(A Case study in Quiberon Bay, France)
(Evaluasi Biologi dan Nilai Ekonomi dari Pengaruh Predator Terhadap Kematian Tiram : Studi Kasus di Teluk Quiberon, Prancis)
Ika Meidy Deviarni
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
STATEMENT
I, Ika Meidy Deviarni, here by declare that the thesis entitled:
Biological and Economic Evaluation of The Impact of Predators on Oyster Mortality (A Case study in Quiberon Bay, France)
contain correct result from my own work and that it has not been published ever
before. All data sources and information used factual and clear methods in this
project, and has been examined by the advising commitee and the external
examiner.
Bogor, September 2011
IKA MEIDY DEVIARNI. Biological and Economic Evaluation of The Impact of Predators on Oyster Mortality (A Case study in Quiberon Bay, France). Under the supervision of Prof. Dr. Ir. Fransiska R. Zakaria, M.Sc and Dr. Ir. Hartrisari Hardjomidjojo, DEA
Pacific oysters have been cultured in several country as a result,
Crassostrea gigas has become the leading species in world culture, with an estimated production of 4.6 million tons in 2006. France producing around 130
000 tons of the cupped oyster C. gigas annually and a remaining 1 500 tons of the
flat oyster Ostrea edulis. Quiberon Bay has a large production of oyster, approximately 15 000 tons annually which contributes to oyster production in
France. These small scale industry activities contributes to largely to France
economy.
Since the year of 2000 and especially 2006, the oyster mortality rates
have reached quite unusual, estimated at 60% mortality in a breeding cycle ending
in late 2006, according to the response of 51 companies interviewed by the CRC.
This mortality brought a great impact on the France's economy. So, in this study,
we observed the effects of oyster mortality in terms of biology and economics.
The objectives of this study are to analyse RISCO data on oyster
mortality, to determine effect of predators on oyster culture, mapping the
distribution of different types of predators and to calculate the economic analysis
of oyster mortality. RISCO project is conducted by Ifremer with their partners. As
present in the study area, this project is held in Quiberon Bay, France and the 15
station’s of oyster culture. The observations of this research have been conducted
monthly (8 surveys) in May until December 2010. Each month, there are several
analyses of RISCO project which have been done, one of them is analysis oyster
growth and mortality (Spat and Adult).
In each station, we counted the number of predators (starfishes and
gastropods) on the cage and in each bag we calculated the numbers of dead
analysis. In each dead oyster, we also observed the boring oyster by oyster drill
and parasitism by Polydora.
The result of this research showed that there were a potential role of two
species of starfishes, Asterias rubens and Marthasterias glacialis and two species of gastropods, Ocenebra erinacea and Ocenebrillus inornatus. Marthasterias glacialis indicated a great impact on mortality of adult oysters. The relation between oyster's mortality and the presence of gastropods depended on time and
according to reproductive cycle. Several months show clearly a predation of
oysters and others show a concentration of gastropods for spawning. Predators on
oyster culture also indicated a great impact in economic value. The economic
value on oyster culture model applied in RISCO project shows that BSP was
higher than BAP. Based on economic value analysis on RISCO project shows that
BSP was higher than BAP. Economic loss in BAP greatly happen in station 2 and
5, approximately €81.28 - €81.83. On the contrary, station 15 indicate has the
higher loss in BSP approximately €54.92 or 363 oysters and for the rest, the
Copyright are protected by law,
1. It is prohibited to cite all of part of this thesis without reffering to and
mention the sources;
a. Citation only permitted for the shake of education, research, scientific,
writing, report writing, critical writing or reviewing scientific problem.
b. Citation does not inflict the name and honor of Bogor Agricultural
University.
It is prohibited to republish and reproduce all part of this thesis without written
Biological and Economic Evaluation of The Impact of Predators
on Oyster Mortality
(A Case study in Quiberon Bay, France)
(Evaluasi Biologi dan Nilai Ekonomi dari Pengaruh Predator Terhadap Kematian Tiram : Studi Kasus di Teluk Quiberon, Prancis)
Ika Meidy Deviarni
Thesis submitted to Graduate School of Bogor Agricultural University (IPB), Indonesia in partial fulfillment of the requirements for the degree of Magister Professional in Master Professional Management of Small and Medium
Industries Study Program and Magister Professional in Master Management of Ressources Biology Study Program in Universite de Bretagne Sud, France.
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
Research Title : Biological and Economic Evaluation of The Impact of Predators on Oyster Mortality (A Case study in Quiberon Bay, France)
Name : Ika Meidy Deviarni
Student ID : P054090225
Study Program : Master Professional Management of Small and Medium Industries
Approved by Advisory Board,
Prof.Dr.Ir. Fransiska R. Zakaria, M.Sc Supervisor
Dr.Ir. Hartrisari Hardjomidjojo, DEA Co-Supervisor
Endorsed by,
The Head of Master Professional Management of Small and Medium Industries, Bogor Agricultural University
Dean of Graduate School Bogor Agricultural University
Prof. Dr. Ir. Musa Hubies, MS, Dipl.Ing, DEA Dr. Ir. Dahrul Syah, M.Sc.Agr
Date of Examination: Date of Graduation:
In the name of God, the Most Beneficient, the Most Merciful. There are
many people I should thank in regard to this work no doubt I will not be able to
name them one by one. To these I can but beg forgiveness. I wish to thank the
following:
1. My supervisor Prof. Dr. Ir. Fransiska R. Zakaria, M.Sc and my
Co-supervisor Dr. Ir. Hartrisari Hardjomidjojo, DEA for their guidance,
comment and constructive critism through my thesis.
2. Prof. Dr. Ir. Musa Hubies, MS, Dipl.Ing, DEA, The Head of Master
Professional Management of Small and Medium Industries for his
kindness and providing academic assistance. And also MPI staff Miss
Vera, Mr. Haer and Mr. Harris.
3. Prof. Dr. Ir. Irwan Katili, DEA and Dr. Ir. Naresworo Nugroho,M.Si for their official endorsement, kindly permission, allowed me to attend
Double Degree Programe Indonesia-Prancis (DDIP).
4. All staff and Lecture in Universite de Bretagne Sud, France for their
constant guidence, continues encouragement, and valuable suggestion
during my study in France.
5. All MPI students year 2009 and All Biotechnology students year 2010, I
really appreciate our togetherness and how we support each other to finish
our study.
6. Great thanks to My musician, always accompany me during my thesis,
thanks for your love and understanding for me.
