gene causing dominant white spotting (KIT) could reduce male fertility and also could lead to abnormality during spermatogenesis (Phung et al. 2011; Fontanessi et al. 2010a; Yoshida et al. 2001). To address concerns related to this, the first study was performed to investigate if buffalo’s coat color pattern had any effect on the quality of epididymal sperm. Beside the fresh sperm, frozen-thawed sperm was analyzed to determine the impact of cryopreservation. The results will serve as a basis for further studies, which will ultimately be needed for a successful artificial insemination program.
3
formation of spotted buffalo epididymal sperm banks for artificial insemination (AI) purpose to increase the birth rate.
Artificial insemination is a reproductive technology that has been widely used in other domestic animal breeding programs. It is a proven and effective method to increase the number and quality of livestock production (Morrell & Rodriguez-Martinez 2011; Purohit et al. 2003). The efficiency and success rate of AI in spotted buffalo can be improved by genetic information related to coat color variation.
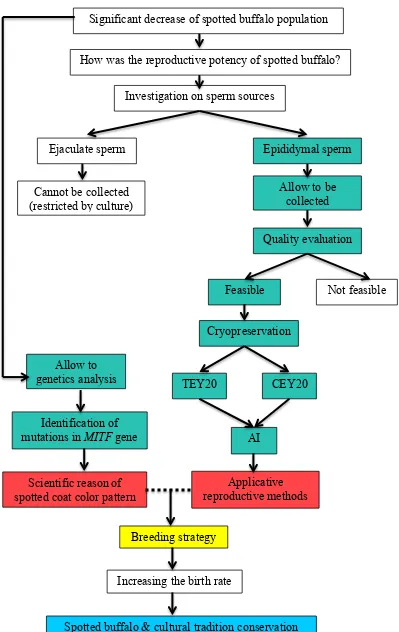
The third study was performed to identify the causative mutations in MITF gene that are associated with spotted coat color pattern in swamp buffaloes. This information can be useful to understand the inheritance pattern of buffalo coat color variation in order to increase the birth rate of spotted buffaloes. Moreover, it can also be used as a marker for diagnostic test to select buffalo embryos before transferred, based on their coat color pattern. The workflow of studies in this dissertation is summarized in Figure 1.2.
General Objectives
The aims of this dissertation were to;
1. Investigate the applicative reproductive technology that can be useful to increase the birth rate of spotted buffalo.
2. Identify causative mutations in MITF gene that are associated with spotted coat color pattern in swamp buffalo.
Output
The outputs of this study will be useful for a breeding strategy by using the frozen-thawed epididymal sperm in chemically defined extender for AI and to take into account the genetic basis of the spotted coat color pattern in swamp buffalo.
Hypotheses
The examined hypotheses in this dissertation were:
1. Coat color pattern affects the epididymal sperm quality.
4
3. Causative mutations in the MITF gene are responsible for spotted coat color pattern.
Novelties
The novelties of this study are:
1. Identification and proof of an alternative source for spotted buffalo sperm, which can be used for reproductive technology application in swamp buffalo.
2. Identification and proof of alternative sperm extenders by using chemically defined components, which can be obtained in rural Indonesian areas.
5
Figure 1.2 The framework of study. Green boxes: experiments, red boxes: outputs, yellow box: implementations, and light blue box: outcome.
How was the reproductive potency of spotted buffalo?
Investigation on sperm sources
Ejaculate sperm Epididymal sperm
Cannot be collected (restricted by culture)
Quality evaluation
Not feasible Feasible
AI TEY20
Cryopreservation
CEY20
Applicative reproductive methods Allow to
genetics analysis
Identification of mutations in MITF gene
Scientific reason of spotted coat color pattern
Allow to be collected
Breeding strategy
Significant decrease of spotted buffalo population
Increasing the birth rate
2
SPOTTED COAT COLOR PATTERN AND ITS EFFECT
Binomial : Bubalus bubalis (Linnaeus, 1758)
The global buffalo population has been estimated to consist of a total of 168 million buffaloes. The populations are distributed in Asia (161 million), Africa (3.7 million), South America (3.3 million), Australia and Europe (Michelizzi et al. 2010). Buffalo is an economically important domestic animal used for milk and meat production, as well as a draft animal in traditional agricultural system.
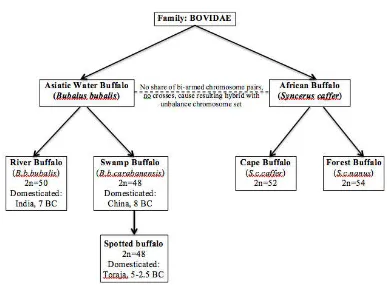
The water buffalo (Bubalus bubalis) has been domesticated into two subspecies; river buffalo (Bubalus bubalis bubalis) and swamp buffalo or the carabao (Bubalus bubalis carabanensis) (Castillo, 2004). Former studies showed that swamp buffalo was classified as a subspecies of water buffalo and domesticated in China in 8 BC (Figure 2.1). Based on the study on cytochrome b, the divergence time of river versus swamp buffalo is from 10,000 to 1.7 million years and it is inferred that domesticated buffaloes originated from at least two populations (Kiersten et al. 2004; Kumar et al. 2007).
It is known that swamp buffalo exhibits several coat color phenotypes, i.e solid, white or spotted coat color pattern. The solid phenotype has light grey to black coat color, while the white phenotype appears to be like a dilution phenotype rather than an extreme form of white spotting.
8
Figure 2.1 A compilation data of buffalo classification and domestication time period (sources: Michelizzi et al. 2010; Michelizzi et al. 2011; Kumar et al. 2007; Zhang et al. 2008). Spotted buffalo belongs to swamp buffalo type and it is domesticated in Toraja during 5-2.5 BC.
Table 2.1 Classification of swamp buffalo based on coat pattern and iris color*
Coat color type Coat color pattern Iris color
Saleko Black spot spread out on the white-based coat color pattern of the body.
