SUPLEMENTATION OF ANTIOXIDANTS IN RATIONS
CONTAINING RICH POLYUNSATURATED FATTY
ACIDS TO IMPROVE REPRODUCTIVE
STATUS OF LOCAL EWES
DEVIDE MARIC HERSADE
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGO
RSTATEMENT OF RESEARCH ORIGINALITY
I hereby declare that this thesis entitled “Supplementation of Antioxidants in Rations Containing Rich Polyunsaturated Fatty Acids (PUFA) to Improve Reproductive Status of Local Ewes” is really and true my idea under the guidance and direction of the supervising committee and has not been submitted in any form to any college or other universities. Sources of information which are derived or cited either form published or unpublished scientific papers from other writers have been mentioned in the script and listed in the Bibliography at the end part of this thesis.
Along with this, I delegate all right reserved of my papers to the Bogor Agricultural University
Bogor, August 2015
RINGKASAN
DEVIDE MARIC HERSADE. Suplementasi Antioksidan dalam Ransum Kaya Asam Lemak Tidak Jenuh untuk Meningkatkan Status Reproduksi Induk Domba Lokal. Dibimbing oleh DEWI APRI ASTUTI dan KOMANG GEDE WIRYAWAN.
Penelitian ini dirancang untuk mengevaluasi pengaruh dari suplementasi antioksidan dalam ransum tinggi asam lemak tidak jenuh sehingga dapat digunakan untuk meningkatkan status reproduksi induk domba lokal. Penelitian ini menggunakan Rancangan Acak Lengkap dengan tiga perlakuan dan lima ulangan. Perlakuan berdasarkan ada atau tidaknya suplementasi serta perbedaan antioksidan di dalam ransum. Perlakuan tanpa suplementasi antioksidan sebagai kontrol (CON), suplementasi antioksidan alami asal ekstrak ampas teh hitam (EATH), dan suplementasi antioksidan vitamin E sintetik (VES). Penelitian ini dibagi menjadi dua tahap yaitu prenatal (bunting) dan postnatal (setelah beranak hingga sapih). Peubah yang diamati adalah konsumsi nutrien induk, pertambahan bobot badan harian (PBBH) induk dan anak, performa reproduksi induk, status metabolit darah induk, status faal induk, malondialdehyde, dan kualitas susu. Data yang diperoleh diuji secara statistik dengan ANOVA dan perbedaan rataan antar perlakuan diuji lanjut Duncan.
Status faal induk pada prenatal dan postnatal tidak dipengaruhi oleh antioksidan. Suplementasi antioksidan tidak mengganggu palatabilitas induk pada prenatal dan postnatal. PBBH induk pada prenatal tidak dipengaruhi oleh perlakuan yang diberikan, sedangkan pada postnatal EATH dan VES memiliki PPBH induk dan anak yang lebih tinggi dibandingkan CON (P<0.05). Awal masa kebuntingan induk pada semua perlakuan memiliki jumlah embrio yang sama, tetapi setelah beranak, jumlah anak domba yang dilahirkan pada EATH lebih banyak dari CON, tetapi lebih sedikit dari VES. Perbedaan jumlah anak yang dilahirkan mengindikasikan adanya kehilangan embrio selama kebuntingan dimana CON paling banyak dan VES paling sedikit. Jumlah anak yang lebih banyak pada VES menyebabkan bobot lahir anak lebih rendah (P<0.05) dan tingkat kematian lebih tinggi dibandingkan perlakuan lain. Selama postnatal, jumlah anak yang mati pada EATH lebih sedikit dibandingkan CON, tetapi lebih banyak dibandingkan VES. Semua perlakuan memproduksi dominan anak domba jantan, tetapi CON paling banyak dan VES paling sedikit. Kadar kolesterol dan glukosa plasma induk EATH dan VES lebih tinggi dibandingkan CON pada prenatal dan postnatal (P<0.05). Suplementasi antioksidan pada ransum induk belum dapat menurunkan tingkat oksidasi di dalam tubuh induk selama prenatal (kebuntingan), sedangkan pada postnatal, EATH dan VES lebih rendah dibandingkan CON (P<0.05). Kandungan nutrien susu induk EATH dan VES lebih tinggi dibandingkan CON (P<0.05). Kesimpulan, ekstrak ampas teh hitam dapat digunakan sebagai antioksidan untuk menggantikan vitamin E sintetik pada ransum yang mengandung asam lemak tidak jenuh sehingga dapat meningkatkan status reproduksi induk domba lokal.
SUMMARY
DEVIDE MARIC HERSADE. Supplementation of Antioxidants in Rations Containing Rich Polyunsaturated Fatty Acids (PUFA) to Improve Reproductive Status of Local Ewes. Supervised by DEWI APRI ASTUTI and KOMANG GEDE WIRYAWAN.
This research was designed to evaluate the effect of antioxidants in high polyunsaturated fatty acids (PUFA) diets to improve reproductive status of ewes. The experiment used completely randomized design with three treatments and five replicates. The treatments based on ration with and without antioxidant (CON). Different types of antioxidants, natural antioxidant form black tea waste extract (BTWE) and commercial vitamin E synthetic (VES). This research was divided into two phases: namely prenatal (pregnancy) and postnatal (after birth until weaning). Variables observed were dry matter intake (DMI) of ewes, average daily gain (ADG) of ewes and lambs, reproductive performance, blood metabolites of ewes, physiological status of ewes, malondialdehyde, and milk quality. Data were tested using Analysis of Variance (ANOVA) and the different among treatment means were examined using Duncan Multiple Range Test.
Physiological status of ewes were not affected by antioxidant treatments. DMI in prenatal and postnatal were not affected by treatments. ADG of ewes in prenatal were not affected by treatments, whereas in postnatal, both antioxidant treatments (BTWE and VES) had higher ADG on ewes and lambs compared with CON (P<0.05). During pregnant, all treatments had the same number of embryos, whereas in postnatal, litter size in BTWE was higher than CON and lower than VES. The results indicate that percentage loss of embryos in CON was the highest and VES was the lowest. High litter size at VES had caused lower average birth weight (P<0.05) and higher mortality at birth than the other treatments. After weaning, mortality of BTWE was lower than CON and higher than VES. All treatments produced male dominant of lambs, but CON was the most and VES was the least. Both antioxidant treatments resulted in glucose and cholesterol levels higher than CON during the prenatal and postnatal (P<0.05). In prenatal, both antioxidant treatments were not able to reduce level of oxidation in the body of ewes (as: malondialdehyde parameter), whereas in postnatal, both antioxidant treatments were lower compared to CON (P<0.05), so that ewes with antioxidant treatments had lower level stress than control. Both antioxidant treatments had better milk quality than CON (P<0.05). In conclusion, black tea waste extract could be used as antioxidant to replace vitamin E synthetic in high PUFA rations to improve reproductive status of local ewes.
Copyright@2015, Bogor Agricultural University
All Right Reserved
It is prohibited to cite all or part of this thesis without referring to and mentioning the source. Citation only permitted for the sake of education, research, scientific writing, report writing, critical writing or reviewing scientific problems. Citation does not inflict the name and honour of Bogor Agricultural University.
Thesis
as one requisite to obtain a degree Magister Sains
at
Study Program of Nutrition and Feed Science
SUPLEMENTATION OF ANTIOXIDANTS IN RATIONS
CONTAINING RICH POLYUNSATURATED FATTY
ACIDS TO IMPROVE REPRODUCTIVE
STATUS OF LOCAL EWES
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2015
2:
FOREWORD
There are no words higher than the noblest benediction of gratitude for all things that I have received from HIS generosity during my life especially during the implementations of my thesis entitled “Supplementation of Antioxidants in Rations Containing Rich Polyunsaturated Fatty Acids (PUFA) to Improve Reproductive Status of Local Ewes” as one requisite to obtain a Magister Sains (M.Si) degree at Study Program of Nutrition and Feed Science. The results of research has been accepted to publish at Pakistan Journal of Nutrition in 2015 with the same title with my thesis. Also, I expressed my gratitude to Prof Dr Ir Dewi Apri Astuti, MS as my supervisor and Prof Dr Ir Komang Gede Wiryawan as my co-supervisor for giving a lots of advanced advice, guidance, time, energy, and thought to this thesis so that it can be done well. I would like also to express my thanks to Dr Ir Dwierra Evvyernie MS MSc and Prof Dr Ir Yuli Retnaini MS as chairman and vice-chairman of Nutrition and Feed Science, Dr Ir Idat Galih Permana, MScAgr as an examiner of close exam whom give a lots of constructive criticism and useful suggestion during my close exam.