Lastly, I dedicated this thesis to my big family, my Beloved father
Suwardy, My Lovely Mother Suprihartini, and my Beloved brother Wahyu Dwi
CURRICULUM VITAE
Ika Meidy Deviarni was born in Pontianak, West
Kalimantan, Indonesia at May 3rd, 1982. She was graduated from Brawijaya University, Faculty of Fisheries, majoring
Technology Fish Processing in 2005. She entered in IPB
Graduate School with Double Degree Indonesia-France
Program in 2009. She enrolled in Magister Professional in
Master Professional Management of Small and Medium Industries Study
Program in Bogor Agricultural University, continued her second year in
Universite de Bretagne Sud majoring Gestion des Bioressources in 2010 and
finally completed her master study in 2011. Her final thesis is “Biological and
Economic Evaluation of The Impact of Predators on Oyster Mortality (A Case
1 Introduction...1
List of Table
Table 1. Oyster’s Predators of Crassostrea gigas...10
Table 2. Oyster’s Predators of Crassostrea virginica...12
Table 3.Oyster’s Predators of Ostrea edulis...13
Table 4. Variance analysis of gastropods...38
Table 5. Variance analysis of Starfishes...38
Table 6. Variance analysis of BAP...40
Table 7. Variance analysis of BSP...42
Table 8. Variance analysis of BAP-%BSP...42
Table 9. Variance analysis of BAP-%BSP and gastropods...43
Table 10. Variance analysis of BAP-%BSP and Ocinebrillus...43
Table 11. Variance analysis between BAP-%BSP and starfish...45
Table 12. Variance analysis of Asterias and BAP-%BSP per month...46
Table 13. Variance analysis of Marthasterias and BAP-%BSP per month...46
Table 14. Oyster presence on BAP...48
Table 15. Economic value on BAP...48
Table 16. Oyster presence on BSP...49
Figure 1. Oyster culture...4
Figure 2. Factors affecting oyster mortality...6
Figure 3. The images oyster’s predators of Crassostrea gigas...11
Figure 4. The images oyster’s predators of Crassostrea virginica...12
Figure 5. The images oyster's predators of Ostrea edulis...13
Figure 6. Ocenebra erinacea...14
Figure 16.a. Chart of France, b. Quiberon Bay (Menier, et. al., 2010)...31
Figure 17. Risco Stations...32
Figure 18.Sedimentology of Quiberon Bay (Menier, 2010)...32
Figure 19. Experimental Design of Oyster Culture...34
Figure 20. Station RISCO...36
Figure 21. a. The presence of Gastropod in Quiberon Bay, b. The presence of Starfish in Quiberon Bay ...37
Figure 22. Mean density of gastropod and starfish per month...38
Figure 23. Oyster’s mortality in on-bottom culture (BAP adult)...39
Figure 24. Matrix of BAP...40
Figure 25. Oyster mortality in on-bottom culture (BSP adult)...41
Figure 26. Matrix of BSP (adult)...41
Figure 27. Interaction gastropods between BAP and BAP-%BSP per month...44
Figure 28. Interaction between starfishes BAP and BAP-%BSP...45
Figure 29. Interaction Marthasterias in BAP and BAP-%BSP...46
Figure 30. Interaction between the presents of Asterias and Marthasterias in BAP and BAP-%BSP...47
6 Conclusion and Suggestion
6.1 Conclusion
The present study confirm that the highest mortality of oysters is happened in the cultured of adults oysters exposed to predations on the bottom (BAP) and it happened greatly in the deeper zone (almost 100%). Indeed, we have identified that there was an impact of predators, gastropods (Ocenebra erinacea and
Ocinebrillus inornatus) and starfishes (Asterias rubens and Marthasterias
glacialis), which existed in Quiberon Bay.
Identification with the statistic, showed that starfishes (Asterias rubens and
Marthasterias glacialis) gave significantly great impact with oyster mortality. In
each month and station, Marthasterias consumes about 10 oysters and the both starfishes consume 11 oysters. The impact of gastropods is clear apart from in autumn during the reproduction phase of O. inornatus. The relation between oyster's mortality and the presence of gastropods were depend on time and according to reproduction cycle. Several months show clearly a predation of oysters and others show a concentration of gastropods for spawning.
Based on economic value analysis on oyster culture model at RISCO project shows that BSP was higher than BAP. Economic loss in BAP greatly happen in station 2 and 5, approximately €81.28 - €81.83. On the contrary, station 15 indicate has the higher loss in BSP approximately €54.92 or 363 oysters and for the rest, the economic value was varies between €29.67-€40.30.
6.2 Suggestion
Several suggestion for this study, (1) in the oyster culture, it would be better to use a cage (2) to reduce the predator, it is better to use an empty cage to attract gastropod and during the time of reproduction (spring and autumn), lift up the cage and throw the eggs of gastropods, (3) it is better to use a cage which is
57
Reference
Allen, P.L. 1983. Feeding Behaviour of Asterias Rubens (L.) On Soft Bottom Bivalves: A Study in Selective Predation. J. Exp. Mar. Biol. Ecol., 1983, Vol. 70, pp 79-90.
Anderson, M.J. and Connell, S.D. 1999. Predation of Fish on Intertidal Oysters. Marine Ecology Progress Series. 187:203-211
Arin. G and Arin. A., 1976. Farming the European Flat Oyster (Ostrea edulis) in the Classical Style in Brittany, France. A Multi Disciplinary Treatise. Development in Aquaculture and Fisheries Science 3. Elsevier Scientific Publishing Company. Amsterdam-Oxford-New York. 73-106
Arzul. I., Langlade. A, Chollet. B., Robert. M., Ferrand, S., Omnes. E., Lerond. S., Couraleau. Y., Joly. J.P., Francois. C., and Garcia. C., 2011. Can the Protozoan Parasite Bonamia ostreae Infect larvae of Flat Oysters Ostrea edulis ?. Veterinary Parasitology. In Press. 2011. http://archimer.ifremer.fr/doc/00028/13898/11061.pdf.
Barker. M. F. and D. Nichols (1983). Reproduction, recruitment and juvenile ecology of the starfish, Asterias rubens and Marthasterias glacialis. Journal of the Marine Biological Association of the United Kingdom, 63: 745-765 Barthelemy, G. 1991. Les Principaux Prédateurs et Compétiteurs de la
Conchyliculture. C.I.C.Section Régionale de Bretagne-Sud avec la collaboration Scientifique de la Station IFREMER de la Trinité/ Mer
Baud, J.-P, Gerard. A, and Naciri-Graven. Y. 1997. Comparative growth and mortality of Bonamia ostreae-resistant and wild flat oysters, Ostrea edulis, in an intensive system. I. First year of Experiment. Marine Biology. 130: 71-79
Beaudesson, P. 1992. Bibliographie Ocenebra erinacea. Trinite sur Mer
Bennet-Clark. H. C. 1976. Marine Mussels: Their Ecology And Physiology. International Biological Programme 10 Edited by B. L. BAYNE, Cambridge University Press, 1976. Pp. xvii+506. Exp Physiol.October 1976. 61(4):359
Bisker, R and Castagna M. 1987. Predation on Single Spat Oysters Crassostrea-virginica Gmelin by Blue Crabs Callinectes-sapidus Rathbun and Mud Crabs Panopeus-herbstii Milne-Edwards. Journal of Shellfish Research. 6 (1): 37-40
Brown, K.M., 1997. Size-spesific aspects of the foraging ecology of the southern oyster drill Stramonita haemastoma (Kool 1987). Journal of Experimental Marine Biology and Ecology. 214: 249-262.
Buestel D., 2007. History, Status and Future of Oyster Culture in France. Oysters Research Institute News. 20 (6):1-28.
Chandler, E. A., McDowell, J. R. and Graves, J. E. (2008), Genetically monomorphic invasive populations of the rapa whelk, Rapana venosa. Molecular Ecology, 17:4079–4091.
Capace.C., Guelorget.O., Vergne. Y. and Ouignard. J.P., 2007. Reproductive Biology Of The Common Eagle Ray Myliobatis Aquila (Chondrichthyes: Myliobatidae) From The Coast Of Languedoc (Southern France, Northern Mediterranean. Vie et Milieu. 57(3):125-130.
Christian. C., 1976. Etude du régime alimentaire de l'Aigle de mer Myliobatis aquila (L., 1758) des côtes tunisiennes. Oxford Journals. Life Sciences. ICES Journal of Marine Science. (1976) 37(1): 29-35
Choi, K.S. 2008. Oyster capture-based aquaculture in the Republic of Korea. In A. Lovatelli and P.F. Holthus (eds). Capture-based aquaculture. The Global overview. FAO Fisheries Technical Paper. No. 508. Rome, FAO. pp. 271– 286.