Mostly white
Bonga White spot is mostly on the face, neck and nape, spread out on the black-based coat color pattern. The white spot can also found on the tail and legs.
Mostly white
Lotong Boko Black spot on the back of the white-based coat color pattern. The black spot can also be found on the face.
Mostly white
Toddi’ Solid coat color pattern with a small white spot on the forehead.
Black
9
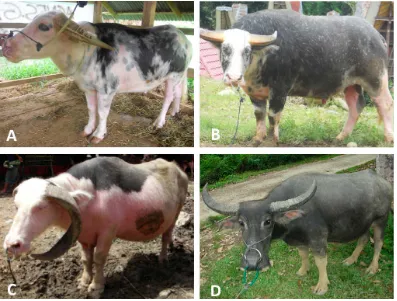
Figure 2.2 Different type of coat color pattern in swamp buffalo. A: Saleko; B: Bonga; C: Lotong Boko and D: Solid.
Epididymal Sperm
Epididymis is a part of male reproductive system that is divided into three regions: caput (head), corpus (body) and cauda (tail). Epididymis located stick on the testicles (Figure 2.3). Sperm undergo a maturation process and are stored before ejaculation in the epididymis. Therefore, the sperm concentration in cauda epididymis is much higher than in the ejaculate.
10
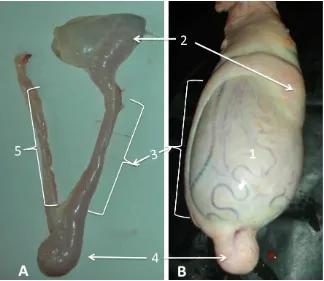
Figure 2.3 Testicle and epididymis of buffalo bull. A is the epididymis, consists of caput (2), corpus (3) cauda (4) and vas deferens (5). B is testicle (1) including the epididymis.
Spotted Buffalo Bulls and Their Fertility
There was no data available related to spotted buffalo reproduction as well as causative mutations underlying the spotted coat color pattern. The spotting phenotype in swamp buffalo is similar to the one in dairy cattle. In dairy cattle, the white spotting phenotype and the degree of spottedness are regulated by mutation in KIT gene (Tazzoli et al. 2007; Liu et al. 2009; Fontanessi et al. 2010a). Mutation in this gene can also lead to abnormality in spermatogenesis and reduce male fertility (Phung et al. 2011; Yoshida et al. 2001). This leads to a question related to the quality and fertility of spotted buffaloes sperm. This study was performed to investigate if the swamp buffalo’s coat color pattern had any impact on the quality of epididymal sperm.
Materials and Methods
Epididymal Sperm Collection
11
other five had solid coat color pattern. The 12 spotted buffaloes were classified into three types, i.eSaleko (n=5), Bonga (n=4) and Lotong Boko (n=3).
Cauda epididymides were isolated from scrotum, testicles and ligaments, an hour after death. Epididymal sperm was collected by incision and flushing the tissues using a commercial soya-lecithin based extender (Lone et al. 2011).
Sperm Cryopreservation and Thawing
Some parts of the fresh semen were cryopreserved. The frozen sperm was later thawed and analyzed to ensure that the cryopreservation process only caused an acceptable degradation of sperm quality.
Fresh epididymal sperm was diluted in a commercial soya-lecithin based extender (Andromed®) into 100x106 cell ml-1 and packed in 0.25 ml plastic straws. Straws were equilibrated at 4°C for 3 hours before freezing. The freezing process was started by placing the straws 10 cm above liquid nitrogen for 15 based on the following parameters: sperm concentration, progressive motility, viability, morphology, and membrane integrity. Sperm concentration was counted using a Neubauer chamber under a 100 magnificent light microscope.
For the evaluation of progressive motility, a drop of sperm was placed on a glass slide and covered with a cover slip. No stain was added. Progressive motility was estimated visually in at least eight randomly chosen microscopic fields under the magnification levels of 100 and 400 of a light microscope.
Sperm viability and morphology was evaluated using eosin-nigrosin stain. With the stain, viable sperm cells (white – no absorption) could be distinguished from non-viable cells (red – stain absorption). About 200 sperms in different fields on the slide were counted randomly under the light microscope at a magnification level of 400.
12 Boko and Solid. The repetition was 5, 4, 3 and 5 times per each type, respectively. The data obtained were analyzed by one-way analysis of variance (ANOVA) with statistic models as follows (Montgomery 2001):
Yij = µ + Ti +
ε
ijNote
Yij : observations
µ : the grand mean of the observations
Ti : treatment effect, a deviation of the grand mean
ε
ij : normally distributed zero-mean random errorsThe calculation used Statistical Product and Software Solution version-13 (SPSS Inc., Chicago, IL, USA).
Results and Discussion
Fresh Epididymal Sperm
Among all evaluated parameters of the fresh epididymal sperm samples, no difference of the quality was found (Table 2.2). Independently of the coat color pattern, the quality of fresh epididymal sperm was always similar. The quality of epididymal sperm collected from both swamp buffaloes with spotted and solid coat color pattern were qualified for AI and exceeded the requirement of Indonesian National Standardization for buffalo semen (progressive motility ≥
30%, SNI 01-4869.2-1998).
Our data showed that both solid and spotted buffaloes have similar sperm concentration. This suggested that both types undergo normal spermatogenesis process in their testicles. The abnormal morphology was detected (6.8-7.5%) within the normal range in epididymal sperm. Distal cytoplasmic droplet was normally detected, even though the epididymal sperm had been through the maturation process in the caput and corpus epididymis.
13
Table 2.2 The quality of fresh epididymal sperm
Parameters Spotted Solid (n=5)
Saleko (n=5) Bonga (n=4) Lotong Boko (n=3)
Even though the ejaculation does not occur, new sperm cells are frequently produced in the testicles every 54 days (Perera 2011). The old and aged sperm leads to the apoptosis process. Therefore, the sperm in cauda epididymis still has a good progressive motility. In this study, the progressive motility of spotted and solid buffalo epididymal sperm was within the range of 71 to 73%.