I would like also to express my thanks to my best team of research (Alfi, Ridha, Riri, Dinar, Santa, Hana, Fandi, Lutfi, Asta, Febrina, Mr Asep, Mr Sugi, Mr Darmawan) for giving time, good collaboration, teamwork, comradeship and a lot of help to the success of this research. My very special gratitude also goes to Mr Supri, Mrs Ade, Mr Yulianri, Mr Teguh, Mr Tekad, Mrs Kokom, Ms Ochi, Ms Dila, Mr Andre, Ms Nur, Mr Widhi, Ms Ella, Mr Donald, Ms Wenny, and Ms Silvi for all the prayer and support. My gratitude also goes to my graduate classmate from generation 2012, 2013, 2014, Gita Swara Pascasarjana (GSP), Perwira 77’s board, AADGC’s board, and to all of them whose names are not mentioned here for all the prayer and support.
Infinite gratitude goes to my beloved parents Mr Herman Soehardjo and Mrs Sari Dewi, my younger brother Jonathan Julian Hersade, Ricky Septian Hersade, and Rully Anugrah Supratama for all that has been given to me so that the thesis was completed. The authors thanks not only to Directorate General of Higher Education of Indonesia for Beasiswa Fresh Graduate, but also Dr. Ir. Lilis Khotijah, MSi and Dilla Mareistia Fassah, SPt, MSc for the project of my thesis.
Author has been working maximally in completion process of this thesis, but author realizes that it might be still there the shortage. Therfore, constructive (suggestion and critictism) from the reader are gladly accepted. Hopefully this thesis can be usefull for all of us and for development of animal science and knowledge.
Bogor, August 2015
TABLE OF CONTENTS
LIST OF TABLE xvii
LIST OF FIGURE xvii
LIST OF APPENDIX xix
INTRODUCTION
Background 1
Purpose of the Research 2
Scope and Limitation of Study 2
RESEARCH METHODOLOGY
Date and Location 2
Materials
Ewes and Ration of Research 3
Methods
Maintenance Procedure 4
Antioxidant Preparations 4
Measurement of Antioxidant Activity 5
Temperature and Humidity 5
Measurement of Physiological Status of Ewes 5
Nutrient Intake Calculation of Ewes 5
Average Daily Gain and Feed Efficiency of Ewes and Lambs 6 Evaluation of Reproductive Performance of Ewes 6
Blood Metabolites of Ewes 6
Milk Composition of Ewes 7
Data Analysis 7
RESULTS AND DISCUSSION
Temperature and Humidity 8
Physiological Status of Ewes 9
Dry Matter Intake and Average Daily Gain of Ewes 10
Reproductive Performance of Ewes 14
Blood Metabolites of Ewes 16
Milk Composition of Ewes 19
CONCLUSION 20
SUGGESTION 20
REFERENCES 20
APPENDIX 26
LIST OF TABLE
1. Ration formulation, nutrient composition, and antioxidant activity 3 2. The average of daily ambient temperature and relative humidity 8
3. Physiological status of ewes 10
4. Nutrients intake and performance of ewes 12
5. Reproductive performance of ewes 14
6. Milk composition of ewes 19
LIST OF FIGURE
1. Time schedule of research 4
2. Ambient temperature and relative humidity. 8
3. Dry matter intake of ewes. 13
4. Cholesterol levels ewe’s of plasma. 17
5. Glucose levels of ewe’s plasma. 17
LIST OF APPENDIX
Appendix 1. ANOVA of Respiration Rate in Prenatal 26 Appendix 2. ANOVA of Respiration Rate in Postnatal 26
Appendix 3. ANOVA of Pulse Rate in Prenatal 26
Appendix 4. ANOVA of Pulse Rate in Postnatal 26
Appendix 47. ANOVA of Glucose Levels of Plasma in ending postnatal 32 2Appendix 48. DMRT of Glucose Levels of Plasma in ending postnatal 32 Appendix 49. ANOVA of Glucose Levels of Plasma in weaning 32 Appendix 50. DMRT of Glucose Levels of Plasma in weaning 32 Appendix 51. ANOVA of Malondialdehyde of Plasma in prenatal 32 Appendix 52. ANOVA of Malondialdehyde of Plasma in postnatal 32 Appendix 53. DMRT of Malondialdehyde of Plasma in postnatal 32 Appendix 54. ANOVA of Malondialdehyde of Plasma in weaning 33 Appendix 55. DMRT of Malondialdehyde of Plasma in weaning 33
Appendix 56. ANOVA of Total Solid of Milk 33
Appendix 57. DMRT of Total Solid of Milk 33
Appendix 58. ANOVA of Fat of Milk 33
Appendix 59. DMRT of Fat of Milk 33
Appendix 60. ANOVA of Protein of Milk 33
Appendix 61. ANOVA of Lactose of Milk 34
1
INTRODUCTION
Background
Sheep breeding programs is upstream sector in sheep farming business. Sheep breeding programs managed properly has potential for sustainability of the sheep population, increasing the number of lambs and reproductive performance of ewes, and economic advantages for livestock sector. Economic advantages in sheep breeding programs should be managed in the proper management (Grau-Sologestoa 2015). Reproductive status of ewes such as reproductive performance, body weight, blood metabolite status, and milk quality become the determining factors in sheep breeding programs.
Local sheep in Indonesia have good reproductive status genetically (Udo and Budisatria 2011). Reproductive status is not only influenced by genetics but also nutrients that were consumed by the ewes. Nutrients consumed affect the reproductive status of ewes. NRC (2007) recommends the ewe’s energy on late pregnancy is 1.5 times higher than maintenance. Nutrients consumption under the prerequisite requirements for reproducing ewes caused disruption of reproductive hormone status (Tsiplakou et al. 2015), disruption the quality and quantity of oocyte (Abecia et al. 2014), disruption of fetal growth (Field et al. 2015), reduced fetal adiposity by late pregancy (Wallace et al. 2015), decreasing birth weight and organ size of lambs (Dunford et al. 2014), decreasing postnatal growth and development of lambs (Williams-Wyss et al. 2014), and affect the milk yield and composition (Pulina et al. 2012)
Polyunsaturated fatty acid (PUFA) is one of nutrients that is essential for ewes in reproductive phase. Effect of PUFA for ewes in prenatal can accelerate oocyte maturation and embryos development (Gaffarillaleh et al. 2014), increase the percentage of pregnancy (El-Nour et al. 2012), increase the number of embryos (Shahneh et al. 2008), and decrease the risk of death the embryo (Mattos et al. 2002). Effect of PUFA for ewes in postnatal can accelerate lamb’s ability for milking so it had better IgG (Capper et al. 2006), increase body weight of lambs and affect the milk yield and composition (Ferreira et al. 2014), and increase cholesterol in plasma that can be used as precursor for steriod hormone (Akbarinejad et al. 2014). PUFA can be derived from vegetable oils such as sunflower oil. Sunflower oil contains 51.2% linoleic acid and 38.23% oleic acid (Ali et al. 2014).
PUFA consumed by sheep will undergo process of biohydrogenation by rumen microbes and it becomes saturated fatty acids (SFA) by 80-92% (Chilliard et al. 2000), so it should be protected. The main fatty acid substrate for biohydrogenation in animals that supplemented with dietary lipids, Linoleic Acid (LA) in the form of triacylglycerols will usually be the main substrate for biohydrogenation. Linoleic Acid (LA) metabolism in the rumen involves the transient formation of Conjugated Linoleic Acid (CLA), mainly cis-9, trans-11-18:2 or rumenic acid, which is then converted to Vaccenic Acid (VA), and finally stearic acid (Saturated Fatty Acids/SFA) (Lourenco et al. 2010).
2
unprotected (Gobert et al. 2009). Antioxidants were classified into two types such as natural and synthetic. Natural antioxidant such as black tea was safe to be used because it is derived from plant extracts (Yanishlleva et al. 2006). Tea is second favorite beverage and consumed 2/3 people in the world (Bhuyan et al. 2009). Theaflavins content in black tea waste possess antioxidant properties. (Bhuyan et al. 2013). Theaflavins have more hydroxyl groups, which are considered to be necessary for exerting scavenging activity, inhibit free radical generation, and chelate transition metal ions (antioxidative properties) (Lucjaz and Skrzydlewska, 2005). Black tea waste extract as a natural antioxidant in ewes can accelerate rate of oocyte maturation (Barakat et al. 2014) and prevent narrowing of the channel fetal sheep (Bubols et al. 2014). Vitamin E synthetic has been widely produced by factories in Indonesia. Vitamin E is able to capture free radicals and break the chain of processes in the membrane lipid peroxidation by donating a hydrogen atom of an OH group on the ring to free radicals, thus forming free radicals vitamin E more stable and not spoil (Gonzalez-Calvo et al. 2014). Vitamin E as a synthetic antioxidant in ewes can affect fatty acid composition and reduce fat oxidation in sheep milk (Gallardo et al. 2015) and reduce blood level of malondialdehyde (Mohebbi-Fani et al. 2012). Black tea waste extract as natural antioxidant can prevent bio hydrogenation of the polyunsaturated fatty acids (PUFA) in the rumen, but this information is very rare in improving the reproductive status of ewes and then compared with synthetic vitamin E as a positive control.