Crothers. J.H., 1968. The biology of the shore crab Carcinus maenas (L.). I. The background anatomy, growth and life history. Field Studies 2.3: 407–434. Culloty, S.C., Mulcahy, M.F., 1996. Season-, age-, and sex-related variation in the
prevalence of bonamiasis in flat oysters (Ostrea edulis L.) on the south coast of Ireland. Journal Aquaculture. 144: 53-63
Dare, P. J., Davies, G., & Edwards, D. B. (1983). Predation on juvenile Pacific oysters (Crassostrea gigas Thunberg) and mussels (Mytilus edulis L.) by shore crabs (Carcinus maenas (L.)). Fisheries Research Technical Report UK Ministry of Agriculture Fisheries Food Directorate of Fisheries Research. Directorate of Fisheries Research (Gt.Brit.).73: 1-15
Eggleston, D.E. 1990. Foraging Behavior Of The Blue Crab, Callinectes sapidus,
on Juvenile Oysters, Crassostrea virginica: Effects Of Prey Density And Size. Bulletin of Marine Science, 46(1): 62-82.
Eggleston, D.B. 1990 Behavioural Mechanism Underlying Variable Functional Responses of Blue Crabs, Callinectus sapidus Feeding on Juvenil Oyster, Crassostrea virginica. Journal of Animal Ecology. 59 (2): 615-630.
Faasse. M and Ligthart. M., 2009. American (Urosalpinx cinerea) and Japanese oyster drill (Ocinebrellus inornatus) (Gastropoda: MUricidae) flourish near shellfish culture plots in The Netherlands. Aquatic Invasion. 4(2): 321-326 FAO. 1999. The Living Marine Resources Of The Western Central Pacific.
59 Cerasi, S. In: FAO Fisheries and Aquaculture Department [online]. Rome. Updated 8 February 2005. [Cited 22 June 2011]
FAO, 2009. Crassostrea gigas. In Culture Aquatic Species Fact Sheets. Text by. Helm.M.M. Edited and compiled by Crespi and Michael.
Fleury P.G., Bouget J.F., Langlade A., Mazurier J., Philippon X., 2008. Bilan des études méthodologiques 2007 pour le projet d’analyse des risques de mortalité d’huîtres en Baie de Quiberon. Rapport Ifremer.
Frid, C. L. J. 1992. Foraging behaviour of the spiny starfish Marthasterias glacialis in lough Ine, Co. Cork. Journal Marine and Freshwater Behaviour and Physiology.19 (4): 227-239
Gallagher. T, Richardson A, Seed, R, and Jones. T., 2008. The seasonal Movement and Abundance of Starfish, Asterias rubens in Relation to Mussel Farming Practice: A Case Study from the Menai Strait, UK. Journal of Shellfish Research 27 (5):1209-1215
Garcia-Meunier P. (coordinateur), 2004. Etude d’une population invasive de bigorneaux perceurs (Ocinebrellus inornatus) dans le bassin de Marennes-Oléron: Etat des lieux, histoire de l’invasion et caractérisationi génétique. Compétition spatiale et trophique avec les taxons indigènes, Gestion du risque dans les écosystèmes conchylicoles. Rapport du Conseil Général de Charente Maritime, 86 pp.
Gera, S.M., 2009. Egg Capsule Hatching Success in Rapana venosa and
Urosalpinx cinerea in Relation to Temperature and Salinity. Thesis. The Faculty of the School of Marine Science. The College of William and Mary in Virginia.
Gollasch,S. 2009. Carcinus maenas (crustacean). Global Invasive Species Database. Ingo Narberhaus & IUCN/SSC Invasive Species Specialist Group (ISSG). http://www.issg.org/database/species/ ecology.asp? fr=1&si=114
Grangeré K., Caractérisation du comportement trophique d’Ocenera erinacea et
Handcock, D.A., 1954. The Destruction of Oyster Spat by Urosalpinx cinerea
(Say) on Essex Oyster Beds. J. Cons. Int Explor. Mer. 20 (2): 186-196. Handcock, D.A., 1955. The Feeding Behaviour of Starfish on Essex Oyster Beds.
J. Mar. boil. Ass. U.K. 34:313-331.
Handog, L.G. 1990. Oyster Culture. Selected Papers on Mollusc Culture. UNDP/FAO Regional Seafarming Development and Demonstration Project (RAS/90/002). National Inland Fisheries Institute. Kasetsart University Campus. Bangkhen, Bangkok. Thailand.
Harding, J.M., Kingley-Smith. P, Savini. D, and Mann. R., 2007. Comparison of Predation Signatures Left by atlantic Coast Oyster Drills (Urosalpinx cinerea Say, Muricidae) and veined rapa Whelks (Rapana venosa
Valencienciennes, Muricidae) in bivalve prey. Journal of Experimental Marine Biology and Ecology. 352 (1): 1-11
Hayward, PJ. and Ryland J.S., 1995. Handbook of the Marine Fauna of North-West Europe. Oxford University Press Inc., New York. United State.
Henderson, J and O'Neil, J. 2003. Economic Values Associated with Construction of Oyster Reefs by the Corps of Engineers. ERDC TN-EMRRP-ER-01.September 2003.
Hughes, R.N and Elner, R.W. 1979. Tactics of a Predator, Carcinus maenas, and Morphological Responses of the Prey, Nucella lapillus. Journal of Animal Ecology. 48(1):65-78
Jardas. I, Anti. M, And Pallaoro. A., 2004. Diet Composition Of The Eagle Ray,
Myliobatis Aquila (Chondrichthyes: Myliobatidae), In The Eastern Adriatic Sea. Cybium, 28(4): 372-374
Joly, J-P, Bouget. J-P and Hirata. T., 2002. Le gastropode predateur Rapana venosa. Point sur le connaisances et observation en Laboratoire. Direction des Ressources Vivantes- Departement Ressources Aquacoles. Laboratoire Conchylicole de Bretagne.
Karhan, S., Kalkan, U and Yokeş M.B., 2007. First record of the Atlantic starfish,
Asterias rubens Echinodermata: Asteroidea) from the Black Sea. JMBA2 - Biodiversity Records. Published on-line. 4 May 2007.
Kerckhof, F.; Vink, R.J.; Nieweg, D.C.; Post, J.J.N. (2006). The veined whelk
Rapana venosa has reached the North Sea Aquat. Invasions 1(1): 35-37 Khanna, D.R and Yadav,P.R. 2005. Biology of Echinodermata. Discovery
Publishing House. India.
Kroeck, M.A and Montes. J. 2005. Occurrence of the haemocyte parasite
Bonamia sp. in flat oysters Ostrea puelchana farmed in San Antonio Bay (Argentina). Disease of Aquatic Organisms. 63: 231-235
Landers, W.S., Rhodes, Jr. E.W., 1970. Some factors Influencing Predation by the Flatworm, Stylochus ellipticus (Girard), on Oysters. Chesapeake Science. 11 (1): 55-60.
Lavoie, R. E. 2005. Oyster culture in North America - history, present and future. The 1stInternational Oyster Symposium Proceedings, Oyster Research
61
Lovatelli A, 1988. Network of Aquaculture Centres in Asia, Status of oyster
culture in selected Asian countries.
http://www.fao.org/docrep/field/003/AB716E/AB716E00.htm#TOC
Manzi, J.J., 1970. Combined Effects of Salinity and Temperature on the Feeding, Reproductive, abd Survival Rates of Eupleura caudata (Say) and
Urosalpinx cinerea (Say) (Prosobranchia: Muricidae). Biological Bulletin. 138 (1): 35-46.
Marteil, L. 1976. La Conchyliculture Française. Deuxieme Partie. L’ostreiculture et La Mytiliculture. Institut Scientifique et Technique des Peches Maritimes. Nantes.
Marteil, L, Dardignac-Corbeil, M-J, Le Dantec, J, Raimbault, R, Hamin, P, Delreil, J-P, His, E, Gras, P, Berthome, J.-P, Razet, D, Grizel, H, Auger, Ch, and Kopp, J. 1979. La Conchyliculture Française. Troisieme Partie. L’ostreiculture et La Mytiliculture. Institut Scientifique et Technique des Peches Maritimes. Nantes.
Martin, C. 1978. Etude du Captage sur Tuiles du Naissain d’Ostrea edulis Linne en Fonction de Divers Parametres en Baie de Quiberon. Station Marine d’Endoume.