Viability parameter gives an idea of what kind of reproductive technology should be applied to utilize the sperm. A non-motile sperm does not mean that it is a non-viable cell. These days, sperm with less progressive motility or completely non-motile still can fertilize the oocyte using assisted reproductive technology, called intra-cytoplasmic sperm injection. The viability of fresh epididymal sperm in this study ranged within 82-86%, it was suggested that they could be stored frozen before AI.
Parallel to progressive motility and viability, membrane integrity of epididymal sperm within all groups was similar within the range of 83-86%. Membrane integrity is positively related to its progressive motility, viability and fertility of the sperm (Akhter et al. 2011a). High percentage of membrane integrity reflects a real number of sperm with normal progressive motility and viability. Sperm can undergo a premature capacitation in a certain condition and this causes hyper-motility of the sperm. This is an unexpected situation, since hyper-motility happens due to the non-intact membrane. Non-intact membrane leads to the death of the cells. Moreover, this will reduce the fertility of the sperm and the success rate of AI, significantly.
Frozen-thawed Epididymal Sperm
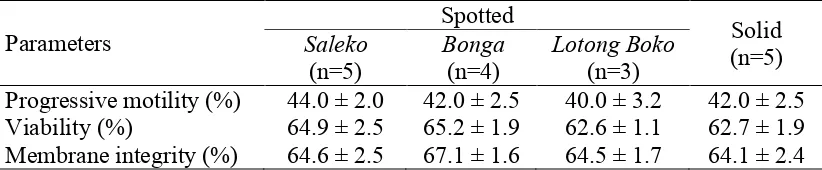
The quality of frozen-thawed epididymal sperm was also similar among all samples (Table 2.3). The progressive motility and other parameters were similar among all coat color pattern classes. The progressive motility of frozen-thawed epididymal sperm among all coat color pattern classes was ≥40%. Despite degradation from 72% (fresh) to 42% (frozen-thawed) in progressive motility, sperm stored and thawed from liquid nitrogen still qualify for AI, according to the levels defined in the Indonesian National Standard for buffalo semen (SNI 01-4869.2-1998).
14
frozen-thawed epididymal sperm within each group in this study was also similar. This result was parallel to membrane integrity of frozen-thawed epididymal sperm of spotted buffalo in preliminary study (67% in Yulnawati et al. 2010).
Table 2.3 The quality of frozen-thawed epididymal sperm
Parameters
Spotted
Solid (n=5) Saleko
(n=5)
Bonga (n=4)
Lotong Boko (n=3)
Progressive motility (%) 44.0 ± 2.0 42.0 ± 2.5 40.0 ± 3.2 42.0 ± 2.5 Viability (%) 64.9 ± 2.5 65.2 ± 1.9 62.6 ± 1.1 62.7 ± 1.9 Membrane integrity (%) 64.6 ± 2.5 67.1 ± 1.6 64.5 ± 1.7 64.1 ± 2.4
It is known that KIT gene plays a central role in melanogenesis, melanoblast migration, proliferation and leads to dominant white spotting phenotype in many species. Pleiotropic effects of KIT can also affect gametogenesis and haematopoiesis in mice (Besmer et al. 1993; Shibanuma et al. 2002; Steingrímsson et al. 2004). In spotted buffalo, our results showed that there was no direct correlation of spotted coat color pattern to the quality of epididymal sperm. Allegedly, established functional the pleotropic effect of KIT in gametogenesis and male fertility does not appear to be perturbed in spotted buffalo. This study also proved that the quality of fresh and frozen-thawed epididymal sperm of spotted buffalo is qualified for being used in AI program.
Conclusion
3
THE QUALITY AND FERTILITY OF FROZEN-THAWED
known for protecting the sperm plasma membrane from premature capacitation and acrosome reaction (Senger, 1999). Therefore, the identification of chemical composition of extenders is needed to get the most efficient and effective extenders for spotted buffalo epididymal sperm.By today, no specific extender is commercially available yet for buffalo (epididymal) sperm. Commercial extenders for cattle semen have been used to cryopreserve epididymal sperm of African buffalo (Synceraus caffer) and spotted buffalo (Bubalus bubalis carabanensis) (Herold et al. 2004; Yulnawati et al. 2010). Therefore, a specific extender is required to maintain the quality of frozen-thawed epididymal sperm.
Furthermore, the access to the commercial extenders is very limited and expensive in the area where spotted buffalo exists. The active period of storage of commercial extenders is also relatively short, less than 6 months. This makes them unapproachable for small-scale spotted buffalo semen banks.
The chemically defined extender is an alternative to be optimized to preserve spotted buffalo epididymal sperm. Chemically defined extender is inexpensive, easy to prepare and always fresh. It seems to be more applicative the cryopreservation of spotted buffalo epididymal sperm.
Several chemically defined sperm extenders, i.e Tris-based, soya milk-based, skim milk and Citrate-based egg yolk, have been used for semen cryopreservation in other species (Sansone et al. 2000; Andrabi 2009; Akhter et al. 2011b; Lone et al. 2012). The results showed that Tris-based egg yolk could maintain the sperm quality higher than other extenders. While Citrate-based egg yolk could maintain the sperm quality better than soya milk and skim milk extenders. Both Tris-based and Citrate-based extenders were qualified for spotted buffalo epididymal sperm.