Purpose of the Research
Through this research was designed to evaluate the effect of antioxidant (black tea waste extract and vitamin E synthetic) in high polyunsaturated fatty acids (PUFA) diets to improve reproductive status of ewes.
Scope and Limitation of the Study
This research focussed on 500 ppm supplementation of natural antioxidant from black tea waste extract and commercial vitamin E synthetic (Lutavit® E50, BASF, Netherland) in ration of ewes and its relation to reproductive status of ewes in prenatal (during pregnancy) and postnatal (birth until weaning) for tropical area.
RESEARCH METHODOLOGY
Date and Location
3
Materials
Ewes and Ration of Research
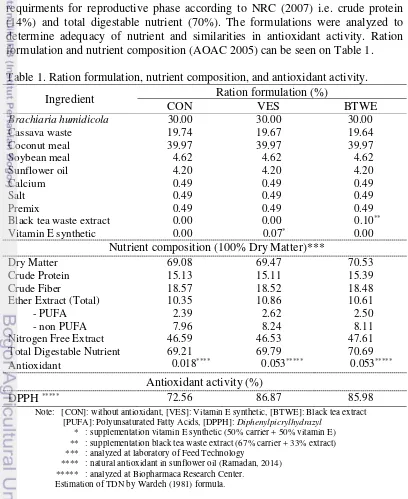
In this research, fifteen local ewes (31.83±0.29 kg of BW) randomly allocated to three treatments. Each treatments had five replications. Three treatments in this research were ration without antioxidant as control (CON), ration with 500 ppm natural antioxidant from black tea waste extract (BTWE), and ration with 500 ppm commercial synthetic vitamin E (VES). According to Gobert et al. (2009), 500 ppm of vitamin E and tea extract can optimally protect of unsaturated fatty acids in rumen, so that the blood plasma has 8% higher PUFA compared to without antioxidant. Ration formulation in this research refers to recommendation of ewe requirments for reproductive phase according to NRC (2007) i.e. crude protein (14%) and total digestable nutrient (70%). The formulations were analyzed to determine adequacy of nutrient and similarities in antioxidant activity. Ration formulation and nutrient composition (AOAC 2005) can be seen on Table 1. Table 1. Ration formulation, nutrient composition, and antioxidant activity.
Ingredient Ration formulation (%)
Nitrogen Free Extract 46.59 46.53 47.61
Total Digestable Nutrient 69.21 69.79 70.69
Antioxidant 0.018**** 0.053***** 0.053*****
Antioxidant activity (%)
DPPH ***** 72.56 86.87 85.98
Note: [CON]: without antioxidant, [VES]: Vitamin E synthetic, [BTWE]: Black tea extract [PUFA]: Polyunsaturated Fatty Acids, [DPPH]: Diphenylpicrylhydrazyl
* : supplementation vitamin E synthetic (50% carrier + 50% vitamin E) ** : supplementation black tea waste extract (67% carrier + 33% extract) *** : analyzed at laboratory of Feed Technology
**** : natural antioxidant in sunflower oil (Ramadan, 2014) ***** : analyzed at Biopharmaca Research Center.
4
Methods
Maintenance Procedure
During the research, ewes were kept in 3 x 1.5 m2 individual cages with two
boxes for ration (concentrate and forage) and a bucket of water. Ration was given 3 times a day (07.00am, 12.00am, and 05.00pm) and water was given ad libitum. Time schedule of research can be seen at Figure 1. In adaptation phase, ewes mated naturally and all of ewes were not given antioxidant (only PUFA). After adaptation phase, evaluation was divided into two phases (prenatal and postnatal) for seven months. Variables observed in prenatal were dry matter intake (DMI), average daily gain (ADG), number of embryos, blood metabolites of ewes, and physiological status. Variable observed in postnatal were DMI of ewes, ADG of ewes and lambs, reproductive performance, blood metabolites of ewes, milk quality, and physiological status.
Time schedule: A. Pregnancy status examination and number of embryos (USG) B. Nutrient intake calculation of ewes
C. Weighing of ewes
D. Evaluation of reproductive performance E. Weighing of lambs
F. Blood glucose and cholesterol sampling G.Malondialdehyde sampling
H. Milk quality evaluation
I. Measurement of physiological status
Fig. 1. Time schedule of research Antioxidant Preparations
Vitamin E as a synthetic antioxidant obtained commercially on the market (Lutavit® E50, BASF, Netherland). Vitamin E synthetic consists of 50% pure vitamin E and 50% carrier.
Black tea as natural antioxidant derived from Research Center Tea and Quinine Gambung, Bandung, West Java, Indonesia. Black tea waste was dried, milled, screened (100 mesh), and stored in an airtight bag. Black tea waste powder was dissolved in ethanol (ratio 1:2) for 24 hours and stirred every eight hours (Rusak et al., 2008). The mixture was filtered using filter paper and then evaporated by vacuum evaporator to obtain black tea waste extract. Black tea waste extract was mixed with cassava flour as a carrier (ratio 1:2).
I
5
Measurement of Antioxidant Activity
Measurement of antioxidant activity was done before the antioxidant treatments was began. An antioxidant activity was analyzed at Biopharmaca Research Center (Bogor Agricultural University, Indonesia). Measurement of antioxidant activity using diphenylpicrylhydrazyl (DPPH) method according to Molyneux (2004). The 1.5 ml of antioxidant solution was prepared in 50 mM phosphate buffer (pH 7.5) and mixed with 1.5 ml of 100 µM methanolic solution of DPPH and kept at room temperature for 30 min. The decrease in absorbance was monitored at 515 nm. DPPH radical scavening capacity was calculated using following equation:
DPPH scavening effects (%) = A -A
A x
Ao and A1 correspond to the absorbances at 515 nm of the radical (DPPH)
in the absence and presence of antioxidant respectively. Temperature and Humidity
Temperature and humidity were recorded on prenatal and postnatal using a digital thermometer at 07.00am, 12.00am, and 05.00pm everyday (July 2013-January 2014).
Measurement of Physiological Status of Ewes
Physiological status observed were respiration rate, pulse rate, and rectal temperature which were recorded at 07.00am, 12.00am, and 05.00pm every week in prenatal and postnatal. Respiration rate was recorded by calculating the inhalations and exhalations during 60s through the nose respiration. The pulse rate was recorded by a stethoscope that was placed on the left side of the thorax for 60s (systole and diastole count in 1 rate). Rectal temperature was measured by a digital thermometer that was placed in rectal until the temperature on the thermometer became stable.
Nutrient Intake Calculation of Ewes
The feed consumption based on dry matter (dry matter intake) was calculated from differences between feed offered and remaining feed then coverted into dry matter. Evaluation of feeding was done everyday on prenatal and postnatal.
Dry matter intake (g/h/d) = feed offered-remaining feed x dry matter Nutrients intake evaluated were crude protein (CP), crude fiber (CF), ether extract (EE), Total Digestible Nutrient (TDN), Nitrogen Free Extract, and antioxidant. Nutrient intake was calculated from the percentage value of nutrient content within ration multiplied by dry matter intake.
Nutrient intake (g/h/d) = nutrient content of feed
6
Average Daily Gain (ADG) and Feed Efficiency of Ewes or Lambs Calculation
Body weight was obtained by weighing the whole ewes or lambs of each treatment every week in prenatal and postnatal. Average daily gain (g/h/d) was obtained from calculation of the differences in body weight (before and after) divided by 7 days.
Average daily gain (g/h/d) = body weight week n – body weight week
n-7
Feed efficiency was used to evaluate the effectiveness of ration to produce better body weight during the research.
Feed efficiency= average daily gain
dry matter intake/day
Evaluation of Reproductive Performances of Ewes
Number of embryos was calculated by ultrasonography (USG) at the beginning of prenatal. Reproductive performances in postnatal were litter size, percentage loss of embryos, type of birth, sex ratio of lambs, birth weight (average and total), average daily gain (ADG) of lambs, weaning weight (average and total), mortality at birth and mortality before weaning.
Blood Metabolites of Ewes
Blood Sampling
Blood sample was taken by using a 5 ml syringe from jugular vein and immediately inserted into tubes containing EDTA. Blood sample was centrifuged for 15 minutes at 3000 rpm to obtain the plasma. Plasma was used to analyze glucose, cholesterol, and malondialdehyde (MDA).
