Attentional status of faces for people with
autism spectrum disorder
Article in Autism · June 2011
DOI: 10.1177/1362361311409257 · Source: PubMed
CITATIONS
10
READS
56
3 authors:
Anna Remington
University College London
17PUBLICATIONS 333CITATIONS
SEE PROFILE
Ruth Campbell
University College London
207PUBLICATIONS 8,590CITATIONS
SEE PROFILE
John Swettenham
University College London
67PUBLICATIONS 6,391CITATIONS
SEE PROFILE
Attentional status of faces for
people with autism spectrum
disorder
Anna Remington
Developmental Science, University College London, London, UK
Ruth Campbell
Developmental Science, University College London, London, UK
John Swettenham
Developmental Science, University College London, London, UK
Abstract
In recent years there has been a growing interest in the role of attention in the processing of social stimuli in individuals with autism spectrum disorders (ASD). Research has demonstrated that, for typical adults, faces have a special status in attention and are processed in an automatic and mandatory fashion even when participants attempt to ignore them. Under conditions of high load in a selective attention task, when irrelevant stimuli are usually not processed, typical adults continue to process distractor faces. Although there is evidence of a lack of attentional bias towards faces in ASD, there has been no direct test of whether faces are processed automatically using the distractor-face paradigm.
In the present study 16 typical adults and 16 adults with ASD performed selective attention tasks with face and musical instrument distractors. The results indicated that even when the load of the central task was high, typical adults continued to be distracted by irrelevant face stimuli, whereas individuals with ASD were able to ignore them. In the equivalent non-social task, distractors had no effect at high load for either group. The results suggest that faces are processed in an automatic and mandatory fashion in typical adults but not in adults with ASD.
Keywords
face processing, selective attention, perception
Autism spectrum disorder (ASD) is a congenital and lifelong condition characterized by behavioural rigidities, deficits in communication and problems with social interaction Autism 16(1) 59–73 ÓThe Author(s) 2012 Reprints and permissions: sagepub.co.uk/journalsPermissions.nav DOI: 10.1177/1362361311409257 aut.sagepub.com
Corresponding authors:
(American Psychiatric Association, 1994). One impairment that has been documented is the lack of expertise for face processing – a fundamental component of social interaction. Although such deficits may not be specific to faces, may involve more general perceptual impairments and may not be universal (Behrman et al., 2006; Jemel et al., 2006), the impairments that have been demonstrated may offer a useful insight into the condition. The typical brain has both cortical (e.g. fusiform face area, FFA) and subcortical networks (e.g. amygdala) that specialize in face processing (Johnson, 2005) yet most of the behavioural and neuroimaging studies that have been performed with individuals with ASD have highlighted reduced or absent activation in the FFA (Critchley et al., 2000; Hubl et al., 2003; Pierce et al., 2001; Schultz et al., 2000).
It has been suggested that the origin of this deficit is a reduced level of orienting towards faces. Typical infants are born with a tendency to look towards the faces of others (Johnson et al., 1991; Valenza et al., 1996) – possibly underpinned by subcortical structures such as the amygdala, superior colliculus and pulvinar, which then facilitate cortical specialization (Johnson, 2005). In ASD, however, this attentional bias does not seem to be present (Schultz, 2005) and a lack of facial orienting is one of the symptoms most often reported in young children with the condition. Analysis of home videos from first birthday parties have demonstrated that infants who were later diagnosed with ASD looked less at other people than typically developing infants or those later diagnosed with general learning difficulties (Osterling and Dawson, 1994; Osterling et al., 2002). It was also shown that 20-month-old infants looked less at others’ faces than typical children or children with developmental delay (Swettenham et al., 1998) and when watching film of people interacting, eye tracking data has revealed that children with ASD spend more time looking at body parts or objects than at faces (Klin et al., 2002; Speer et al., 2007).
Change blindness paradigms have also been used to assess attention to faces in ASD by examining the speed taken to spot a face change versus an object change in image pairs. The results demonstrate that while typically developing children were faster at detecting face changes than object changes, the ASD group showed no difference between the conditions, again reinforcing the idea that the children with ASD lack the attentional bias towards faces (Kikuchi et al., 2009).