Menier, D. 2010. Cartographie Synthetique des Principeaux Faceus Sedimentaires Seperficiels de La Baie Quiberon et Baie Vilaine. Rapport pour le Conseil General du Morbihan. 41 p.
Menier D, Tessier B, Proust J.-N, Baltzer A, Sorrel P and Traini C., 2010. The Holocene Transgression as recorded by Incised-Valley Infilling in a Rocky Coast Context With Low Sediment Supply (Southern Brittany, Western France). Bull. Soc. Geol. Fr. 2010. 181 (2: 115-128)
Minchin. D.1987. Sea-water temperature and spawning behaviour in the seastar
Marthasterias glacialis. Journal Marine Biology. 95(1): 139-143.
Miossec. L, Le Deuff. RM and Goulletquer. P,. 2009. Alien Species Alert:
Crassostrea gigas (Pacific Oyster). ICES Cooperative Research Report No. 299. 42 pp.
Nawawi. M.Y. B.H. 1993. A Guide to Oyster Culture in Malaysia. Fisheries Research Institute, Department of Fisheries. Ministry of Agriculture, Penang, Malaysia. BAY OF BENGAL PROGRAMME, Madras, India. Newel, R.I.E, Kennedy, V.S and Shaw K.S., 2007. Comparative vulnerability to
predators, and induced defense responses, of eastern oysters Crassostrea virginica and non-native Crassostrea ariakensis oysters in Chesapeake Bay. Marine Biology. 152:449–460.
Papineau. C. 1978. Element de la Biologie D’Ocenebra erinacea. Aplication a la protection des cultures de Pectinides en rade de Brest. Raport de Stage Realise au Centre Oceanologique de Bretagne. DEA D’Oceanographie Biologique. Paris VI. Annee 1977-1978.
Perry. H., 2011. Carcinus maenas. USGS Nonindigenous Aquatic Species Database, Gainesville, FL. http://nas.er.usgs.gov/queries/factsheet.aspx? SpeciesID=190 RevisionDate: 4/25/2008
Pratt D.M, 1974. Attraction to Prey and Stimulus to Attack in the Predatory Gastropod Urosalpinx cinerea. Marine Biology. 27 (37:45).
Pauley, G.B, Van der Raay. B., and Troutt. D., 1988. Pacific Oyster. Species Profile: Life Histories and Enviromental Requirements of Coastal Fishes and Invertebrates (Pacific Northwest). Perform for Coastal Ecology Group and US. Department of the Interior. Fish and Wildlife Service. Biological Report 82(11.85). September 1988.
Penney. A.J. and Griffiths. C.L. 1984. Prey selection and the impact of the starfish
Marthasterias glacialis (L.) and other predators on the mussel
Choromytilus Meridionalis(Krauss). Journal of Experimental Marine Biology and Ecology. 75(1) :19-36.
Pigeot. J,Miramand. P, Garcia-Meunier. P, Guyot. T and Séguignes. M, 2000. Presence d’un nouveau predateur de l’huitre creuse, Ocinebrellus inornatus
(Récluz, 1851), dans le bassin conchylicole de Marennes-Oléron. Science de la vie/ Life Sciences. 323. 697-703.
Prince William Sound Regional Citizens “Advisory Council”. (2004). “Fact Sheet 3”. Non-indigenous aquatic species of concern for Alaska. Prince William Sound Regional Citizen Advisory Council. Retrieved 2011-01-09
Rodriguez. LF, 2009. Impact of Native predatory whelk on cultivated oyster (Crassostrea gigas) crops in San Quintin Bay, Mexico. Aquaculture Research. 40. 419-427.
Saier, B. 2001 Direct and indirect effects of seastars Asterias rubens on mussel beds (Mytilus edulis) in the Wadden Sea. Journal of Sea Research 46: 29-42.
Sauriau P.-G. 2002. Les bigorneaux perceurs des Pertuis Charentais : Élément de Biologie-Synthèse des moyens de lutte. Rapport de Contrat au Conseil General de Charente-Maritime et à l’Université de la Rochelle : 23 pp. Shelmerdine, R. L. and Leslie, B. (2009). Restocking of the native oyster, Ostrea
edulis, in Shetland: habitat identification study. Scottish Natural Heritage Commissioned Report No. 396
Savini.D, Occhipinti. A, and Ambrogi. 2006. Consumption Rates and Prey Preference of the Invasive Gastropod Rapana venosa in the Northern Adriatic Sea. Hegoland Marine Research. 60 (2):153-159.
63
Sola, L., A. Moretti, D. Crosetti, N. Karaiskou and A. Magoulas et al., 2006. Gilthead seabream Sparus aurata. Proceedings of the WP1 Workshop on Genetics of Domestication, Breeding and Enhancement of Performance of Fish and Shellfish. June 12-17, Viterbo, Italy, pp: 6-6.
Spencer BE,. 1990. Cultivation of Pasific Oyster. Lab. Leafl., Maff Direct. Fish. Res., Lowestoft, (63), 47 pp
Synder S and French N., 2006. Distribution of Asterias on a Rocky Shore: A Study at Moose Point State Park. Unity College Marine Biology Journal. 7: 4-7
Tun. T., 2001. Étude de cas de mortalité massive chez les huîtres perlières. L’huître perlière - Bulletin de la CPS n° 14 :17-20
Valentinčič.T., 1973. Food finding and stimuli to feeding in the sea star
Marthasterias glacialis. Netherlands Journal of Sea Research. 7th European
Symposium on Marine Biology.
Venables, W.N, Smith, D.M and the R Development Core Team. 2010. An Introduction to R. Note on R: A Programming Environtment for Data Analysis and Graphics Version 2.13.0(2011-04-13). R Development Core Team.
Verling E, Crook A.C, Barnes DKA and Harrison SSC., 2003. Structural dynamics of a sea-star (Marthasterias glacialis) population. Journal of the Marine Biological Association of the United Kingdom. 83: 583-592.
Villalobos. F. B, Tyler. P.A, and Young. C.M., 2006. Temperature and pressure tolerance of embryos and larvae of the Atlantic seastars Asterias rubens and
BAP-%BSP = Number dead Oysters (BAP) – ((Number life oysters in BAP +
Number dead Oysters in BAP) X Percentage dead oysters in BSP)
ABSTRACT
IKA MEIDY DEVIARNI. Biological and Economic Evaluation of The Impact of Predators on Oyster Mortality (A Case study in Quiberon Bay, France). Under the supervision of Prof. Dr. Ir. Fransiska R. Zakaria, M.Sc and Dr. Ir. Hartrisari Hardjomidjojo, DEA
Since the year of 2000 and especially 2006, the oyster mortality rates have
reached quite unusual, estimated at 60% mortality in a breeding cycle ending in
late 2006 in Quiberon Bay. This mortality brought a great impact on the France's
economy since oyster cultures are activities of collective small scale industry of
great importance economic. So, in this study, we observed the effects of oyster
mortality in terms of biology and economics value.
The impact of predators in Quiberon Bay France, was examined. The
objectives of this study are to analyse Risks in Shellfish Farming (RISCO) located
in Quiberon Bay data on oyster mortality, to determine effect of predators on
oyster culture, to map the distribution of different types of predators and to
calculate the economic analysis of oyster mortality.
We investigated the potential role of two species of starfishes, Asterias rubens and Marthasterias glacialis and two species of gastropods, Ocenebra erinacea and Ocenebrillus inornatus. Marthasterias glacialis indicated a great impact on mortality of adult oysters. The relation between oyster's mortality and
the presence of gastropods were time dependent and according to reproductive
cycle. Several months show clearly a predation of oysters and concentration of
gastropods for spawning. Predators on oyster culture also indicated a great impact
in economic value. The economic value on on oyster culture model applied at
RISCO project shows that BSP was higher than BAP. Economic loss in BAP
greatly happen in station 2 and 5, approximately €81.28 - €81.83. On the contrary,
station 15 indicate has the higher loss in BSP approximately €54.92 or 363 oysters
and for the rest, the economic value was varies between €29.67-€40.30.