Analysis of Plasma Glucose
Glucose was analyzed using the Glucose kit® (Cat. No. 112 101, reg. No. AKL 20,101,803,460). Ten µl of plasma were transferred into test tube using micropipette, then 1000 µl of the glucose reagen kit were added and stirred with vortex (10s) and incubated for 10 min at 25oC. The same method was done to
prepare mixture of blank and standard of glucose (sample of plasma was replaced with aquadest for blank and standard kit for glucose). The mixtures was analyzed by using the spectrophotometer UV-Vis Genesis® 10S with wavelength 500nm. The data shown were the absorbance value of each sample and standard. Glucose levels was calculated by formula:
7
Analysis of Plasma Cholesterol
Cholesterol was analyzed using the Cholesterol kit® (Cat. No. 101 592, reg. No. AKL 10,101,803,466). Ten µl of plasma were transferred into test tube using micropipette, then 1000 µl of the cholesterol reagen kit were added and stirred with vortex (10s) and incubated for 10 min at 25oC. The same method was done to
prepare mixture of blank and standard of cholesterol (sample of plasma was replaced with aquadest for blank and standard kit for cholesterol). The mixtures were analyzed by using the spectrophotometer UV-Vis Genesis® 10S with wavelength 500nm. The data shown were the absorbance value of each sample and standard. Cholesterol levels was calculated by formula:
Plasma cholesterol (mg/dL) = absorbance sample absorbance standard x
Analysis of Malondialdehyde
Blood sample for analysis of malondialdehyde was taken at the end of prenatal, during postnatal, and weaning. Malondialdehyde was measured by using the method of TBARS (Rice-Evans et al. 1991). Briefly, 100 µl of pancreatic homogenates or malondialdehyde (MDA) standard were pippeted into test tubes containing 1.5 ml of 20% (w/v) glacial acetic acid (pH 3.5), 200 µl of 8.1% (w/v) sodium dodecyl sulfate SDS, 1.5 ml of 0.8% (w/v) Thiobarbituric acid TBA and 700 µl of distilled water. The test tubes were incubated at 95oC for 60 min with a
marble on the top of each test tube. After incubation, the testtubes were cooled and then centrifuged at 3000 rpm for 10 min and amount of MDA formed was spectrophotometrically measured at 532 nm.
Malondialdehyde (mg100/mL) = x 100
Milk Composition of Ewes
Milk compositions were total solids, fat, lactose, and protein. Milk sample was taken by milking manually two times a day during postnatal and analyzed by using a milk analyzer (Master Pro Milkotester®) at laboratory of Dairy Nutrition (Bogor Agricultural University, Indonesia).
Data analysis
8
RESULTS AND DISCUSSION
Temperature and Humidity
Thermal comfort is quantified as the thermal neutral zone. The optimal thermal environment promotes maximum performance and provides the least stress for the ewes. High ambient temperature, high direct and indirect solar radiation and humidity are environmental stressing factors that impose strain on animals (Etim et al. 2014). The thermal comfort zone for ruminants is between 18oC and 25oC (Marai et al. 2007) and relative humidity is lower than 50% (Etim et al. 2013). Climate in Bogor was classified as tropical climate type A. Ambient temperature in Bogor ranges between 25-35oC and relative humidity ranges between 45-85%. When temperature exceed 25oC and relative humidity is higher than 50%, ruminants will suffer heat stress.
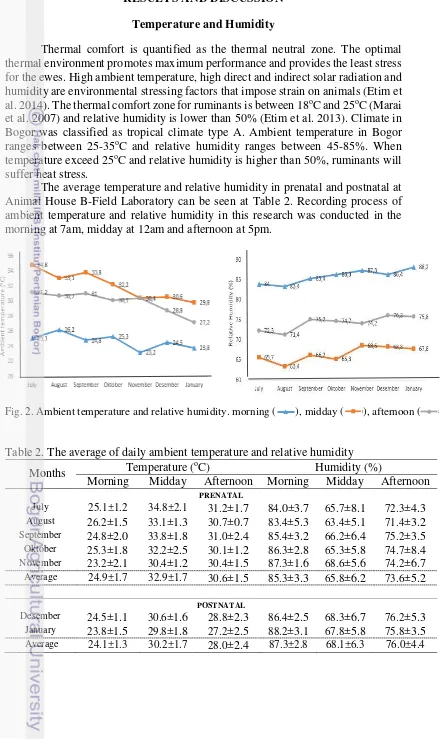
The average temperature and relative humidity in prenatal and postnatal at Animal House B-Field Laboratory can be seen at Table 2. Recording process of ambient temperature and relative humidity in this research was conducted in the morning at 7am, midday at 12am and afternoon at 5pm.
Fig. 2. Ambient temperature and relative humidity. morning ( ), midday ( ), afternoon ( )
Table 2. The average of daily ambient temperature and relative humidity
Months Temperature (
oC) Humidity (%)
Morning Midday Afternoon Morning Midday Afternoon
PRENATAL
July 25.1±1.2 34.8±2.1 31.2±1.7 84.0±3.7 65.7±8.1 72.3±4.3
August 26.2±1.5 33.1±1.3 30.7±0.7 83.4±5.3 63.4±5.1 71.4±3.2
September 24.8±2.0 33.8±1.8 31.0±2.4 85.4±3.2 66.2±6.4 75.2±3.5
Oktober 25.3±1.8 32.2±2.5 30.1±1.2 86.3±2.8 65.3±5.8 74.7±8.4
November 23.2±2.1 30.4±1.2 30.4±1.5 87.3±1.6 68.6±5.6 74.2±6.7
Average 24.9±1.7 32.9±1.7 30.6±1.5 85.3±3.3 65.8±6.2 73.6±5.2
POSTNATAL
Desember 24.5±1.1 30.6±1.6 28.8±2.3 86.4±2.5 68.3±6.7 76.2±5.3
January 23.8±1.5 29.8±1.8 27.2±2.5 88.2±3.1 67.8±5.8 75.8±3.5
9
In general, the average ambient temperature and relative humidity relatively the same from prenatal (July - November 2013) until postnatal (Desember 2013 – January 2014) (Fig. 2). In the morning of prenatal periode, the average ambient temperature and relative humidity were 24.9±1.7oC and 85.3±3.3%. In the morning of postnatal periode, the average ambient temperature and relative humidity became 24.1±1.3 oC and 87.3±2.8%. The average ambient temperature in the morning was still in the thermal comfort zone, but the relative humidity was higher than comfort humidity according to Etim et al. (2013). In midday of prenatal periode, the average ambient temperature and relative humidity were 32.9±1.7oC and 65.8±6.2%. In the midday of postnatal periode, the average ambient temperature and relative humidity became 30.2±1.7oC and 68.1±6.3%. In afternoon of prenatal periode, the average
ambient temperature and relative humidity were 30.6±1.5oC and 73.6±5.2%. In the midday of postnatal periode, the average ambient temperature and relative humidity became 28.0±2.4oC and 76.0±4.4%. The average ambient temperature and relative humidity in the midday and afternoon has been exceeded from the normal temperature and humidity according Etim et al. (2013).
The high relative humidity during the research caused by the rain that occurs almost every day (in rainy season). The rainfall took place not only in the morning but also afternoon and evening. In midday, the weather was almost cloudy. Meawhile, high relative humidity also caused by the air circulation in Animal House B-Field Laboratory was not so humid. In Bogor, a high ambient temperature and relative humidity are the major constraint on animal reproduction and production. During the early stage of embryonic life, number and placentome size were greatly reduced and cell size decreased by exposure to the high ambient temperature (30-40 oC) compared to thermal comfort zone (18-25 oC) (Marai et al.
2007). The impact of heat stress during prenatal restricts early fetal development, even before the maximal fetal growth phase (Regnault et al. 2000). Heat stress affects many performance of the reproductive system including mating period, gonadotropin profiles, follicular growth, steroidogenesis and oocyte and embryos development (Roth 2009).