16
Materials and Methods
Epididymal Sperm Collection and Cryopreservation
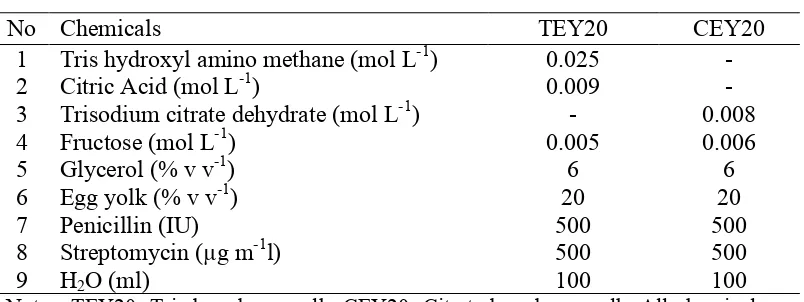
Cauda epididymides were collected from six spotted buffaloes at the biological age ranging between six to ten years. Cauda epididymides were separated from scrotum, testicles and ligaments, an hour after death. Epididymal sperm was collected by incision and flushing the tissues using two different extenders; Tris-based egg yolk (TEY20) or Citrate-based egg yolk (CEY20). The composition of TEY20 and CEY20 extenders were referred to Singh et al. (2007) and Lone et al. (2011), respectively (Table 3.1).
Table 3.1 The composition of sperm extenders
No Chemicals TEY20 CEY20
Epididymal sperm was separately diluted using two different extenders that have been mentioned above into 100x106 cells ml-1. Diluted sperm was packaged into 0.25 ml plastic straws. Straws were equilibrated at 4°C for 3 hours before freezing. The freezing process was started by placing the straws 10 cm above liquid nitrogen for 15 minutes and followed by plunging the straw into liquid nitrogen (-196°C) for storage. Frozen sperm was thawed at 37°C for 30 seconds either for quality evaluation or AI.
Sperm Quality Evaluation
Several quality parameters, sperm concentration, progressive motility, viability, morphology and membrane integrity, were evaluated in fresh samples. The progressive motility, viability and membrane integrity parameters were further evaluated after dilution, equilibration and thawing. The sperm concentration was counted using a Neubauer chamber under a 100 magnificent light microscope.
17
visually in at least eight randomly chosen microscopic fields under 100 and 400 magnificent light microscope.
Sperm viability was evaluated using eosin-nigrosin stain on the glass slide (Therien & Manjunath 2003). No stain was absorbed on viable sperm cells, while the non-viable cells absorbed red stain on the sperm head. About 200 sperms in different fields on the slide were counted randomly under the light microscope at a magnification level of 400.
The functional integrity of sperm membrane was evaluated using hypo-osmotic swelling test (HOST). A drop of 100µl sperm samples was mixed with 900µl hypo-osmotic solution (0.13 g l-1 of fructose, and 0.07 g l-1 of Na Citrate, refer to Rodriquez-gil et al. 1994), and incubated at 38ºC for 45 minutes. Then, a small drop of mixed solution was placed on a glass slide and covered with a cover slip. Sperm cells with curled or swollen tail indicated an intact membrane, while sperm with straight tail indicated a damaged. Two hundred sperms were counted randomly under a 400 magnificent light microscope.
Artificial Insemination for Testing the Fertility
Thirty cows were injected using double dosages of prostaglandin (PGF2α)
hormone to synchronize the estrous and ovulation periods. The interval of each injection was 11 days. Cows were divided into two groups (15 cows per group). One group was inseminated using frozen-thawed epididymal sperm in TEY20, while cows from the other group were inseminated using the one in CEY20. The frozen-thawed epididymal sperm of an individual spotted buffalo bull was used to avoid bias of AI results due to male individual factor. Estrous signs were observed 72-84 hours after the second injection of PGF2α. Artificial insemination was
performed about 8 to 12 hours after the estrous signs appear. The pregnancy was confirmed by rectal palpation at day 120 after insemination.
Statistical Analysis
Each individual animal was considered as a repetition. Results were expressed as the means ± standard error mean (SEM). Data were analyzed using t-test. Calculation used Statistical Product and Software Solution version-13 (SPSS Inc., Chicago, IL, USA).
Results and Discussion
Fresh Epididymal Sperm
18
was distal cytoplasmic droplet. The abnormal morphology of fresh epididymal sperm in this study was 7%. Abnormal morphology in the interval of 8-10% did not give any significant effect on fertility (Bearden & Fuquay 1997).
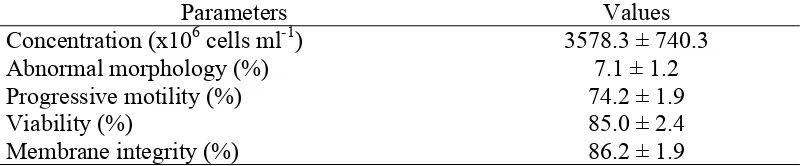
The progressive motility of fresh spotted buffalo epididymal sperm in this study was 74.2%. Sperm with more than 70% of motile progressive and less than 15% of abnormal morphology was required for being cryopreserved (Hafez & Hafez 2000).
Table 3.2 The quality of fresh epididymal sperm of spotted buffalo (mean ± sem)
Parameters Values Concentration (x106 cells ml-1) 3578.3 ± 740.3 Abnormal morphology (%) 7.1 ± 1.2 Progressive motility (%) 74.2 ± 1.9 Viability (%) 85.0 ± 2.4 Membrane integrity (%) 86.2 ± 1.9
Viability and membrane integrity of fresh epididymal sperm in this study was 85%. The viability of fresh epididymal sperm was 77.2 to 84.5% in cattle (Turri et al. 2012) and 91.1% in river buffaloes (Barati et al. 2009). Meanwhile, the membrane integrity of epididymal sperm in our study (86.2%) was higher than 72.7% in river buffalo (Singh et al. 2007). The viability and membrane integrity of epididymal sperm should be protected from damage by an extender that contains all sperm needs to overcome extreme temperature changes during cryopreservation.
Frozen-thawed Epididymal Sperm
There was no significant difference of progressive motility, viability and membrane integrity of epididymal sperm that was evaluated after dilution, equilibration and thawing (Table 3.3). The progressive motility of frozen-thawed epididymal sperm either in TEY20 or CEY20 was above 30%, and this level was exceeded the Indonesian National Standardization for buffalo semen quality for AI (SNI 01-4869.2-1998).