BAP-%BSP = Number dead Oysters (BAP) – ((Number life oysters in BAP +
Number dead Oysters in BAP) X Percentage dead oysters in BSP)
1 Introduction
1.1 Background
Oysters have proved highly amenable to aquaculture, and today
exploitation of wild populations contributes little to worldwide oyster production.
In the United States, five species of oysters are cultivated on the Pacific Coast of
the United States : the native oyster Ostrea lurida, the Atlantic oyster Crassostrea
virginica, the Kumamoto oyster Crassostrea sikamea, the European flat oyster
Ostrea edulis, and the Pacific oyster Crassostrea gigas. The Pacific oyster is by far the dominant species, with a production level in the range of 5 million kg per
year. The Pacific oyster has been the most important cultivated oyster since 1977.
Beside in the Pacific Coast, Pacific oyster also well developed in Washington
State, Willapa Bay, Oakland Bay, and Samish Bay (Lavoie, 2005).
In Asia, oysters have been traditionally appreciated as seafood. Such as in Malaysia, they found on the market in fresh form, or as shucked meat, frozen
meat, dried or canned. Three genera of commercially important oysters are found
in Malaysia, Crassostrea, Saccostrea, and Ostrea. The genus Crassostrea
comprises of two species, C. iredalei and C. heicheri, whereas the genus Ostrea
has only one species (O. folium). C. heicheri, C. iredalei and Saccostrea spp. are
usually harvested in Malaysia for human consumption. The species most valued
for culture is C. iredalei (Nawawi, 1993).In Korea, the Pacific Oyster (C. gigas),
Korean kang-gul (C. rivularis), Korean pawit-gul (C. nippona), spiny oyster (C.
echinata) and the densely lamellated oyster (C. denselamellosa) are grown, however the Pacific oyster is the main species for commercial farming (Park et
al., 1988). On the other hand, in Indonesia, oysters are not famous. There aren’t
many oysters farming. Even though, we have Pinctada maxima in east Indonesia
(Tun, 2001).
In France, oyster culture began in the middle of the 19th century. The
indigenous species Ostrea edulis was replaced first with Crassostrea angulata,
then Crassostrea gigas. Right now the top producer and consumer of oysters are
in Europe, producing around 130 000 tons of the cupped oyster C. gigas annually and a remaining 1 500 tons of the flat oyster O. edulis (Buestel et al., 2009). Quiberon Bay has a large production of oyster, approximately 15 000 tons
annually which contributes to oyster production in France (Fleury et al., 2008).
There are 81 oyster-farmers (22% of companies in South Brittany) exploit 2643
ha of marine farming in deep water or 50% of the production area in the southern
Brittany. As raw material, oyster farmer sell the oyster directly to the consumer,
supermarket or export to other countries. More than 50% of the production of the
Bay is sold directly in Marennes-Oléron shippers.
Pacific oyster have been cultured in several country, as a result, C. gigas
has become the leading species in world culture, with an estimated production of
4.6 million tons in 2006. Because C. gigas does not require additional food to
sustain its growth, this species is relatively inexpensive and easy to produce. Its
capacity to adapt to various environmental conditions and temperature
fluctuations, coupled with its rapid growth and resistance to highly turbid areas,
contributes to its success (Miossec et al., 2009).
In oyster culture, there are several factors that could influence growth
and mortality the oyster, such as predator, disease, parasite, and environmental
conditions such as salinity, temperature, etc. This study focus on mortality which
is caused by the predator, which is regularly happened in oyster farming beside
other factors.
Since the year of 2000 and especially 2006, the oyster mortality rates (C.
gigas) have reached quite unusual number estimated at 60% mortality in a breeding cycle ending in late 2006, according to the response of 51 companies
interviewed by the Comité Régional de la Conchyliculture de Bretagne Sud
(CRC). This mortality brought a great impact on France's economy. So, in this
study, we observed the effects of oyster mortality in terms of biology and
economics.
RISCO-QB (Risks in shellfish farming, in Quiberon Bay) is a project
3
oyster culture in Quiberon Bay. RISCO is a “Pôle Mer Bretagne” project with a
collaboration between CRC (Comité Régional de la Conchyliculture de Bretagne
Sud), Ifremer La Trinite sur Mer and different partners including UBS Laboratory
Géoscience Marine et Géomorphologie du Littoral (G.M.G.L) Université de
Bretagne Sud, Vannes, Université de Nantes – Laboratoire LEMNA: Laboratoire
d’Économie et Management de Nantes, Cochet Environnement and CER du
Morbihan (Réseau nautile, Vannes)
1.2 Objectives
The objectives of this study are to analyse RISCO data on oyster mortality,
to determine effect of predators on oyster culture, to map the distribution of
different types of predators and to calculate the economic analysis of oyster
2.1 Oyster Culture
Oyster farming is an aquaculture (mariculture) practice in which oysters are raised for human consumption. Generally, there are three methods in oyster farming (Marteil et al., 1979):
1. On-bottom culture: shellfish or oyster are deposited on the ground or on bottom in the intertidal zone, or beyond the low tide of the seas in deep water.
2. Off-bottom culture: it is only practice on off- bottom at low tide. The oysters are placed in racks through pockets for example. All the oysters rest on racks or tables that keep it up of a few decimeters.
3. Suspended culture: the oysters are constantly immersed in the culture as in deep water, but it does not rest on the ground and are gathered into bins or pockets, bonded in the bars or ropes, etc. Suspended between the water surface and depths varying from floating or fixed facilities which is dominant in the sea. In figure 1, we can see methods of oyster culture.
Figure 1.Oyster culture
Commonly, in Asia there are two methods which have been done in oyster culture, there are (Webster, 2007):
5
1. The Floating raft, in example: China. They use bamboo to support the oysters while they are growing.
2. Stick culture, Sticks are placed vertically in soft mud bottom, catching spat from the water column.
In Indonesia, several methods of oyster culture have been experimented.
The raft or floating method was tested in Banten Bay, Java, in the early 1970's as well as the rack method in the estuarine waters of Pamanukan, Java. The stake and rock methods are also used. Oyster culture in Indonesia is largely experimental at present (Lovatelli, 1988).
In Malaysia, three culture methods are practiced. The raft or floating method is employed for oysters grown in riverine conditions where siltation is relatively heavy and tidal range is considerable. The pole and rack methods are intertidal fixed-culture methods used for both spat collection and grow-out. The bottom oyster culture method as it is practiced in the Muar River requires no special grow-out grounds(Lovatelli, 1988).
In China, several methods are being practiced: (1) Bottom method. The oyster C. rivularis is cultured mainly on the bottom of estuaries of low salinity, (2) Stake method with bamboo, (3) Stone-bridge method. This method is preferred for sandy muddy bottoms, (4) Raft method, and (5) Bottom method.
Choi (2008) found that in Korea, the culture techniques are bottom culture and long lines and rafts were introduced and the culture area subsequently expanded from the intertidal area to deeper waters offshore. There are 14 species of oysters that have been identified in Korean waters, although only Crassostrea
gigas is extensively used in oyster industry.
In Europe, there are several techniques in oyster culture: on-bottom
culture (5%) is done by the hanging oyster’s fixed on ropes or in baskets from special frames in the Mediterranean lagoons or on lines in the open sea. The Brittany coastline is highly varied with numerous favorable bays and estuaries, and it has a longstanding tradition of flat oyster culture. Oysters are cultivated in mesh bags (70%) or in deep waters (Buestel, et al. 2009). In the Bay of Quiberon,
deep water culture has increased, with 100 farms producing about 10,000 t per year from more than 2600 ha of concessions (Buestel, 2007).