Physiological Status of Ewes
10
Table 3. Phyiological status of ewes
Parameters --- Prenatal --- --- Postnatal ---
CON VES BTWE CON VES BTWE
Respiration rate (breath/min) 62.0±5.4 60.0±4.6 61.0±9.4 54.0±8.4 51.0±7.2 52.0±9.4 Pulse rate (beat/min) 94.0±4.8 96.0±5.6 92.0±3.2 84.0±6.4 84.0±7.2 82.0±5.4 Rectal temperature (oC) 38.9±0.3 38.8±0.2 38.8±0.3 38.9±0.3 38.9±0.2 38.9±0.1
Note: [CON]: without antioxidant, [VES]: Vitamin E synthetic, [BTWE]: Black tea extract
In sheep, heat loss by the increase in respiration rate is the principal way of heat dissipation because sweating is prevented by the presence of wool coat (Marai et al. 2007). Antioxidant treatments (natural and synthetic) did not affect the respiration rate in prenatal and postnatal as seen at Table 3. Respiration rate in prenatal and postnatal were affected by the ambient temperature and relative humidity during the research as seen at Table 2. The respiration rate displayed by the ewes in the research indicated they were subjected to medium level of heat stress in prenatal and low level of heat stress in postnatal. Low thermal stress has respiration rate ranges between 40-60 breaths per minute, nevertheless medium thermal stress has respiration rate ranges between 60-80 breaths per minute (Silanikove2000). Respiration rates in all treatments were lower than the research using flaxseed oil on lactating ewes under high ambient temperature (28-35 oC) (90-100 breaths per min) as reported by Caroprese et al. (2012) but higher than the research without using oil on ewes in thermal neutral zone (<25 oC) (20-30 breaths per min) by Indu et al.(2014).
Pulse rate in prenatal and postnatal did not affected by antioxidant treatments (natural and synthetic) as seen at Table 3. This result was due to the high relative humidity that happened during the research (Table 2). Pulse rate results was higher than the research without using oil on ewes in thermal neutral zone (<25 oC)
(60-70 beats per min). Pulse rate increases on exposure to high environmental temperature (Marai et al.2007). The increases of blood flow from the core to the surface give a chance for more heat to be lost by sensible (loss by conduction, convention, and radiation) and insensible (loss by diffusion water from the skin) (Marai et al.2007).
Antioxidant treatments (natural and synthetic) did not affect the rectal temperature in prenatal and postnatal as seen at Table 3. Rectal temperature were lower than the research using flaxseed oil on lactating ewe under high ambient temperature (28-35 oC) (39.47-39.70oC) as reported by Caroprese et al. (2012). Rectal temperatures vary between 38.3 and 39.9 oC under thermo-neutral condition (Marai et al. 2007). An increase in the ambient air temperature from 18 to 25 oC is accompanied by significant increases in rectal temperature in sheep (Marai et al. 2007).
Dry Matter Intake and Average Daily Gain of Ewes
11
high ambient temperature and relative humidity. Reducing feed intake is a way to decrease heat production in warm environments because the heat increament of feeding, especially in ruminants, is an important source of heat production (Kadzere et al.2002). This effort of ewes is to reduce the heat accumulation inside the body, although it must be accompanied by a reduced growth as consequences (Marai et al.2007).
Antioxidant treatments (natural and synthetic) had no negative effect to the DMI in prenatal, it means the palatability of ration was good. The DMI in prenatal for all treatments ranges between 819-829 g/h/d or equal to 2.29-2.42 % body weight. However, the DMI in prenatal was lower than the recommendation of ewe requirements for reproductive phase according to NRC (2007) which is 900 g/h/d or equal to 2.5% of body weight. The DMI in prenatal was the same as the research using 6% sunflower oil on ewes in prenatal (725±125 g/h/d) as reported by Khotijah (2014). The low DMI in prenatal not only caused by the high ambient temperature and relative humidity, but also the growth of fetus in the uterus that limit the ration in the stomach and high fat diets. Feeding high energy density diets during prenatal is associated with a greater decline of dry matter and energy intake as parturition approaches (Hayirli 2001). Fetal growth will suppress the rumen and cause the rumen capacity becomes smaller (Gonzalez-Garcia et al. 2015). According to Czerkawski (1972), high fat diets may reduce the nutrients digestibility in ruminal environment and thus, reduce the dry matter intake.
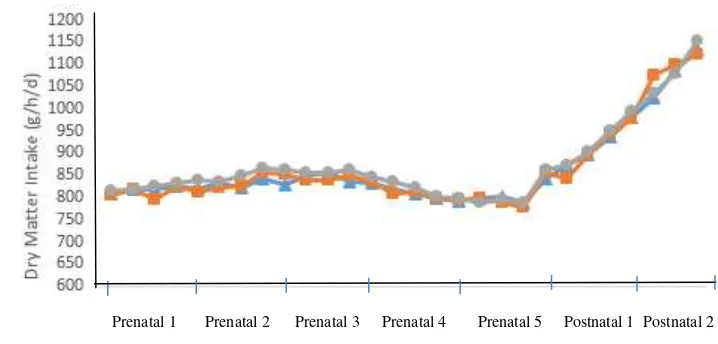
Antioxidant treatments also had no negative effect to the DMI in postnatal but increased compared to prenatal as seen at Table 4. The DMI in postnatal for all treatments ranged between 971-981 g/h/d or equal to 3.00-3.15 % body weight. According to NRC (2007), the DMI of ewes for reproductive phase was 960 g/h/d or equal to 3% of body weight. The DMI in postnatal were the same as the research using 6% sunflower oil on ewes (975±125 g/h/d) as reported by Khotijah (2014). After calving, DMI increased continuously for all treatments (Fig. 3.). The increasing DMI in postnatal was caused by more nutrients required by ewes for lactating. The energy demand after parturition (postnatal) for maintenance of body tissue function and milk production of ewes is up to 3 times higher than before calving (prenatal) (Drackley et al. 2001). The ewes require more nutrients to recover the body condition after lambing and preparation of conception (Kongsted et al. 2013).
12
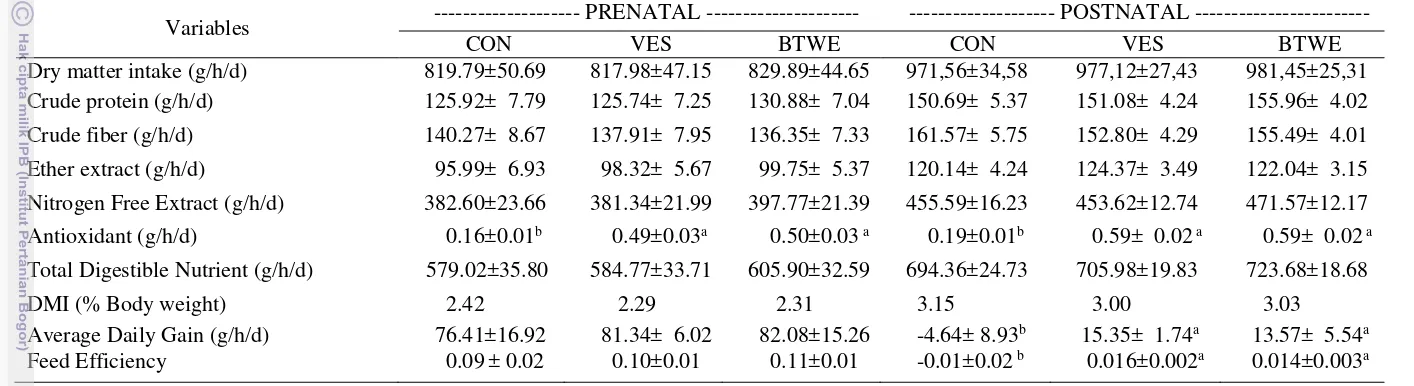
Table 4. Nutrients intake and performance of ewes.
Variables --- PRENATAL --- --- POSTNATAL ---
CON VES BTWE CON VES BTWE
Dry matter intake (g/h/d) 819.79±50.69 817.98±47.15 829.89±44.65 971,56±34,58 977,12±27,43 981,45±25,31
Crude protein (g/h/d) 125.92± 7.79 125.74± 7.25 130.88± 7.04 150.69± 5.37 151.08± 4.24 155.96± 4.02
Crude fiber (g/h/d) 140.27± 8.67 137.91± 7.95 136.35± 7.33 161.57± 5.75 152.80± 4.29 155.49± 4.01
Ether extract (g/h/d) 95.99± 6.93 98.32± 5.67 99.75± 5.37 120.14± 4.24 124.37± 3.49 122.04± 3.15
Nitrogen Free Extract (g/h/d) 382.60±23.66 381.34±21.99 397.77±21.39 455.59±16.23 453.62±12.74 471.57±12.17
Antioxidant (g/h/d) 0.16±0.01b 0.49±0.03a 0.50±0.03 a 0.19±0.01b 0.59± 0.02 a 0.59± 0.02 a
Total Digestible Nutrient (g/h/d) 579.02±35.80 584.77±33.71 605.90±32.59 694.36±24.73 705.98±19.83 723.68±18.68
DMI (% Body weight) 2.42 2.29 2.31 3.15 3.00 3.03
Average Daily Gain (g/h/d) 76.41±16.92 81.34± 6.02 82.08±15.26 -4.64± 8.93b 15.35± 1.74a 13.57± 5.54a
Feed Efficiency 0.09± 0.02 0.10±0.01 0.11±0.01 -0.01±0.02 b 0.016±0.002a 0.014±0.003a
Note: [CON]: without antioxidant, [VES]: Vitamin E synthetic, [BTWE]: Black tea extract
13
Fig. 3. Dry matter intake of ewes. CON ( ), VES ( ), BTWE ( )
The nutrient intakes such as crude protein, crude fibre, ether extract, nitrogen free extract, and also total digestible nutrient (TDN) in prenatal and postnatal were not affected by the treatments. The crude protein intake in this research was the same as the recommendation of ewe requirements for pregnancy (100 g/h/d) and lactation (150 g/h/d) according to NRC (2007). Protein sources in the ration that used in this research is soyabean meal (SBM). A good quality ration should have approximately 30% of the protein source as digestible undergradable protein (DUP) and this is often supplied by soya bean meal (Vipond et al. 2010). The high quality protein in ration is offered to ewes in late pregnancy to maintain body condition, give optimal lamb birth, and sufficient high quality colostrum (Pulina et al. 2006). The ether extract intake was higher than the research using 6% sunflower oil on ewes as reported by Khotijah (2014) which is 158±12.3 g/h/d. The high ether extract caused by the ether extract composition in ration (10.35-10.86%) was higher than Khotijah (2014) which is 8.5%. The crude fiber and nitrogen free extract intake were lower than the research using 6% sunflower oil on ewes reported by Khotijah (2014) which is 159.30±19.19 g/h/d and 534.97±89.78 g/h/d respectively. The TDN intake in this research was the same as the TDN recommendation of NRC (2007) for ewe requirements during pregnancy (520g/h/d) and lactation (720 g/h/d).