In a functional MRI (fMRI) study, Bird et al. (2006) showed that there was a lack of attentional modulation of the neural responses to face stimuli in ASD. On each trial, participants were shown four pictures arranged in a cross formation: one pair of face stimuli and one pair of house stimuli. They were asked to make a same/different judgement about one of the pairs (i.e. vertical or horizontal) and the neural responses to the face and house stimuli when in the attended and unattended positions were compared. It was seen that for typical individuals, attention to the face stimuli resulted in enhanced FFA activation levels. For the ASD group, however, no such modulation was observed. With house stimuli, attention modulated the neural response in house-selective areas for both participant groups. The authors hypothesized that the absence of attentional modulation to social stimuli was due to weaker connectivity between V1 and extrastriate areas in ASD.
the faces even when processing interferes with task performance – faces are extremely difficult to ignore. This finding has led to the suggestion that faces have a special status in attention, being processed in an automatic and mandatory fashion (Farah et al., 1995; Kanwisher et al., 1997). One way in which this has been demonstrated experimentally is by using a selective attention task in which perceptual load is manipulated (Lavie et al., 2003). According to perceptual load theory (Lavie and Tsal, 1994), the processing of irrelevant stimuli in the visual field is dependent on the perceptual load (amount of potentially task-relevant information) of the central task in question. If the perceptual load of a task is low, and does not exceed perceptual capacity, then distractor elements are processed. However, if the perceptual load is high, such that processing capacity is exhausted, then irrelevant distractors are not processed. Maylor and Lavie (1998) neatly demonstrated this effect using a task in which participants were presented with a ring of letters and asked to identify a target (X or N) within the ring. The number of letters in the ring was varied in order to manipulate the perceptual load of the task. Participants were explicitly told to ignore distractor letters that were presented slightly offset from the central display. Distractor letters were either incompatible (X when the target was N, or N when the target was X) or neutral (e.g. T or L) and therefore unrelated to the target letters. If the distractor letters were processed then an incompatible distractor would lead to confusion over which target was present and slow down the target identification response time. The incongruent trials would therefore be slower than the neutral trials. However, if distractor letters were not processed then the time taken to respond in both conditions, incompatible and neutral, would be equivalent. Comparing the response times to neutral and incompatible trials at each level of load revealed that distractor letters appear to be processed at low levels of perceptual load but are no longer processed at high levels of perceptual load.
Interestingly though, the relations between perceptual load and distractor processing is not seen when the distractor elements are faces. Lavie et al. (2003) presented participants with a famous name hidden among a list of non-words and asked them to classify the name as a pop star or politician. Flanking the list were distractor faces that were either the same category as the name (congruent) or the opposite category (incongruent). Participants were explicitly instructed to ignore the faces, and the time taken to classify the name was recorded. The perceptual load of the central task was adjusted by varying the number of non-words in the list. In this task participants were consistently slower to respond to incongruent trials versus congruent trials regardless of perceptual load, that is, distractor faces were processed even when the central task was at the highest level of perceptual load. It was argued that this effect demonstrated that faces have a special status in attention, being processed in an automatic and mandatory fashion.
In the current study we examined whether adults with ASD would process faces regardless of perceptual load in a selective attention task. We used a task similar in structure to Lavie et al. (2003). However, given the potential lack of world knowledge in the clinical group, the task was modified to include anonymous male and female face distractors and participants were asked to classify the written target name according to gender. Congruity therefore depended on whether the face and target name were the same or different gender.
using photos and names of musical instruments, was also included and participants were asked to classify the target named instrument according to instrument category (string or wind) whilst ignoring photos of instrument flankers. It was predicted that for both the ASD and typical comparison group there would be no evidence of distractor interference using these non-social stimuli at the highest level of perceptual load.
Experiment 1
Methods
Participants.
A total of 16 adults with ASD and 16 comparison adults took part in the study, and all participants gave their informed consent before being included in the study. Participants in the ASD group had received a clinical diagnosis of ASD from a trained, independent clinician who used the criteria listed in theDiagnostic and Statistical Manual of Mental Disorders, Fourth Edition(American Psychiatric Association, 1994). Diagnosis was then confirmed by assessment with Module 4 of the Autism Diagnostic Observational Schedule (Lord et al., 2002). None of the participants had any other mental or neurological disorder. Groups were matched for non-verbal IQ using a subscale from the Wechsler Abbreviated Scale for Intelligence (WASI, Wechsler, 1999) and reading ability was verified using the National Adult Reading Test (NART, Nelson, 1982) (Table 1).Independent samples t-tests showed that the WASI and NART scores of the two groups were not significantly different (allp values>.05).