In The United States, oysters can be grown either on or off the bottom of the ground. Additionally, off-bottom techniques are being developed in Maryland on the Chesapeake Bay involving floatation devices made from PVC frames on which these racks or bags can be placed (Webster, 2007).
2.2 Factors affecting the oyster mortality
Oyster culture is affected by factors like temperature and salinity, water circulation, the presence and condition of substrate, productivity of appropriate algal food, presence of predators and disease and protection from ice or storm that might damage culture facilities. The oyster farmers control many of these factors, thus enhancing larval and spat production in the hatcheries. Whether oyster cultivation begins in a hatchery or in nature, eventually small oysters are used in either on-bottom or off-bottom (suspended) culture (FAO, 2004).
7
1. Temperature
Pacific oysters live and grow in water with temperatures of 4 to 24OC, and
are able to survive air temperatures as low as -4OC when exposed by the tide.
Optimum temperature for water transport through adult oysters appears to be about 20OC (Pauley et al., 1988). The growth usually begins in April, reaching a
peak in July and August, declines to allow level by November and December (Spencer, 1990).
2. Salinity
Although oyster grow well at wide range of salinity, flat oyster prefer salinity in the higher levels near to 30 psu and pacific oyster prefer low levels near to 25 psu (Spencer, 1990). Pauley et al. (1988) observed that optimum salinity for maximum water transport through the body of C. gigas is 25-30 psu, and pacific oyster become increasingly sensitive to salinities below 20 psu.
3. Water circulation
Water circulation plays a paramount role in providing condition for feeding and cleansing, as well as for successful reproduction and for dispersal of oyster larvae. In the area less than15 m deep with unstratified waters and having moderately rapid water exchange, that areas provide good condition oyster 1988). Trays, particularly those with a small mesh, may quickly clog with silt.
This can smother the oyster or cause them to grow more slowly because of the poor exchange of water (Spencer, 1990).
5. Algal food
hour through its body cavity, depending on its size, sea temperature and other environmental and biology factors. Some microscopic algae produce toxins which accumulate in the flesh of mussel, oysters and clams. Shellfish or oysters containing the toxin can induce paralytic shellfish poisoning (PSP) in human who eat them (Spencer, 1990).
6. Predator and disease
Unprotected oysters and other small bivalves are eaten by various predators, especially drills, starfishes, crabs, fishes and to a lesser extent, by birds (Spencer, 1990). Introduction of Pacific oysters is not thought to have brought pathogens with them that have resulted to post-spawning physiological stress in warm water when oyster are densely crowded. However, their transport to some countries for direct relaying in the sea has been inadvertently accompanied by a number of pests and parasites including the Japanese oyster drill, Ocenebrillus
inornatus, the oyster flatworm, Pseudostylochus ostreophagus, and copepod
parasite, Mytilicola orientalis. Bacterially related diseases of larvae and early juveniles are not uncommon in hatcheries and are most frequently attributed to
Vibrio spp (FAO, 2009).
2.3 Oyster Culture in the Quiberon Bay
The French coastline, which is around 5500 km long, provide favorable environments for mollusk development, particularly oyster which have always been appreciated by the French. The coast of Brittany is highly varied with numerous bays and estuaries that favour cupped oyster culture. Such as in the Bay of Quiberon, there is large-scale development of cupped oyster cultivation on the bottom in deep water (Buestel et al., 2007).
9
2.4 Economic value of oyster culture
Oyster farming is an aquaculture (mariculture) practice in which oysters are raised for human consumption. Oyster habitat quality is affected by number of physical factors, such as water depth, dissolved oxygen, salinity, proximity to the other reefs and biological variables, such as the presence of living oysters on the
reef (Henderson and O'neil, 2003).
According to FAO (2011) the French aquaculture industry is an old and established sector, one of the first to develop among the EU countries; 243 907 tonnes were produced in 2004 placing it as the second highest producer in terms of volume in Europe. Marine production is dominated by molluscs; mainly oyster with 106 750 tonnes and mussels with 74 100 tonnes generating a gross income of about €600 million produced by the work of 20 000 people in 3 700 farms. Freshwater production is concentrated on trout with 36 611 tonnes produced by 500 farms, most of which produce less than 200 tonnes/year each.
The market for shellfish is essentially domestic; Pacific oysters are sold mainly on the French market but also in Italy (4 000 tonnes), Belgium (700 tonnes) and Germany (400 tonnes). France also imports (in 2004) oysters from Ireland (1 600 tonnes), Great Britain (400 tonnes) and Spain (300 tonnes). Consumption of oysters is mainly seasonal, with 70 percent of the yearly production marketed between November and January. The positive trade balance for shellfish produced in France about €12 million, France's trade balance for mussels meanwhile is negative with a net import worth about €60 million due to a large volume of imported product from Netherlands (17 100 tonnes), Ireland (13 000 tonnes), Spain (7 500 tonnes) as well as other European countries. The main export markets are Spain (2 500 tonnes), Germany (500 tonnes) and Italy (400
2.5 Bibliographical Synthesis of Biological Features of The Major Oyster
Predators
Even after many decades of study, predation remains one of the major hurdles to successful field marine culture of mollusks in many areas of the world. In the table 1 below, we have some of oyster’s predators.
Table 1. Oyster’s Predators of Crassostrea gigas
Predator Place Reference
Gastropod, Ocinebrellus inornatus Pacific-coast of North America in 1924 (Washington State), Coast of
Gastropod, Whelk, Macron trochlea San Quintin Bay, Mexico •Rodriguez, 2009
Gastopod, The veined whelk (Rapana
venosa) •Miossec et.al., 2009
Gastropod,Ocenebra erinacea Fal, Helford River, England •Spencer, 1990
Star fish or ‘’five fingers’’ (Astrerias
Flatworm, Pseudostylochus ostreophagus Alaska •Prince William Sound Regional Citizens “The Advisory Council”, (2004)
11
Ocinebrillus inornatus (http :// elrinconmarinosgasteropodosRapana venosa 2. iespana . es / Thaididae . htm , 2011)
Ocenebra erinacea Cancer productus(en.wikipedia.org, 2011)
Carcinus maenas
(http :// www . glaucus . org . uk / c - maenas . htm , 2011)
Asterias rubens (http :// www . scuba - equipmentTetractenos spp. - usa . com / marine / FEB 05/ , 2011)
Table 2. Oyster’s Predators of Crassostrea virginica
Predator Place Reference
Gastropod, oyster drill, Stramonita haemastoma (Kool 1987) In United state •Brown, 1997
Gastropod, oyster drills (Urosalpinx cinerea, and veined rapa
whelks (Rapana venosa) •Harding, et.al., 2007
Crustacea, Blue Crabs, Callinectes sapidus Virginia, USA •Eggleston, 1990a
•Eggleston, 1990b
Mud Crabs (Panopeus herbstii ) and Blue Crabs (Callinectus sapidus)
Mud crabs (Rhithropanopeus harrisii, Eurypanopeus depressus, Dyspanopeus sayi, and Panopeus herbstii), the blue crab Callinectes sapidus, and two sizes of polyclad Xatworms (Stylochus ellipticus and Euplana gracilis)
Chesapeake bay •Bisker and Castagna, 1987
•Newel, et.al., 2007
Flatworm, Stylochus ellipticus Connecticut •Landers and Rhodes, 1970
Stramonita haemastoma
13
Table 3.Oyster’s Predators of Ostrea edulis
Predator Place Reference
Gastropod, Murex erinacous In the Golf du Morbihan, France •Arin and Arin., 1976
Star fish or ‘’five fingers’’ (Astrerias
Fish, Myliobatis Aquila and the fish locally called “guele pavée” (Pagrus pagrus)
The Golf du Morbihan, France • Arin and Arin, 1976
The parasitic protozoans, Marteilia
Fungus, Ostracoblabe implexa. The Golf du Morbihan, France • Arin and Arin, 1976
Necora puber (http :// www . marlin . ac . uk / taxonomyidentification . php ?