Average daily gain was affected by dry matter intake and nutrient intake during the research (Table 4). Reducing feed intake of ewes is to reduce the heat accumulation inside the body, although it must be accompanied by a reduced growth as consequences (Marai et al. 2007). In prenatal, the average daily gain (ADG) (ranged between 76.41-82.08 g/h/d) and feed efficiency of ewes (ranged between 0.09-0.11) were not affected by antioxidant treatments as seen at Table 4. The ADG in prenatal was lower than the research using 6% sunflower oil on ewes in prenatal (112±27.5 g/h/d) as reported by Khotijah (2014). In prenatal, the average daily gain (ADG) of ewes was refractive. The ADG of ewes was not only influenced by the ewe’s body weight but also the number of embryos and fetal growth (Kenyon et al. 2012).
In postnatal, the average daily gain (ADG) and feed efficiency of ewes in CON (-4.64±8.93 g/h/d and -0.01±0.02 respectively) were lower (P<0.05) than VES (15.35±1.74 g/h/d and 0.016±0.002 respectively) and BTWE (13.57±5.54 g/h/d and 0.014±0.003 respectively) as seen at Table 4. In postnatal, the ADG of
14
ewes in this research was influenced by level stress of ewes. Malondialdehyde as an indicator levels of stress in BTWE and VES were lower than CON (Fig.3). High oxidation levels caused the ewes become stressed and disrupt the physiological function in the body including the recovery of body condition after lambing (Sejian et al. 2014). In prenatal, all treatments had similar DMI and ADG, so that the feed efficiency were the same in all treatments. In postnatal, all treatments had similar DMI but both antioxidant treatments had better ADG compared to CON, so that both antioxidant had better feed efficiency.
Reproductive Performance of Ewes
Reproductive performance of ewes become one of the determining factors in sheep breeding programs. In early, pregnancy ewes in all treatments had almost the same number of embryos (2.40-2.80 embryos per ewes) as seen at Table 5. It means that PUFA had the same ability to produce number of twin embryos (Shahneh et al. 2008). Supplementation fat to flushing diets in ewes may have a significant impact on animal reproductive performance through an increase in plasma concentrations of cholesterol and progresterone, and also an increased supply of lipoproteins involved in the regulation of streiodogenesis system in ovaries (Williams 1996). The increase levels of blood progresterone concentration may be due to an increase in levels of cholesterol available which is a precursor for progresterone biosynthesis by corpus luteum (Kia and Safdar 2015). The number of embryos was the same as the research using 6% sunflower oil on ewes in prenatal (2.0-2.5 embryos per ewes) as reported by Khotijah (2014).
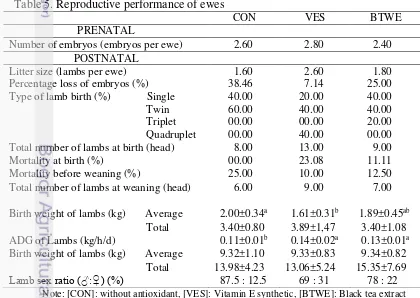
Table 5. Reproductive performance of ewes
CON VES BTWE
Note: [CON]: without antioxidant, [VES]: Vitamin E synthetic, [BTWE]: Black tea extract
15
Reproductive performances of ewes in this research were influenced by the antioxidant. Antioxidant activity in VES and BTWE were higher than CON, it was characterized by high level of DPPH of ration as seen at Table 1. The higher antioxidant activity in VES and BTWE were expected to produce more PUFA after passed through the rumen than CON. Polyunsaturated fatty acid (PUFA) is one of nutrients that is essential for ewes in reproductive phase. Effect of PUFA for ewes in prenatal can accelerate embryos development (Gaffarillaleh et al. 2014), increase the percentage of pregnancy (El-Nour et al. 2012), increase the number of embryos (Shahneh et al. 2008), and decrease the risk of death the embryo (Mattos et al. 2002).
The other reproductive performances of ewes were influenced by type of birth. CON had type of birth in single (40%) and twin (60%). VES had type of birth in single (20%), twin (40%), and quadruplet (40%). BTWE had type of birth in single (40%), twin (20%), and triplet (20%). The litter size in BTWE (1.8) was higher than CON (1.6), but lower than VES (2.6) (Table 5). The litter size was the same as the research using 6% sunflower oil on ewes in postnatal (1.4-2.6) as reported by Khotijah (2014).
The number of embryos at prenatal in all treatments were the same, but the litter size was different due to a different percentage of loss embryos as seen at Table 5. The percentage loss of embryos in BTWE (25%) was lower than CON (38.46%), but higher than VES (7.14%). The percentage of loss embryos is influenced by two factor: external (ambient temperature) and internal (oxidative stress and nutrient status). During the early stage of embryonic life and number were greatly reduced by exposure to the high ambient temperature compared to thermal comfort zone (Marai et al. 2007). The levels of oxidation in ewe’s body in prenatal were the same for all treatments, nevertheless VES was better to protect embryos compared to CON and BTWE. The high metabolism process will produce high free radical in the body and affecting the cell membrane and DNA integrity of oocytes and embryos (Barakat et al. 2014). Within the body, oocytes and embryos can be protected from oxidative stress by free radical and enzymatic scavenging antioxidant from vitamin E that exist within the follicular and oviductal fluid (Gupta et al. 2010). The higher antioxidant activity in VES and BTWE produce more PUFA after passed through the rumen than CON. Colesterol plasma in BTWE and VES was higher than CON (Fig. 4). Low colesterol plasma as a precursor hormone causes deficiency of steroid hormone that causes the death in early embryos and embryos will absorbed in uterine wall (Salem et al 2006). The lower percentage of loss embryos in VES dan BTWE indicated the success of vitamin E and black tea waste extract as antioxidant to keep the number of embryos.
The mortality at birth in BTWE (11.11%) was higher than CON (0%), but lower than VES (23.08%), nevertheless the mortality before weaning in BTWE (12.5%) was higher than VES (10%), but lower than CON (25%). Mortality at birth was correlated with birth weight. Lambs with small birth weight had low body resistance causing the mortality (Everett-Hincks et al. 2014). High mortality in CON was influenced by the quality of milk consumed by lambs during postnatal (Table 6). Lambs only consume milk form their mother in postnatal period before consuming solid ration. Nutrient content in their mother’s colostrum and milk affects the condition and endurance of lambs (Novoa-Garrido et al. 2014).
16
total birth weight of lambs (range 3.40-3.89 kg) was not affected by antioxidant treatments as seen at Table 5. The birth weight of lamb were the same as the research using 6% sunflower oil in ewes in postnatal (1.7-3.1 kg/h for single and 1.5-2.1 for twin, triplet and quadruplet) as reported by Khotijah (2014). Dominant type of twin births in VES caused lower average birth weight than dominant type of single birth in CON. Lamb types of twinning will compete in absorbing nutrients from ewes during growth of embryo in uterus, while lambs born singly can fully absorb nutrients from the ewes (Kenyon et al. 2012). Capacities of the uterus also affect the weight of lambs born as a twin (Sharma et al. 2012). Limitations of capacities from uterus characterized by total of body weight at birth were similar in all treatment but has a different type of birth.