Stimuli.
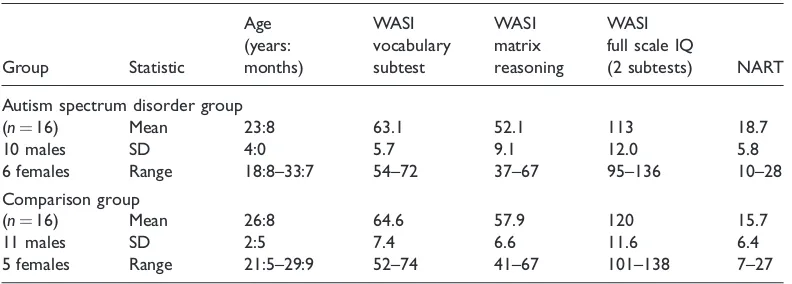
Stimuli were created using Microsoft Visual Basic (version 6), run on a custom-built desktop computer, and displayed on a ProLite 15 inch flat LCD screen (resolution of 12801024 pixels, 2 ms response rate). Viewing distance was 60 cm. Stimuli were presented in black against a grey background. Following the presentation of a fixation cross in the centre of the screen for 500 ms, a target name was presented in centre of the screen. In order to manipulate the perceptual load of the task, the name was presented alone (set size 1) or among a list of one, three or five non-words (set sizes 2, 4 and 6, respectively) (see Figure 1). On each trial, a distractor face (from a set of 12 faces: 6 male, 6 female) measuring 4.1 3.3 visual angles was presented at the side of the list of words, 5 fromTable 1 Descriptive statistics for each group in experiment 1
Age WASI WASI WASI
6 females Range 18:8–33:7 54–72 37–67 95–136 10–28
Comparison group
(n¼16) Mean 26:8 64.6 57.9 120 15.7
11 males SD 2:5 7.4 6.6 11.6 6.4
5 females Range 21:5–29:9 52–74 41–67 101–138 7–27
fixation. These distractor faces were either congruent (same sex as the target name) or incongruent (opposite sex as target name). Four blocks of 192 trials were created with each set size and distractor condition appearing equally. Condition presentation order and target position within the list of words was also counterbalanced. A set of six male (John, James, David, Henry, Thomas, Michael) and six female forenames (Lucy, Mary, Sarah, Jane, Katie, Sophie) were used as the target words and a set of 12 non-words were used as the non-target elements.
Procedure.
Participants were told to indicate with a button press whether the name hidden among the non-words was male or female. They were explicitly told to ignore the face stimuli and to perform the task as quickly as possible. For each trial, the key-press, response time(milliseconds from stimulus onset time) and accuracy were automatically recorded. All stimuli and procedures were approved by the University College London ethics committee and were therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Results
Data reduction.
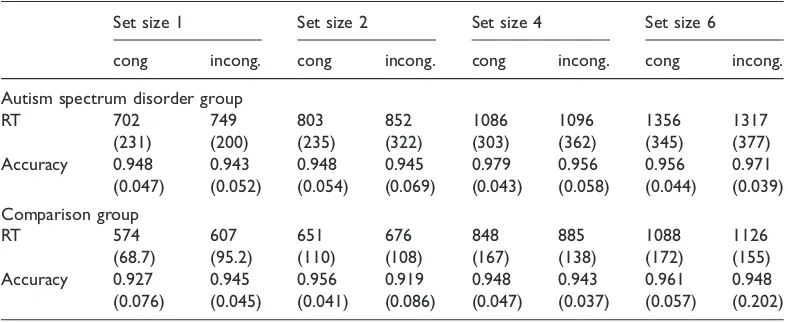
The median reaction time for correct responses and error rates for each distractor condition at set size 1, 2, 4 and 6 were calculated. All incorrect trials were excluded from reaction time analyses. For each participant, the median reaction times for correct responses to congruent and incongruent trials at each set size were calculated (Table 2). Where group/condition averages are stated, these are the mean of the median reaction times of each participant in that group or condition.Accuracy data.