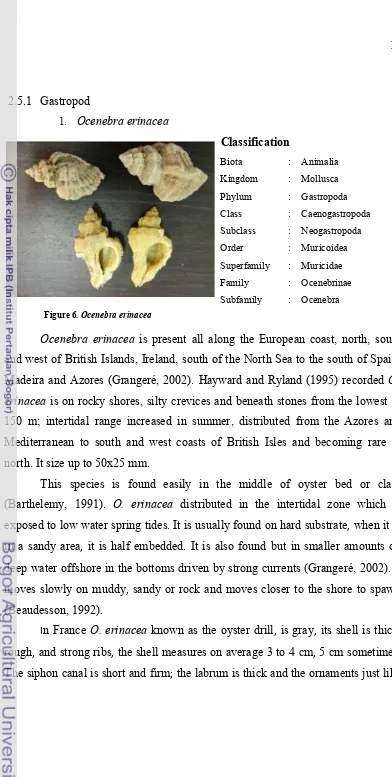
Figure 6. Ocenebra erinacea
and west of British Islands, Ireland, south of the North Sea to the south of Spain,
Madeira and Azores (Grangeré, 2002). Hayward and Ryland (1995) recorded O.
erinacea is on rocky shores, silty crevices and beneath stones from the lowest to
150 m; intertidal range increased in summer, distributed from the Azores and Mediterranean to south and west coasts of British Isles and becoming rare in north. It size up to 50x25 mm.
This species is found easily in the middle of oyster bed or clam (Barthelemy, 1991). O. erinacea distributed in the intertidal zone which is exposed to low water spring tides. It is usually found on hard substrate, when it is
in a sandy area, it is half embedded. It is also found but in smaller amounts on
deep water offshore in the bottoms driven by strong currents (Grangeré, 2002). It
moves slowly on muddy, sandy or rock and moves closer to the shore to spawn (Beaudesson, 1992).
In France O. erinacea known as the oyster drill, is gray, its shell is thick,
rough, and strong ribs, the shell measures on average 3 to 4 cm, 5 cm sometimes.
15
the rest of the shell. In the retracted position, a lid closes tightly the opening of the
shell. The animal may move slowly crawling over a flat part of the foot pedal sole.
Usually living at the limit of the low-tide, it has good resistance to prolonged
emersion (Marteil. et al, 1976).
At the reproduction level, almost inactive in winter, the oyster drill begins
his sexual activity from a temperature of 10o C, correspond to the Brittany coast to
the period from March to April. The sexes are separate (Barthelemy, 1991). The water temperature probably plays an important role in the reproduction of
Ocenebra erinacea. At the beginning of egg laying in 1977 and 1978 it was 9o C,
it also reported a temperature of 9o C at the beginning of spawning. Muricids
gastropods are known to lay eggs enclosed in capsules firmly bonded to
substrates. Of these capsules can emerge either planktonic larvae or juveniles with a morphology and behavior similar to those of adults (Sauriau, 2002).
The oyster drill need a hard substrate to lay the egg capsule. It is on the
rocks in the intertidal zone. There are also lying on shells. In deep water, the egg
capsules are laid on empty shells (scallops, slipper limpets, oysters) (Papineau, 1978).
O. erinacea has a single spawning peak in spring. During that time, it deposits most of its capsules (Garcia-Meunier, 2004). The larvae have planktonic phase extremely short, on average 2-3 days and a maximum of 5 days. The hatched larva has a morphology comprising a shell and velum (an organ which allow this species to swim in open water) and only in consequence of the loss of
the velum it becomes benthic (Sauriau, 2002). The oyster drill have proliferation. Barthelemy (1991) said that spawning may take several days; each female can deposit 30 to 40 capsules, each capsule containing 10 to 160 eggs. The females have a negative geotropism and tend to up out of 1 to 2 meters before lying. Its activity decreases gradually in autumn (Beaudesson, 1992).
In this species, females are slightly larger than males. The predatory
activity of O. erinacea is independent of production. It increases continuously from March until the summer, and then decreased in autumn (Grangeré, 2002). On
the other hand, the smaller prey seemed to be more attractive to borers, that is to say that they place with the small predator prey. They observed that the first attacks were quickly place on small prey (4 days) while on large prey they had to wait 14 days before observing predatory behavior. Behavior of the predators shows that both species behave differently. Indeed, the species O. erinacea has mainly attacked the oysters that present in the water intake (Grangeré, 2002).
2. Ocinebrellus inornatus
The muricid gastropod,
Ocinebrellus inornatus, was formely
17
South Japan. It was introduced along the Pacific coasts of North America in 1924 (Washington State). It was also present on the coasts of Colombia (1931), Oregon (1930-1934) and California (1941) (Pigeot, 2000). In 2007, this gastropod was found in Netherland (Faasse and Ligthart, 2009).
Like the European oyster drill Ocenebra erinacea, Ocinebrellus inornatus
is a carnivorous species preferentially feeding on bivalves and which may cause serious damage on cultivated oysters. Ocinebrellus inornatus differs from
Ocenebra erinacea by shell ornamentation. The last whorl of O. inornatus
presents only three to four varices at the surface of its otherwise smooth shell, whereas the local species displays five to seven small varices at the finely laminate surface of the shell. Also, the thin outer lip is less crenated in O.
inornatus than in O. erinacea. In France, O. inornatus lives in the same sites as O.
erinacea, with preferences for mediolittoral rocky shores in the middle of the bay
of Marennes-Oléron. At low tide, the adults live beneath flat stones. Their density reaches ten to twelve individuals per linear meter. At exceptionally favorable sites, as many as 800 individuals of O. inornatus were found per square meter (Pigeot, 2000).
The reproductive cycle of Ocinebrellus inornatus does not include any planktonic larval stage. Similar to Ocenebra erinacea, females produce 30–40 egg capsules each containing 10–15 larvae, which are benthic at hatching (Pigeot,
2000). This species possesses two peaks of spawning of smaller amplitude: first, in spring and second, in autumn. The development of the capsules is
approximately three weeks (Garcia-Meunier, 2004).
Numerous females oyster drills were observed during the breeding period, in autumn and winter. They live in the same habitat as adults, but preferentially in silty crevices of the stone. Adults of Ocinebrellus inornatus feed on the oyster
Crassostrea gigas by perforating its valve. In the bay of Marennes-Oléron, O.
inornatus seem very well fitted to its new environment and reached larger shell
2000). Sexual dimorphism was noted for females O. inornatus (Garcia-Meunier, 2004).
Sauriau (2002) showed that the development of embryos in their capsule
during the entire period of their development: the shell appears in the 5th week of
development and hatched (7th week in laboratory conditions), the juvenile has a
shell which the peristome has good shape but with the incomplete apex. The
embryo does not have a velum, an organ body which characterizes the planktonic larvae phase. The larva hatches so that the capsule cannot swim but has all the
characteristics of a future adult. Therefore a direct development and the hatching
larva lead a benthic life phase.
3. Comparison Ocenebra erinacea and Ocinebrillus inornatus
Grangeré (2002) showed the O. erinacea had a higher pressure of predation in the invasive species O. inornata. In fact, O. erinacea showed a clear preference on the smaller oysters (20g), instead of average (50g) and large (80g). Even though behavior of the predators shows that both species behave differently, the species O. inornata attacked on above and below the water intake (in the same proportions), so it seems that this species has a more opportunistic behavior.