The average daily gain (ADG) of lambs in CON was lower than VES and BTWE (P<0.05). Lamb’s ADG was higher in both antioxidant treatments, and it was influenced by quality of milk produced by their mother (Table 6). Nutrient content of ewe’s milk on both antioxidant treatments was better than CON. Availability of higher nutrient of milk will increase lamb’s ADG (Kotsampasi et al. 2014). ADG of lambs were higher in antioxidant treatments causing average and total lamb’s body weight at weaning were the same in all treatments. The average and total weaning weight of lambs were the same in all treatments as seen at Table 5. The average daily gain of lambs was the same as the research using 6% sunflower oil on ewe in postnatal (120±30 g/h/d) as reported by Khotijah (2014).The average and total weaning weight of lambs were the same as the research using 6% sunflower oil in ewes in postnatal (10±4 kg/ewe) as reported by Khotijah (2014).
All treatments produced more male lamb than female which CON had the highest male percentage (87.5%) while VES was the least (69%). Results of sex ratio in line with Khotijah et al. (2013) which used a source of unsaturated fatty acid (sunflower oil) with the same level (6%). Extra nutrient derived from breakdown of PUFA enhance the development of the male embryos, but inhibit the female embryonic growth and development (Akbarinejad et al. 2012). Environmental factor like reproductive secretions including follicular, tubal, and vaginal fluid pH can affect the sex ratio and X (female) and Y (male) chromosome population (Paasch et al. 2007). Ratio of male lambs was lower in antioxidant treatments due to ion exchange activity between free radicals and antioxidant that affect the acid-base balance. Ion exchange causes the uterus become acidic (increasing ion H+) (Parraguez et al. 2013). Acid condition decrease the loss of potassium from Y-sperm that may cause physiological significance and maintain intracellular sperm from loss of swimming (Paasch et al. 2007). The genital pH acidity may active speed of X-bearing sperm to reach fertilizing side earlier than Y-bearing sperm.
Blood metabolites of ewes
17
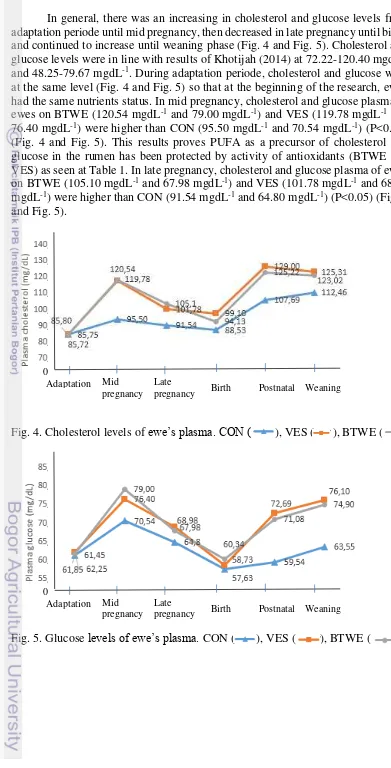
In general, there was an increasing in cholesterol and glucose levels from adaptation periode until mid pregnancy, then decreased in late pregnancy until birth, and continued to increase until weaning phase (Fig. 4 and Fig. 5). Cholesterol and glucose levels were in line with results of Khotijah (2014) at 72.22-120.40 mgdL-1 and 48.25-79.67 mgdL-1. During adaptation periode, cholesterol and glucose were
at the same level (Fig. 4 and Fig. 5) so that at the beginning of the research, ewes had the same nutrients status. In mid pregnancy, cholesterol and glucose plasma of ewes on BTWE (120.54 mgdL-1 and 79.00 mgdL-1) and VES (119.78 mgdL-1 and
76.40 mgdL-1) were higher than CON (95.50 mgdL-1 and 70.54 mgdL-1) (P<0.05) (Fig. 4 and Fig. 5). This results proves PUFA as a precursor of cholesterol and glucose in the rumen has been protected by activity of antioxidants (BTWE and VES) as seen at Table 1. In late pregnancy, cholesterol and glucose plasma of ewes on BTWE (105.10 mgdL-1 and 67.98 mgdL-1) and VES (101.78 mgdL-1 and 68.98 mgdL-1) were higher than CON (91.54 mgdL-1 and 64.80 mgdL-1) (P<0.05) (Fig. 4 and Fig. 5).
Fig. 4. Cholesterol levels of ewe’s plasma. CON ( ), VES ( ), BTWE ( )
Fig. 5. Glucose levels of ewe’s plasma. CON ( ), VES ( ), BTWE ( )
Adaptation Mid pregnancy
Late
pregnancy Birth Postnatal Weaning
0
Adaptation Mid pregnancy
Late
pregnancy Birth Postnatal Weaning
18
At birth, the cholesterol and glucose plasma of ewes in BTWE (94.3 mgdL-1
and 60.34 mgdL-1, respectively) and VES (99.10 mgdL-1 and 58.73 mgdL-1, respectively) were higher than CON (88.53 mgdL-1 and 57.63 mgdL-1, respectively) (P<0.05) (Fig. 4 and Fig. 5). In birth, the decreasing of cholesterol and glucose levels in all treatments because more nutrients were required to produce milk, recover body condition after birth, and thermoregulations so that decreased nutrients availability in blood circulation.
In postnatal, the cholesterol and glucose plasma of ewes in BTWE (129 mgdL-1 and 72.69 mgdL-1, respectively) and VES (125.22 mgdL-1 and 71.08 mgdL
-1, respectively) were higher than CON (107.69 mgdL-1 and 59.54 mgdL-1,
respectively) (P<0.05) (Fig. 4 and Fig. 5). In postnatal, cholesterol and glucose levels in antioxidant treatments were increased dramatically compared to CON (Fig. 4 and Fig. 5). The increased was related to the level of stress in ewes (Fig. 6), so that antioxidants had influential role in postnatal. At weaning, the cholesterol and glucose plasma of ewes in BTWE (125.31 mgdL-1 and 76.10 mgdL-1, respectively)
and VES (123.02 mgdL-1 and 74.90 mgdL-1, respectively) were higher than CON (112.46 mgdL-1 and 63.55 mgdL-1, respectively) (P<0.05) (Fig. 4 and Fig. 5).
Decreasing the levels of cholesterol at weaning in both antioxidant treatments indicated the use of cholesterol for synthesis of reproductive hormones (preparation for estrus cycle and conception). Glucose levels of plasma was affected by the heat stress (t: 24.9-32.9oC, h: 65.8-87.3%). Plasma cortisol can increase during acute heat stress because of its hyperglycaemic action, which increases glucose availability during stress to meet the high energy demand (Matteri et al.2000). The low glucose levels in CON caused by the increasing of utilization glucose for thermoregulation. The increase of PUFA was protected by the antioxidant is connected to increased mobilization of body fat reserves necessary to meet the energy requirements for the thermoregulation.
19
Fig. 6. Malondialdehyde levels of ewe’s plasma. CON ( ), VES ( ), BTWE ( )
Milk composition of ewes
Sunflower supplementation is used in dairy ewe ration as a means of increasing the energy density of the ration and modifying milk composition (Pulina et al.2006). Milk compositions were observed in this research such as total solid, lactose, fat, and protein were presented at Table 6.
Table 6. Milk composition of ewes
CON VES BTWE
Total solid (%) 17.33±0.59 b 18.61±0.35 a 18.92±0.18 a Fat (%) 6.83±0.26 b 7.56±0.11a 7.63±0.37a Protein (%) 6.75±0.28 6.68±0.16 6.80±0.14 Lactose (%) 3.75.±0.15 b 4.37±0.28 a 4.49±0.23 a
Note: [CON]: without antioxidant, [VES]: Vitamin E synthetic, [BTWE]: Black tea extract Different superscript in the same row is significant (P<0.05).
20
CONCLUSION
Physiological status of ewes were still categorized as medium thermal stress in prenatal and low thermal stress in postnatal periode for all treatments. The supplementation of black tea extracts and vitamin E synthetic with a dose of 500 ppm in high polyunsaturated fatty acids ration did not disturb DMI and have more efficient to decrease level of oxidation for lactating ewes, multiple births, restore body weight of ewes after lambing, and maintain the level of cholesterol and glucose plasma. Black tea extracts and vitamin E synthetic had better milk quality than control. Black tea extracts could be used as antioxidant to replace vitamin E synthetic in ration with high PUFA for ewes to reproductive status especially in postnatal periode.
SUGGESTION
1. Evaluation the effect of vitamin E synthetic and black tea waste extract which be given directly with high polyunsaturated fatty acids to protect polyunsaturated fatty acids during the storage period.