Analysis of variance (ANOVA) was performed on the number of errors for each group across each condition. There were no significant main effects or interactions between the error rates for both groups under each condition (all p values>.05). The error rates are consistently low across all conditions for both participants with ASD and comparison participants.Reaction time data.
An ANOVA was performed on the mean median correct reaction times with group (ASD vs. control) as the between-subjects factor and distractor congruency (congruent vs. incongruent) and set size (1, 2, 4 and 6) as within-subject factors.There was a main effect of set size (F(3,90)¼369.69,p<.001,Zp 2
¼0.93) and of distractor congruency (F(1,30)¼11.94, p¼.002, Zp2¼0.29). Inspection of the means revealed that reaction times increased with set size (as the task became more difficult) and were longer for incongruent trials. The main effect of congruency did not interact with set size (F(3,90)¼2.03, p¼.116, Zp2¼0.06) or group (F(3,90)¼1.27, p¼.268, Zp2¼0.04). There
Table 2 Overall mean, median reaction times (RTs, ms), accuracy rates (proportion correct) and standard deviations (SD) for the two groups under congruent (cong.) and incongruent (incong.) distractor conditions at each set size for experiment 1
Set size 1 Set size 2 Set size 4 Set size 6
cong incong. cong incong. cong incong. cong incong.
Autism spectrum disorder group
RT 702 749 803 852 1086 1096 1356 1317
(231) (200) (235) (322) (303) (362) (345) (377)
Accuracy 0.948 0.943 0.948 0.945 0.979 0.956 0.956 0.971
(0.047) (0.052) (0.054) (0.069) (0.043) (0.058) (0.044) (0.039) Comparison group
RT 574 607 651 676 848 885 1088 1126
(68.7) (95.2) (110) (108) (167) (138) (172) (155)
Accuracy 0.927 0.945 0.956 0.919 0.948 0.943 0.961 0.948
was also no significant interaction between set size and group (F(3,90)¼3.09, p¼.063, Zp2¼0.09).
There was a main effect of group (F(1,30)¼10.88,p¼.003, Zp2¼0.27) due to generally slower reaction times of the ASD participants.
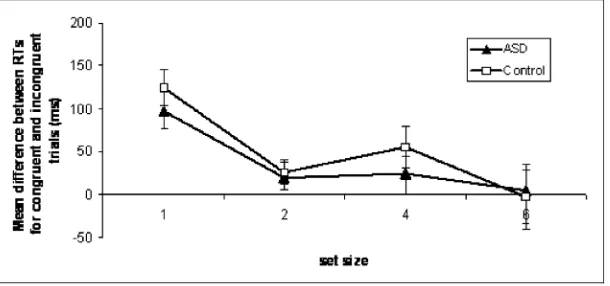
The three-way interaction between group, set size and distractor congruency was also significant (F(3,90)¼3.22,p¼.039,Zp2¼0.10) indicating that the two groups were showing different congruency effects at the various set sizes (Figure 2).
Post hoc t-tests (with Bonferroni–Holm adjustments to the significance level) were used to further investigate the significant three-way interaction. In the comparison group, there was a significant effect of congruency at all set sizes (set size 1, t(15)¼3.06 p¼.008; set size2, t(15)¼4.32, p¼.001; set size 4, t(15)¼2.51, p¼.024; set size 6, t(15)¼2.27, p¼.039) indicating that typical adults continue to be distracted by faces at all levels of perceptual load.
In the ASD group, there was significant effect of congruency at set size 1(t(15)¼3.97, p¼.001) and 2 (t(15)¼3.26, p¼.005) but not for set size 4 (t(15)¼0.68, p¼.509) or 6 (t(15)¼–0.84, p¼.414) demonstrating that, unlike typical adults, ASD individuals only process faces at low levels of load; when the perceptual load of the central task was increased no congruency effect was observed.