The Tris-based extender is very commonly used in almost every species due to its efficiency and effectiveness, as well as Citrate-based extender (Singh et al. 2007; Lone et al. 2011). Tris-based egg yolk extender has also been used to cryopreserve buffalo ejaculated sperm. The progressive motility of frozen-thawed ejaculated sperm of Nili-Ravi buffalo in Tris-based extender was 48.8% (Waheed et al. 2012). While the progressive motility of frozen-thawed epididymal sperm of African buffalo was 56.4% in Triladyl™ (Tris-based) versus 44.1% in AndroMed® (soya-based) extenders (Herold et al. 2004).
19
Citrate-based extender was found sufficiently clear for motility assessment compared to commercially milk-based extender (Sansone et al. 2000).
Table 3.3 The quality of epididymal sperm during cryopreservation and after thawing
Parameters Post Dilution Post Equilibration Post Thawing TEY20 CEY20 TEY20 CEY20 TEY20 CEY20
The cold shock effect during cryopreservation influences the ratio of cholesterol/phospholipids, the content of lipids in the lipid bilayer membrane, the degree of hydrocarbon chain saturation and the ratio of protein/phospholipid on the plasma membrane (Medeiros et al. 2002). Moreover, it also reduces membrane permeability to water and solutes, and injures the acrosomal membranes (Purdy 2006), as well as mitochondrial sheath and axoneme (Salamon & Maxwell 2000). Decreasing temperature that occurs extremely during cryopreservation induces the formation of intracellular ice crystals as well as osmotic and chilling injury of sperm cells (Isachenko et al. 2003). Ice crystal formation cause damaged on the cytoplasmic part of sperm cells. It leads to negative effects on the cytoskeleton and genome-related structures and on sperm tail. It also reduces the motile ability and fertility due to compromising the integrity of plasma membrane (Harshan et al. 2005; Andrabi et al. 2008). It is known that plasma membrane integrity is important for motility and viability of sperm cells, as well as an indicator of fertilizing ability (Mehmood et al. 2009).
20
Pregnancy Rate of AI Using Frozen-thawed Epididymal Sperm
The pregnancy rate following artificial insemination using frozen-thawed epididymal sperm in TEY20 was 47% (7 out of 15 cows), while in CEY20 was 40% (6 out of 15 cows). The produced offspring of AI program in this study is shown in Figure S1&S2 (see the Supplements). Our results showed that frozen-thawed epididymal sperm of spotted buffalo in either TEY20 or CEY20 had a good fertility and yield a good success rate of pregnancy. The pregnancy rate of AI using frozen-thawed epididymal sperm in other species varied from 16.7% in Spanish ibex (Santiago-Moreno et al. 2006), 22.2% in European bison (Kozdrowski et al. 2011), 50% in cows (Guerrero et al. 2010), 55.8% in ewes (Álvarez et al. 2012), 56% in red deer (Soler et al. 2003) to 66.6% in mares (Papa et al. 2008).
The results of this study showed that buffer was not the only factor that influenced the ability of extenders to maintain the quality and fertility of frozen-thawed sperm epididymal sperm. The combination of the right amount of all constituents affected the ability of chemically defined extenders in maintaining the quality and fertility of frozen-thawed epididymal sperm of spotted buffalo.
Conclusion
4
MITF
MUTATIONS ARE CAUSING SPOTTED COAT
COLOR IN SWAMP BUFFALOES
Introduction
Coat Color Genetic Studies
Many studies in coat color pigmentation have been conducted. It is known that more than 150 identified coat color-associated genes have been discovered, which influence pigmentation in various ways. Different genes can be responsible for highly similar coat colorations in different individuals of a species or in different species (Cieslak et al. 2011).
The primary process of pigmentation in mouse, including development of the melanocyte, the differentiation of melanocyte as it generates melanosomes, the regulation of type of melanin that is deposited upon the melanosome (red or yellow phaeomelanin, black or brown eumelanin) and the transport of melanosome within the pigment cell and transfer to the neighbouring keratinocytes are regulated by over 300 gene loci (Lamoreux et al. 2010). Six out of those loci are also important in pigmentation process in cattle and other ruminants. They are genes in A, B, C, D, E, S and R loci (Millar et al. 2000).
Agouti (A) locus is responsible for melanin distribution and its expression. The absence of melanin causes white color expression. Agouti signaling protein (ASIP) gene is located on chromosome 1 in cattle (Royo et al. 2005).
Brown (B) locus is responsible for black coat color. TYRP1 is a type I membrane-bound protein that is expressed in both melanocytes and the retinal epithelium, and is involved in the distal eumelanic pathway (Gratten et al. 2008).
TYRP1 is located on chromosome 8 in cattle. Previous studies showed that the mutations in TYRP1 could alter the density of eumelanin pigment deposited in melanosomes, leading to a dilution in overall coloration, as well as color variations in several domestic mammals, including mice, cat and cattle (Zdarsky
et al. 1990; Berryere et al. 2003; Schmidt-Kuntzel et al. 2005).
Albino (C) locus is related to TYR gene located on chromosome 29 in cattle. It is involved in the enzymatic process that converts tyrosine to melanin pigments in cattle and all other mammals (Bertolotto et al. 1998). This gene is regulated by a transcription factor called microphthalmia-associated transcription factor encoded by the MITF gene in Mi locus (Mahalingam et al. 1997).
Dilution (D) locus has correlation SILV gene on chromosome 5 in cattle. A non-synonymous mutation located in the first exon of SILV (c.64A>G) has been identified as the causative mutation for coat color dilution in Charolais breed (Gutierrez-Gil et al. 2007).