O. erinacea moves closer to the shore to spawn and its predation activity
decreases gradually in autumn (Beaudesson, 1992). Ithas a single spawning peak in spring, during that time, it deposits most of its capsules and O. inornatus
possesses two peaks of spawning of smaller amplitude: first, in spring and second,
Figure 9. Urosalpinx cinerea recorded in this country (United State) on the oyster beds of the river Black-water, Essex. In America, Urosalpinx occurs in very large numbers from Cape Cod to Florida, and was carried more recently oysters to San Francisco. Urosalpinx has entirely replaced the native rough tingle, Ocenebra erinacea, it has spread and multiplied by cold winters (Handcock, 1954). Hayward and Ryland (1995) recorded that U. cinerea on the oyster, from lower intertidal to 12 m, hibernating in mud at 7o C or less, and it size up to 40 x 20 mm.
The oyster drill U. cinerea, a carnivorous gastropod native to the East coast of the North America, is an important predator of oysters and has been inadvertently introduced with Crassostrea virginica in Great Britain (Pratt, 1974). It is also exists in France, in the Arcachon basin and was discovered in Brest on oyster beds (Marteil, 1976). Urosalpinx drilled Balanus balanoides, B. eburneus, Crassostrea virginica, Crepidula fornicata, C. plana, Mercenaria mercenaria,
Modiolus demissus, Mya arenia, Mytilus edulis, Spisula solidissima, and Yoldia
limitula (Pratt, 1974).
Marteil (1976) showed that although Urosalpinx looks like Ocenebra
labrum less thick and ornamented. Its size is also smaller and rarely exceeds 3 cm. The size and shape of the egg cases are different from those of Ocenebra, the shorter incubation period (8-9 weeks against 12 to 13). Cole (1942) observed that females of Urosalpinx grow more quickly than males and reach a larger size.
Urosalpinx reaches a much greater average size in Britain than in its natural
habitat on the Atlantic coast of the U.S.A.
Urosalpinx has no free-swimming larval stage, the eggs being deposited in
capsules from which fully formed young tingles emerge. Spawning begins when the water temperature in its seasonal rise reaches 12° C. Adult females may deposit an average of 25 egg capsules at a single laying, but it is possible that further capsules are deposited later in the season. The bulk of the spawn is deposited in May and June on British beds, but a few freshly laid capsules may sometimes be found in August and September. The average period of incubation is about eight weeks. Young tingles usually begin to emerge early in July. The average number of young emerging from each capsule is 11–74 (Cole, 1942). Gera (2009) found that historically, U. cinerea was a pest to the oyster industry in the Chesapeake Bay; a single U. cinerea may consume in excess of 20 to 200 oysters in a season, depending on size (water temperature 19oC to 28oC; size range
of oysters not noted).
Manzi (1970) showed that the initiation and rate of egg capsule deposition appear to be greatly influenced by temperature, although other factors, such as food, availability of suitable substratum, and population density, may be contributory regulators of spawning intensity. Gera (2009) observed that the copulation occurs year round and egg capsule deposition occurs as water temperatures rise above 20oC in May through early November, with a brief pause
in egg capsule deposition observed in August. Each U. cinerea egg mass contains 1 to 22 egg capsules. Incubation U. cinerea of embryos ranges from 18 days (at 23oC to 29oC) to 78 days (at 15oC to 30oC). Sexual maturity is reached within the
21
5. Rapana venosa
Rapana whelk, Rapana venosa, is a native mollusk to the Sea of Japan, Yellow sea, Eastern China sea and the gulf of Bohai (Seyhan et al., 2003). Chandler et al (2008) observed that in the 1940s, Rapana venosa were discovered in the Black Sea. Additionally, in 1997 R. venosa were discovered in the Bay of Quiberon (Joly et al., 2002), in Chesapeake Bay (USA) in 1998, in the Rio de la Plata between Uruguay and Argentina in 2000, and Kerckhof et al. (2006) showed that the veined whelk R. venosa, was first recorded in July 2005 in the Dutch part of the North Sea, and in September 2005 in the central southern North Sea (the wider Thames estuary). It may exceed 160 mm in length (Harding et al. 2007)
Seyhan et al (2003) observed that feeding behavior of Rapana have been studied previously. Rapana prefer hard sand bottom habitats but will also invade hard substrate where food is abundant. They mainly feed on bivalve mollusks, but the preference of their prey depends on the diversity of the available prey items. For example preferred prey can vary from hard clams to oyster, soft clam and
mussel (Mytillus edulis). On average of 50 g Rapana in the Eastern Black Sea marine ecosystem consume 0.17-0.30 g mussel in a day. Savini et al. (2006) also showed an average consumption of about 1 bivalve prey per day (or 1.2 g wet weight per day). Predation was species and size selective towards small specimens of Indo-Pacific invasive clam. Anadara inaequivalvis; consumption of the two commercial species was lower.
R. venosa are highly fecund and their eggs hatch as planktonic veliger
larvae that can be carried in ballast water, characteristics that make them effective invasive species. R. venosa are dioecious and adult females lay large mats of egg cases from April through September. Each egg case contains approximately 100 to 3000 eggs, and a female can lay up to 500 egg cases in each mat. Additionally, females may produce over 10 different egg mats per year (Chandler et al, 2008).
Harding et al. (2007) observed that the number of embryos observed in egg cases produced by Chesapeake Bay Rapana whelks ranged from 123 embryos in a 7.4 mm high egg case to 3,673 embryos in a 33.5 mm high egg case. Rapa
Figure 11. Asterias rubens
Asterias rubens, widespread across the English Channel and the Atlantic
coast, is a well known predator of bivalves, particularly mussels from natural beds or livestock (Barthelemy, 1991). Karhan et al., (2007) observed that three specimens of Atlantic starfish, Asterias rubens are here reported as the first record from the Black Sea, after being first reported from the Bosphorus Strait in 1996. Typically found in edge of various beds of shells, the bottom sandy-muddy, but bivalves was investigated on a sand bottom in aquaria, a seasonal preference for
Abra alba and Spisula substruncata was noted (Allen, 1983). Saier (2001),
observed that A. rubens feeds opportunistically on a variety of items including slipper limpets, Crepidula fornicata, oysters, infaunal bivalves such as Spisula
occasionally ate spat and adult oyster, but almost always exhibited a preference for mussels and, in the absence of these, for Crepidula, and sometimes even for
Urosalpinx. Asterias rubens are migrating because of lack of food Gallagher et al.
(2008), to changes in the abundance and distribution of the slipper limpet
Crepidula fornicata, to aggregate and spawn in the spring (April to May) when
seawater temperatures are increasing. Increasing seawater temperature has been shown to be important in controlling spawning asteroids.
Bennet-Clark (1976) recorded large invasion of Asterias onto beds of mussels just above low-water mark in Morecambe Bay (England); starfish densities were up to 200-450 animals m-2, and the swarm, at one time, covered
2.25 ha of ground, and may have cleared up to high mussel cover or from deep water to shallow mussel beds during the summer,
when conditions were warm.
25
approximately 90 days for A. rubens. Growth of juvenile starfish was followed for 17 months. Juvenile starfish feed carnivorously at the completion of metamorphosis. Early growth is rapid; however, there is a reduction in the growth rate during winter months. (Valentinčič, 1973). Frid (1992) observed that the spiny starfish, Marthasterias
glacialis, is a common predator in the sub-littoral of Atlantic coasts.
A wide variety of prey consumed by Marthasterias glacialis. Small (<5 cm radius) individuals consumed algae, epifaunal turfs and small gastropods, while larger individuals consumed bivalves and carrion (Frid, 1992). Penney and Griffiths (1984) observed that the diet of the starfish,Marthasterias glacialis, consists of a variety of mollusc species, as well as ascidians and barnacles. Starfish densities are maximal where mussels, Choromytilus meridionalis
(Krauss), are abundant in such areas mussels form the bulk of the diet and indicate that Marthasterias glacialis select mussels of particular sizes. The length of prey
taken is an increasing function of predator arm length. Verling et al., (2003) recorded the larger M. glacialis (>100mm) exploited the variety of food resources