Experiment 2
Our first experiment’s results showed that the typical adults, but not the individuals with ASD, continued to process distractor faces even at high levels of perceptual load. In order to determine whether the effects were specific to faces, we ran a second experiment using meaningful non-face distractors, following Lavie et al. (2003). This task differed from experiment 1 in that it used musical instruments instead of faces. Participants were asked to classify the name of an instrument (string or wind) while ignoring pictures of the instruments that flanked the words. By using photographs of musical instruments, the distractors are meaningful, of similar visual complexity to faces and require subordinate category
discrimination. By running this control task, the specific impact of face stimuli on selective attention in ASD can be assessed.
Methods
Participants.
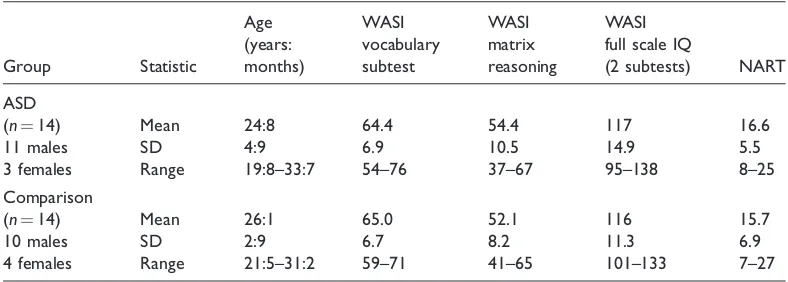
A total of 14 young adults with ASD and 14 comparison adults took part in the study. Diagnostic and matching procedures were identical to those of experiment 1 (Table 3). A new set of participants were recruited here in order to avoid practice effects that would arise if the individuals who had previously performed experiment 1 took part in this subsequent task. These participants were matched closely on age, sex and non-verbal IQ with the corresponding group from experiment 1.Independent samples t-tests verified that there was no difference between the groups on these measures (allpvalues>.4).
Stimuli and procedure.
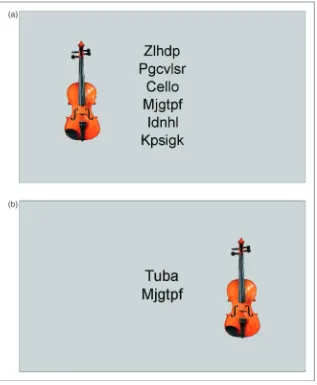
The experimental paradigm was similar to that of experiment 1, but the male and female names and face distractors were replaced by the names of wind or stringed musical instruments and corresponding pictures. Stimuli were presented in black against a grey background. Following the presentation of a fixation cross in the centre of the screen for 500 ms, then name of a target instrument was presented in centre of the screen. The target could be one of six wind instruments (clarinet, horn, saxophone, trombone, trumpet, tuba) or one of six stringed instruments (banjo, bass, cello, guitar, harp, violin). Participants were told to indicate with a button press whether the name of the instrument hidden among the non-words was either a string or a wind instrument. In order to manipulate the perceptual load of the task, the name would be presented alone (set size 1) or among a list of one, three or five non-words (set sizes 2, 4 and 6, respectively) (Figure 3). A set of 12 non-words were used as the non-target elements. The names and non-words were matched in length. On each trial, a distractor instrument measuring 4.1 3.3visual angles (identical size to face distractor from experiment 1) was presented at the side of the list of words, 5 from fixation. These distractor instruments were either congruent (same instrument class as the target name) or incongruent (opposite instrument class to the target name). Four blocks of 192 trials were created with each set size and distractor condition appearing equally. For set sizes 2 and 4, the position of the target within theTable 3 Descriptive statistics for each participant group in experiment 2
Age WASI WASI WASI
3 females Range 19:8–33:7 54–76 37–67 95–138 8–25
Comparison
(n¼14) Mean 26:1 65.0 52.1 116 15.7
10 males SD 2:9 6.7 8.2 11.3 6.9
4 females Range 21:5–31:2 59–71 41–65 101–133 7–27
list was counterbalanced. Following the completion of the experimental trials, the pictures of the instruments were presented to each participant and they were asked to both name the instrument and indicate which group (string or wind) it belonged to. This was to ensure that all participants had the relevant knowledge to meaningfully complete the task.
Results
Data reduction.
The median correct reaction time and error rates for each distractor condition at set sizes 1, 2, 4 and 6 were calculated. All incorrect trials were excluded fromfurther analyses. For each participant, the median reaction times for correct responses to congruent and incongruent trials at each set size were calculated (Table 4).