22
color expression) and eumelanin (that is responsible for black or brown color). Any mutations that occur in MC1R influences the melanogenesis (melanocyte synthesis) process and causes different coat color expression (Jackson 1993; Guiterez-Gil et al. 2007; Mohanty et al. 2008).
Spotted (S) locus is located on chromosome 6 in cattle and on chromosome 3 in human. The important candidate gene that exists in chromosome 6 is KIT
(receptor tyrosine kinase) that leads to the white spot on mammalian skin and early embryonic death/abortion (Fontanessi et al. 2010b; Haase et al. 2007; Klungland & Vage 2001; Reinsch et al. 1999). The white face pattern in Simmental and Hereford cattle as well as the degree of white spotting in Holstein dairy cattle is regulated by KIT gene (Tazzoli et al. 2007; Liu et al. 2009; Fontanessi et al. 2010a). Furthermore, the belt spotted pattern in pigs is also regulated by mutations in KIT gene (Fang et al. 2009).
Roan (R) locus is associated with mast-cell growth factor (MGF) gene, a part of KITligand, which is located on chromosome 5 in cattle. Roan coat color is defined as mixture of white and pigmented hairs that does not grey out or fades as the animal ages. Well-known cattle breed that has roan coat color is Belgian Blue and Shorthorn cattle (Seitz et al. 1999). Homozygous dominant of MGF gene is expressed as fully pigmented, while the homozygous recessive cattle expresses white coat color and relates to White Heifer disease. In the heterozygous individual a rough mixture of white and colored regions is found on the coat.
MITF Gene
The microphthalmia-associated transcription factor (MITF) gene is located on chromosome 22 in cattle referenced genome (Figure 4.1). MITF is a member of the Myc superfamily of basic helix–loop–helix leucine zipper (bHLH-Zip) transcription factor that is able to form homo- and hetero-dimers with other MiT family members (Tfeb, Tfec and Tfe3) in vitro. MITF encodes a transcription factor that contains both basic helix-loop-helix and leucine zipper structural features (Steingrímsson et al. 2004).
The MITF protein activates transcription of a large number of different target genes in different cell types that have specific function. MITF protein is primarily located in the nucleus and its role is as key transcription factor and regulators of pigment cell-, mast cell-, and osteoclast-specific genes expression (Lin et al. 2002; Steingrímsson et al. 2004; Hershey & Fisher 2004; Phung et al.
2012; 2011).
23
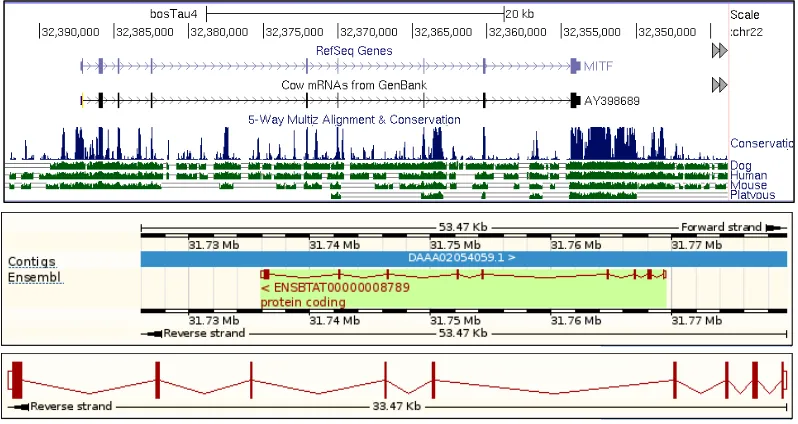
Figure 4.1 The schema of MITF gene that uses cattle genome as reference. It consists of 9 exons, with a 1,602 bp mRNA and 413 residues of translation length (source of figures: UCSC genome browser, http://www.genome.ucsc.edu and Ensemble genome browser, http://www.ensemble.org).
MITF Regulation in Pigmentation
There are at least two potential switches of MITF. First is the potential positive feedback circuit between MITF and MC1R. The activation of MC1R by α-melanocyte stimulating hormone (α-MSH) stimulates the MITF transcription through cAMP responsive element binding (CREB). MC1R itself is a transcriptional target of MITF.α-MSH binds MC1R and induces the activation of the αS-coupled G protein, which then activates adenylyl cyclase. Increased adenylyl cyclase results in an increase of intracellular cAMP synthesis. cAMP binds to the regulatory subunit of protein kinase A, which then liberates the catalytic subunit, which is free to phosphorylate various substrates including the nuclear CREB transcription factor (Figure 4.2). Phosphorylated CREB then binds to the cAMP responsive elements (CRE) DNA-binding site and activates gene expression. SOX10 as well as PAX3 are important melanocyte-specific modulator of this pathway. It was shown that the cAMP-mediated activation of the MITF-M
promoter is dependent on a SOX10- and PAX3-binding site, in addition to the CREB-binding site (Steingrímsson et al. 2004).
The second switch is a similar circuit between MITF and receptor tyrosine kinases (RTK) receptors KIT and mesenchymal-epithelial transition factor (MET). These receptors also appear to be MITF targets, while a stimulatory pathway can be seen from RTK activation through MAPK and CREB to MITF (Lamoreux et al. 2010).
24
activation through the M-box of the TYR, TYRP1 and DCT promoters. The melanogenic effects of cAMP are dependent on the increasing transcription from the MITF-M promoter since a dominant negative MITF protein may interfere with the increase in TYR expression following cAMP induction, but not in the expression of a CRE response element. Lack of MITF expression will not induce
TYR, TYRP1, and DCT expression and the cells will not start producing melanin (Steingrímsson et al. 2004).
Figure 4.2 The roles of action of MITF gene in pigmentation. It shows that MITF
mainly regulates pigmentation through melanogenic enzyme expression of TYR, TYRP1 and TYRP2 genes (this figure is modified based on explanation in Lamoreux et al. 2010 and Steingrímsson et al.
2004).