Accuracy data.
An ANOVA performed on the number of errors for each group across each condition revealed a main effect of set size (F(3,78)¼5.83,p¼.001,Zp2¼0.18) and a main effect of distractor congruency (F(1,26)¼9.43, p¼.005, Zp2¼0.27); the error rates were higher for the lower set sizes and for incongruent trials. The latter observation is logical, while the idea of more errors being made at lower set sizes is a little counterintuitive. This may be a speed-accuracy trade-off, however, given the incongruent distractors were being processed at the lower set sizes, it is more likely that this was negatively influencing the responses (i.e. increasing error rates) at low levels of load. There was a significant interaction between congruency and set size (F(3,78)¼7.90, p<.001, Zp2¼0.23). In line with the reasoning regarding the main effect of set size, this reflects a greater number of errors in the incongruent trials versus congruent trials at the lower set sizes. There was no significant interaction between set size and group (F (3,78)¼2.85, p¼.055, Zp2¼0.10) or between congruency and group (F (1,26)¼0.98, p¼.331, Zp2¼0.04). There was no main effect of group (F(1,26)<0.01,p>.999, Zp2<0.01).The three-way interaction between group, set size and distractor congruency was not significant (F(3,78)¼0.76, p¼.520, Zp2¼0.03) indicating that the two groups were not showing a different pattern of error rates across the various conditions.
Reaction time data.
An ANOVA was performed on the median correct reaction times with group (ASD vs. control) as the between-subjects factor and distractor congruency (congruent vs. incongruent) and set size (1, 2, 4 and 6) as within-subject factors.There was a main effect of set size (F(3,84)¼322.50,p<.001,Zp2¼0.92) and of distractor congruency (F(1,28)¼31.88, p<.001, Zp2¼0.53). These significant effects reflect the fact that reaction times increased with set size (as the task became more difficult) and were longer for incongruent trials. The main effect of congruency did not interact with group
Table 4 Overall mean median reaction times (RTs, ms), accuracy rates (proportion correct) and standard deviations (SD) for the two groups under congruent (cong.) and incongruent (incong.) distractor conditions at each set size for experiment 2
Set size 1 Set size 2 Set size 4 Set size 6
cong incong. cong incong. cong incong. cong incong.
(SD) (SD) (SD) (SD)
ASD
RT 730 827 897 924 1154 1151 1420 1436
(103) (107) (211) (159) (272) (196) (273) (253)
Accuracy 0.976 0.920 0.917 0.935 0.964 0.961 0.979 0.958
(0.035) (0.101) (0.126) (0.084) (0.065) (0.060) (0.036) (0.057) Comparison
RT 731 841 822 856 1060 1109 1344 1382
(143) (206) (139) (139) (170) (198) (205) (244)
Accuracy 0.973 0.884 0.958 0.949 0.967 0.949 0.967 0.961
(F(3,84)¼0.18, p¼.671, Zp2¼0.01). There was also no significant interaction between set size and group (F(3,84)¼0.94, p¼.427, Zp2¼0.03). There was, however, a significant interaction between set size and congruency (F(3,84)¼3.11, p¼.031,Zp2¼0.10). This is a reflection of the fact that there was a congruency effect at low set sizes (when the perceptual load of the task is low) but not at higher set sizes.
There was no main effect of group (F(1,28)¼0.76, p¼.392, Zp2¼0.03); overall, both groups were performing the task at a similar speed. The three-way interaction between group, set size and distractor congruency was not significant (F(3,84)¼0.05, p¼.984, Zp2<0.01) indicating that the two groups were showing the same pattern of congruency effects at the various set sizes (Figure 4). It appears that both groups displayed an effect of distractor type at the lower set sizes, which then disappeared as the perceptual load of the task increased.
Post hoc t-tests (with Bonferroni–Holm adjustment to the significance level) confirmed that in both the comparison and ASD groups, there was only a significant effect of congruency at set size 1 (p<.01), indicating that participants were only processing the distractors at the low level of perceptual load.