The spotted phenotype in swamp buffaloes implicates a defect in the migration/survival of melanocytes, since pigmentation is essentially normal in the pigmented areas. The spotted swamp buffaloes show altered pigmentation in both coat and iris (eye) color. This suggested a number of well-defined coat color genes affecting both coat and eye pigmentation as candidate genes (Phung et al.
2011; Karlsson et al. 2011; Bauer et al. 2009; Fang et al. 2009; Stachurska & Brodacki, 2008; Royo et al. 2005). Among these genes, we hypothesized that the prime candidate gene was the microphthalmia-associated transcription factor (MITF). This gene was associated with spotted coat color phenotypes in mice (Steingrímsson et al. 2003, 2004), dogs (Karlsson et al. 2007), horse (Sundström
25 Spectrophotometer. All DNA samples were sequenced for all nine MITF exons as well as highly conserved intronic and flanking regions based on sequence conservation as defined by comparing the database of 29 mammals (Lindblad-Toh
et al. 2011). Cattle (Bos taurus) genome (UCSC Genome Browser assembly ID: Oct. 2011, Baylor Btau_4.6.1/bosTau7) was used as reference genome sequence.
PCR Amplification and DNA Sequencing
PCR amplifications were prepared in 25 µl reactions, that included 1× PCR buffer II, 2.5 mM of MgCl2, 0.2 mM dNTP, 0.06 U of AmpliTaq® Gold (Applied Biosystems, Foster City, CA), 0.5 µM of Forward and Reverse primer and 10 ng genomic DNA. The PCR profile included an initial denaturation step at 94°C for 10 min, followed by 10 touchdown-cycles (denaturation at 94°C for 30 s, annealing at 65°C for 30 s, followed by extension at 72°C for 30 s), 30 cycles of amplification (denaturation at 94°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 30 s) and an additional extension step of 7 min at 72°C. PCR products were purified with Exociap mix containing alkaline phosphatase, Exo1 buffer and Exo1 at 37°C for 1 h and 85°C for 15 min. The purified PCR products were sequenced using Sanger sequencing. The nucleotide sequences were aligned using Codon Code Aligner (Codon Code Corporation), which was also used for SNPs calling.
cDNA Sequencing
26
cattle (Bos taurus) genome assembly (UCSC Genome Browser assembly ID: Oct. 2011, Baylor Btau_4.6.1/bosTau7).
Transcription/Translational System for PCR-generated DNA
PCR-generated DNA were were transcribed into RNA that was then translated into protein using the TnT® T7 Quick kit (Promega). The TnT® quick master mix was thawed and other components were thawed at room temperature, thereafter stored on ice. The final volume of each reaction was 50 µl included 40 µl TnT® quick master mix, 1 µl of 1mM Methionine, 0.5 µg PCR-generated DNA template, 1 µl T7 TnT® PCR enhance, and nuclease-free water. The reaction was incubated at 30°C for 90 min and stored at -80°C.
Electrophoretic Mobility Shift Assay (EMSA)
EMSAs were performed using LightShift™ Chemiluminescent EMSA kit (Thermo Scientific). Probe and competitor DNAs used in EMSA included wild-type MITF CCAACATGTGCACTCCAC-3') and mutant MITF (5'-CCAAACTGGTCACTCCAC-3'). Wild-type MITF probe was labeled with biotin using Biotin 3' End DNA Labeling Kit (Thermo Scientific). EMSAs were performed by incubating wild-type and mutant MITF proteins in binding buffer (10 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1 mM dithiothreitol, 50 ng/µl Poly (dI.dC), 1 mM EDTA, 5% glycerol, 5 mM MgCl2, 50 mM KCl and 4 pmol unlabelled MITF DNA) on ice for 20 min. Thereafter, 20 fmol Biotin End-Labelled MITF DNA was added to each reaction with the total volume of 20 µl, and the reactions were incubated at room temperature for 20 min. A 5 µl of 5X loading buffer was added to each 20 µl binding reaction. MITF-containing protein complexed with DNA were separated from unbound free DNA by electrophoresis through 5% Criterion TBE gel from Bio-Rad at 120 V for 70 min, and then transferred to a nylon membrane at 100 V for 60 min. Transferred DNA-protein complexes were cross-linked for 15 min with the membrane faced down on a trans-illuminator equipped with 312 nm bulbs. The membrane was exposed using CCD camera.
Statistical Analysis
27
Results and Discussion
A total of 14 SNPs were identified in MITF gene, and two of these independently showed a strong association to the spotted phenotype (Table 4.1). One of these SNPs corresponded to a nonsense mutation (C>T) at position chr22: 32,322,242 in exon 3 (acc.no.HG917421), while the other introduced a donor splice-site mutation (T>A) at position chr22: 32,297,683 (acc.no.HG917423) in intron 8 (Figure 4.3). The phenotype of individual samples used in this study is shown in Figure 4.4.
Table 4.1 Identified SNPs in swamp buffalo MITF gene*
SNP Position Allele Minor Allele Frequency Major Minor Solid Spotted White
Note: *located at chr22: 32,291,319-32,324,807. Nucleotide positions are given according to the cattle reference genome assembly (UCSC Genome Browser assembly ID: Oct. 2011, Baylor Btau_4.6.1/bosTau7)
Twenty-three out of thirty-eight spotted individuals (60.5%) were heterozygous for the nonsense mutation, whereas all solid and all white individuals were homozygous for the wild-type allele. A nonsense mutation is a point mutation in DNA that results in a premature stop codon in the mRNA. The nonsense mutation showed a highly significant association to spotted coat color pattern (P=1.3x10-7; Table 4.2). RT-PCR analysis on mRNA showed that mRNA containing the nonsense mutation was not expressed at a detectable level. This result suggested that mutant mRNA was degraded by the nonsense-mediated decay pathway (Maquat, 2004).
28
There was a considerable phenotypic variability in spotted coat color pattern among the 23 animals that were heterozygous for the nonsense mutation; some showed massive spotted color, while others only had a very small white spot on the forehead. This phenotypic variation was likely caused by genetic variation at loci interacting with MITF.