Discussion
The findings from these experiments highlight how typical adults and high-functioning young adults with ASD differ in their attentional response to social distractor stimuli. The results for the typical adults were similar to those reported by Lavie et al. (2003); the congruency effect of non-social distractors was eliminated at high levels of perceptual load whereas face distractors were processed at all set sizes. In contrast, the individuals with ASD showed the same pattern of results with both the face and non-social distractors. Congruency effects were evident for both faces and instruments at low set sizes but when perceptual load increased there was no evidence of distractor interference for either type of distractor.
This finding provides evidence that faces do not capture attention in adults with ASD in the same manner as for typical adults. In mainstream attention research, the inability to ignore distractor faces at high levels of load is used in support of the idea that face processing
is an automatic and mandatory process and is not subject to general capacity limits. The data presented here question whether faces play a special role in attention for individuals with ASD.
The distractor faces were, however, not always ignored by the individuals with ASD; congruency effects in the ASD group at lower levels of perceptual load demonstrated that gender was processed from the distractor faces and that this influenced the time taken to classify the gender of the written name. We cannot completely rule out the possibility that the group difference was due to a tendency by the typical adults to make overt gaze shifts and fixations on the to-be-ignored faces in the high perceptual load conditions, as we did not collect eye tracking data. However, the task requirements to classify the written name as quickly as possible and to ignore the flanker stimuli meant participants were discouraged from shifting gaze from the central task toward the face. In addition, looks toward the face would be likely to afford a large reaction time cost and one would predict slower reaction times for performance on the central task in the typical adults if they were looking at the faces. In fact, inspection of the mean reaction times and the main effect of group in experiment 1 indicate that the ASD group had a slower reaction time than the typical adults at all set sizes. It would be interesting in future studies to use eye tracking data to examine directly whether there were gaze shifts toward the distractor faces in either group. It is unclear why reaction time was slower in the ASD group as the groups were matched for reading ability and IQ. One possibility is that the element of social processing involved in the central task, classifying the written name in terms of gender, meant the task was more difficult for participants with ASD. Even if this was the case, it would not explain the similarities and differences in the congruency effects at different set sizes compared with typical adults as the social demands of the central task did not change with set size.
When we compared group performance on experiment 2 (non-social distractor stimuli), we found no difference between the groups in the size of the congruency effect at different levels of perceptual load. The congruency effect disappeared at similar (high) levels of perceptual load in both groups. What we have not shown here is evidence of increased perceptual capacity in adults with ASD when non-social stimuli are used as distractors, as might be predicted from our earlier study using a different methodology (Remington et al., 2009), and by a more recent replication demonstrating increased perceptual capacity and conscious awareness of non-social distractors in adults with ASD (Remington et al., manuscript submitted).
ASD. If this is the case it is even more impressive that the typical adults continued to process distractor faces in experiment 1, regardless of perceptual load.
In typical adults, the finding that face distractors are processed irrespective of perceptual load has been interpreted as evidence for the existence of a separate processing capacity for faces (Lavie et al, 2003). Further support for this idea is provided by a study showing that only the addition of other face stimuli, and not additional non-face distractors, can dilute the congruency effect (Jenkins et al., 2003). However, as these authors point out, it is possible that the separate capacity in typical adults is not innately assigned for faces but becomes face-specific because of the strong interest in faces in typical development and the resultant expertise. That is, the separate processing store may be reserved for any specialized interest, which in typical adults is social stimuli, but in ASD may be a non-social object category such as types of trains or buildings. Given that individuals with ASD often have an unusually strong, if not obsessive interest in a specific category of non-social objects, it would be interesting to examine whether a separate processing capacity exists for exemplars of a category of interest, with distractor processing continuing at high levels of perceptual load. This would be one way to test whether the lack of special status for faces in attention in individuals with ASD is a result of the lack of experience attending to faces, something which is clear from early in development and throughout childhood (e.g. Dawson et al., 2004; Klin et al., 2002; Swettenham et al., 1998).
Why is there no separate capacity for faces in adults with ASD? We speculate here that the special status in attention for faces in typical adults could be the result of an innate tendency to preferentially orient to others (and especially faces) and to build up experience and expertise with social stimuli. There is now growing evidence that infants and young children with ASD do not preferentially orient toward social stimuli (e.g. Dawson et al., 2004; Klin et al., 2009; Swettenham et al., 1998) and do not therefore gather as much experience with faces. The existence of a separate capacity for an alternative category of expertise in individuals with ASD would provide some support for this view.
Acknowledgements
This research was supported by a departmental studentship awarded to Anna Remington from the Department of Developmental Science, University College London. We thank Elizabeth An˜ez for her invaluable advice regarding the statistics and we gratefully acknowledge the support of all those who took part in the study.
References
American Psychiatric Association APA (1994)DSM-IV Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American: Psychiatric Association.
Behrman M, Thomas C and Humphreys K (2006) Seeing it differently: visual processing in autism.Trends in Cognitive Sciences10: 258–264.
Bird G, Catmur C, Silani G, Frith C and Frith U (2006) Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage31: 1614–1624. Critchley HD, Daly EM, Bullmore ET, Williams SC, van AT, Robertson DM, et al. (2000)
Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, et al. (2004) Early social attention impairments in autism: social orienting, joint attention, and attention to distress.Developmental Psychology40: 271–283.
Farah MJ, Wilson KD, Drain HM and Tanaka JR (1995) The inverted face inversion effect in prosopagnosia: evidence for mandatory, face-specific perceptual mechanisms. Vision Research35: 2089–2093.
Hubl D, Bolte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, et al. (2003) Functional imbalance of visual pathways indicates alternative face processing strategies in autism.Neurology61: 1232–1237.
Jemel B, Mottron L and Dawson M (2006) Impaired face processing in autism: fact or artifact?Journal of Autism and Developmental Disorders36: 91–106.
Jenkins R, Lavie N and Driver J (2003) Ignoring famous faces: category-specific dilution of distractor interference.Perception & Psychophysics65: 298–309.
Johnson MH (2005) Subcortical face processing.Nature Reviews Neuroscience6: 766–774. Johnson MH, Dziurawiec S, Ellis H and Morton J (1991) Newborns’ preferential tracking of
face-like stimuli and its subsequent decline.Cognition40: 1–19.
Kanwisher N, McDermott J and Chun MM (1997) The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience 17: 4302–4311.
Kikuchi Y, Atsushi S, Tojo Y, Osanai H and Hasegawa T (2009) Faces do not capture special attention in children with autism spectrum disorder: A change blindness study. Child Development80: 1421–1433.
Klin A, Jones W, Schultz R, Volkmar F and Cohen D (2002) Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism.Archives of General Psychiatry59: 809–816.
Klin A, Lin DJ, Gorrindo P, Ramsay G and Jones W (2009) Two-year-olds with autism orient to non-social contingencies rather than biological motion.Nature459: 257–261. Lavie N, Ro T and Russell C (2003) The role of perceptual load in processing distractor
faces.Psychological Science14: 510–515.
Lavie N and Tsal Y (1994) Perceptual load as a major determinant of the locus of selection in visual attention.Perception & Psychophysics56: 183–197.
Lord C, Rutter M, DiLavore PC and Risi S (2002) Autism Diagnostic Observational Schedule. Los Angeles, CA: Western Psychological Services.
Maylor EA and Lavie N (1998) The influence of perceptual load on age differences in selective attention.Psychology and Aging13: 563–573.
Nelson H (1982)The National Adult Reading Test. Windsor: NFER.
Osterling J and Dawson G (1994) Early recognition of children with autism: a study of first birthday home videotapes.Journal of Autism and Developmental Disorders24: 247–257. Osterling JA, Dawson G and Munson JA (2002) Early recognition of 1-year-old infants with
autism spectrum disorder versus mental retardation. Development and Psychopathology 14: 239–251.
Pierce K, Muller RA, Ambrose J, Allen G and Courchesne E (2001) Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain 124: 2059–2073.
Schultz RT (2005) Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience 23: 125–141.
Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. (2000) Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome.Archives of General Psychiatry57: 331–340. Speer LL, Cook AE, McMahon WM and Clark E (2007) Face processing in children with
autism: effects of stimulus contents and type. Autism11: 265–277.
Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A, et al. (1998) The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. Journal of Child Psychology and Psychiatry39: 747–753.
Valenza E, Simion F, Cassia VM and Umilta C (1996) Face preference at birth. Journal of Experimental Psychology: Human Perception and Performance 22: 892–903.