Figure 4.3 The results of Sanger sequencing of MITF DNA products generated from mutant spotted and wild-type swamp buffaloes. Circled bases are the SNPs position. Nonsense mutation occurred when the C base in the wild-type changed into Y (C/T) in mutant individual. Splice-site mutation occurred when the T base in the wild-type changed into W (T/A) in the mutant individual.
29
Table 4.2 The genotype frequencies of the nonsense mutation (chr22: 32,322,242a) and the splice site mutation (chr22: 32,297,683a) in
MITF gene of swamp buffalo with different coat color phenotypes
Phenotype Nonsense mutation Splice-site mutation
CC CT TT* Pb TT AT AA* Pb
Solid 32 0 0 31 1 0
Spotted 15 23 0 1.3x10-7 31 7 0 0.05
White 23 0 0 23 0 0
Note: aNucleotide positions are given according to the cattle reference genome assembly (UCSC Genome Browser assembly ID: Oct. 2011, Baylor Btau_4.6.1/bosTau7). bFisher’s Exact Test (one-tailed) comparing the allele frequencies in solid and spotted animals. *Individual with genotype TT or AA was lethal.
Figure 4.4 Variations of coat and eye color phenotype in swamp buffaloes. A. solid coat color with black iris; B. white coat color with pigmented iris; C. spotted coat color, Saleko type, with white iris color, individual was identified as the carrier of the nonsense mutation; D. spotted coat color, Lotong Boko type, with black iris, individual classified as not to the carrier of either the two mutations; E. spotted coat color, Bonga type, with white iris, individual was identified to be a carrier of the donor splice-site mutation; and F. spotted coat color,
30
Figure 4.5 The mRNA sequences of wild-type versus splice-site mutation. Twenty-four of intron 8 bases (red underline) were added into exon 8 of MITF gene in the splice-site mutant form. An extension of 8 amino acid residues occurred in the exon 8 of MITF gene as the result.
Figure 4.6 Amino acids alignment of splice-site mutation (SS) of spotted buffaloes versus wild-type of solid buffaloes (Wt) and 9 other species. It is shown on the alignment that mutant form has extra 8 amino acid residues compared to wild-type and other species.
The capacity of the mutant MITF protein produced by aberrant splicing to bind in vitro to the well-established MITF target site (5’-CACGTG-3’) was evaluated by performing the electrophoretic mobility shift assays (EMSA) using
31
Figure 4.7 The binding affinity of mutant versus wild-type MITF protein on electrophoretic mobility shift assay (EMSA). a) DNA only; b) 2 µl wild-type protein; c) 4 µl wild-type protein d) 6 µl wild-type protein; e) 2 µl mutant protein; f) 4 µl mutant protein; g) 6 µl mutant protein; h) 2 µl wild-type protein + 2 µl mutant protein; i) 3 µl wild-type protein + 3 µl mutant protein; j) 4 µl wild-type protein + 4 µl mutant protein; k) 4 µl wild-type protein + 400-fold molar excess of unlabelled wild-type DNA.
The splice-site mutation detected using genomic DNA was confirmed by cDNA sequencing. The splice-site mutation leads to aberrant splicing of exon 8. The resulting protein translated from such aberrantly spliced mRNA was expected to disturb the leucine zipper part in the basic helix-loop-helix zipper (bHLH-Zip) domain and would most likely influence dimerization and DNA binding capacity. There was a clear trend that individuals carried splice-site mutation showed a less pronounced spotting phenotype compared with most of animals carrying the nonsense mutation, suggesting that this was a partial loss-of-function allele while the nonsense mutations cause a null allele and the phenotype as a result of haplo-insufficiency. The observation of a solid colored buffalo carrying this splice site mutation suggested that this was either caused by a phenotype misclassification or that this mutation was not fully penetrant.
32
and Mitfmi-ew) were affected by semi-dominant mutations (Steingrímsson et al.
2004).
Figure 4.8 The estimated position of splice-site mutation in MITF gene and the position of helix-loop-helix DNA binding (HLH DNA-bd) and the leucine zipper part in the basic helix-loop-helix zipper (bHLH-Zip) domain. The splice-site mutation is expected to disturb the leucine zipper part in the bHLH-Zip domain because the additional of 8 amino acids in exon 8.
Previous study showed that many variations of MITF mutations were associated with hypopigmentation and white-spotted phenotype in different species. In German Fleckvieh cattle an R210I missense mutation has been identified as being responsible for hypopigmentation, heterochromia irides, colobomatous eyes and bilateral hearing loss (Phillip et al. 2011). Three variant mutations, a proximal melanocyte-specific MITF promoter replacing a thymine with 11 nucleotides, a small deletion in exon 5, and a de novo missense mutation in exon 6, were detected causing splashed white and macchiato in Frances-Montagnes horse (Hauswirth et al. 2012). While in white-spotted dogs, the mutation was shown to be a regulatory mutation located closer to the M promoter (228 bp upstream) of MITF-M gene transcription start site and negatively influencing MITF-M transcription (Karlsson et al. 2007; Baranowska-Körberg et al. submitted).
33
Moreover, eight of thirty-eight spotted individuals did not carry any of the two identified MITF mutations identified in our study (Table 4.2) and none were composite heterozygous for the two mutations as expected for loss-of-function mutations. Thus, there must be at least one additional mutation that remains to be identified affecting white spotting in swamp buffalo.
The fact that three or even more mutations caused spotted coat color pattern in swamp buffalo was consistent with the huge cultural importance these animals have had and still have. A strong positive selection over a considerable time period has led to the accumulation of multiple mutations causing a white spotting phenotype. This is another example of the huge cultural importance of white domesticated animals. Another famous example is white horses that have had a huge impact on human culture worldwide (Rosengren-Pielberg et al. 2008).
Conclusion