2. Management of feeding with different quality of nutrient in ration for ewes which has different number of fetuses.
REFERENCES
Abecia JA, Casao A, Pascual-Alonso M, Lobon S, Aguayo-Ulloa LA, Forcada F, Meikle A, Sosa C, Marin RH, Silva MA, Maria GA. 2014. Periconceptional under nutrition increases quantity and quality of oocyte population, but not cognitive or emotional response of 60-day-old lambs. J. of Anim. Phys. and Nutr. DOI: 10.1111/jpn.12211.
Akbarinejad V, Niasari-Naslaji A, Mahmoudzadeh H, Mohajer M. 2012. Effects of diets enriched in different sources of fatty acids on reproductive performance of Zel sheep. Iranian J. of Vet. Res. 13(4).
Ali MA, Nouruddeen ZB, Latip MRA, Othman NH. 2014. Effect of microwave heating on oxidative degradation of sunflower oil in the presence of palm olein. Sains Malaysiana 43(8):1189-1195.
Alonso DMA, Torres AJFJ, Sandoval CCA, Hoste H. 2010. Tannins in tropical tree fodders fed to small ruminants: J. of Small Rum. Res. 89:164-173.
AOAC. 2005. Official Methods of Analysis, 17th Ed. Association of Official Analytical Chemists, Washington DC, USA.
Barakat IAH, Al-Himaidi A, Rady AM. 2014. Antioxidant effect of green tea leaves extract on in vitro production of sheep embryos. Pakistan J. Zool. 46(1):167-175.
21
Bhuyan LP, Hussain A, Tamuly P, Gogoi RC, Bordoloi PK, Hazarika M. 2009. Chemical characterization of CTC black tea of northeast India: Correlation of quality parameters with tea tester evaluation. J of the SciFoodAgri.89:1498– 1507.
Bhuyan LP, Sabhapondit S, Baruah BD, Bordoloi C, Gogoi R, Bhattacharyya P. 2013. Polyphenolic compounds and antioxidant activity of CTC black tea of North-East India. J. Food Chem. 141:3744-3751.
Bianchi AE, Macedo VP, Franca RT, Lopes STA, Lopes LS, Stefani LM, Volpato A, Lima HL, Painano D, Machado G, da Silva AS. 2014. Effect of adding palm oil to the diet of dairy sheep on milk production and composition, function of liver and kidney, and the concentration of cholesterol, triglycerides, and progesterone in blood serum. J. of Small Rum. Res. 117: 78-83.
Bubols GB, Zielinsky P, Piccoli GL, Nicoloso LH, Vian I, Moro AM, Charao MF, Brucker N, Bulcao RP, Nascimento SN, Baierle M, Alievi MM, Moresco RN, Markoski M, Garcia SC. 2014. Nitric oxide and reactive species are modulated in the polyphenol induced ductus arteriosus constriction in pregnant sheep. J of Prenatal Diag. 34(13): 1268-1276.
Capper JL, Robert G, Wilkinson, Alexander M, Mackenzie, Sinclair LA. 2006. Polyunsaturated fatty acid supplementation during pregnancy alters neonatal behavior in sheep, nutrient physiology, metabolism, and nutrient-nutrient interactions. J of Nutr. 136:397-403.
Caroprese M, Albenzio M, Bruno A, Annicchiarico G, Marino R, Sevi A. 2012. Effect of shade and flaxseed supplementation on the welfare of lactating ewes under high ambient temperatures. J. of Small Rumin. Res. 102: 107-185. Cerri RLA, Juchem SO, Chebel RC, Rutigliano HM, Bruno RGS, Galvao KN,
Thatcher WW, Santos JEP. 2009. Effect of fat source differing in fatty acid profile on metabolic parameters, fertilization, and embryo quality in high producing dairy cows. J. of Dairy Sci. 92:1529-1531.
Chilliard Y, Glasser F, Ferlay A, Bernard L, Rouel J, Doreau M. 2000. Diet, rumen bio hydrogenation and nutritional quality of cow and goat milk fat. Eur. J. Lipid Sci. Tech. 109:828-855.
Czerkawski, JW. 1972. Fate of metabolic hydrogen in the rumen. Proceedings of the Nutrition Society. 31(2): 141-146.
da Costa RLD, Fontes RdS, da Cunha EA, Bueno MS, Quirino CR, Alfonso VAC, Otero WG, Santos LEd, Dias AJB. 2011. Reproductive performance of Santa Ines ewes fed protected fat diet. Pesq. Agropec. Bras. 46(6):663-668.
Drackley JK, Overton TR, Douglas GN.2001. Adaptations of glucose ang long chain fatty acid metabolism in liver of dairy cows during the periparturient period. J. of Dairy Sci. 84:E100-E112.
Dunford LJ, Sinclair KD, Kwong WY, Sturrock C, Clifford BL, Giles TC, Gardner DS. 2014. Maternal protein-energy malnutrition during early pregnancy in sheep impacts the fetal ornithine cycle to reduce fetal kidney micro vascular development. J. of FASEB 11:4880-4892.
22
El-Nour HHM, Nasr SM, Hassan WR. 2012. Effect of calcium soap of fatty acids supplementation on serum biochemical parameters and ovarian activity during out-of-the-breeding season in crossbred ewes. The scientific World J. 2012:1-7.
Etim NN, Williams ME, Evans EI, Offiong EE. 2013. Physiological and behavioural respons of animals to stress: implications for animal productivity. AmericaJ. of Adv. Agric. Res. 1(2).
Etim NN, Oguike MA. 2014. Environmental and management stressors: implications for reproductive and productive performances of farm animals in the tropics. J. of Agric. And Sustain. 5(2):153-170.
Everett-Hincks JM, Mathias-Davis HC, Greer GJ, Auvray BA, Dodds KG. 2013. Genetic parameters for lamb birth weight, survival, and death risk traits. J. of Anim. Sci. 92(7): 2885-2895.
Faulkner, A. 1983. Fetal and Neonatal Metabolism. In: Nutritional Physiology of Farm Animals. J.A.F. Book and P.C. Thomas. Longman Inc. New York. Ferreira EM, Pires AV, Susin I, Gentil RS, Gilaverte S, Parente MdOM, Biehl MV,
Ribeiro CVDM. 2014. Lamb performance, milk production and composition from ewes supplemented with soybean oil partially replaced by fish oil blend. J. of Livestock Sci. 163:51-61.
Field ME, Anthony RV, Engle TE, Archibeque SL, Keisler DH, Han H. 2015. Duration of maternal under nutrition differentially alters fetal growth and hormone concentrations. J. of Domestic Anim. Endo. 51: 1-7.
Gallardo B, Manca MG, Mantecon AR, Nudda A, Manso T. 2015. Effect of linseed oil and natural or synthetic vitamin E supplementation in lactating ewes’ diets on meat fatty acid profile and lipid oxidation form their milk fed lambs. J. of Meat Science 102:79-89.
Grau-Sologestoa I. 2015. Livestock management in Spain from Roman to post-medival times: a biometrical analysis of cattle, sheep/goat, and pig. J. of Arch. Sci. 54:123-134.
Ghaffarilaleh V, Fouladi-Nashta A, Paramio MT. 2014. Effect of α-linoleic acid on oocyte maturation and embryo development of pre pubertal sheep oocytes. J. of Theriogenology 82(5):686-696.
Gupta S, Sekhon L, Kim Y, Agarwal A. 2010. The role of oxidative stress and antioxidants in assisted reproduction. Curr. Women’s Hlth. Rev. 6:227-238. Gobert M, Martin B, Ferlay A, Chilliard Y, Graulet B, Pradel P, Bauchart D,
Durand D. 2009. Plant polyphenols associated with vitamin E can reduce plasma lipo-peroxidation in dairy cows given n-3 polyunsaturated fatty acid. J. of Dairy Sci.: 92(12):6095-6104.
Gonzalez-Garcia E, Tesniere A, Camous S, Bocquir F, Barillet F, Hassoun P. 2015. The effects of parity, litter size, physiological state, and milking frequency on the metabolic profile of Lacaune dairy ewes. J. of Dom. Anim. Endo. 50:32-44.
Hayirli A. 2001. Management of dry matter intake and lipid metabolism to allevate hepatic lipidosis in periparturient dairy cattle. Ph.D. Thesis. Univ. of Wisconsin Madison.
23
Indu S, Sejian V, Naqvi SMK. 2014. Impact of simulated heat stress on growth, physiological adaptability, blood metabolites, and endocrine responses in Malpura ewes under semiarid tropical environment. J. of Anim. Prod. Sci. http://dx.doi.org/10.1071/AN14085
Kadraze CT, Murphy MR, Silanikove N, Maltz E. 2002. Heat stress in lactating dairy cows: a review. J. of Livestock Prod. Sci. 77(1):59-91.
Kenyon PR, Hickson RE, Hutton PG, Morris ST, Stafford KJ, West DM. 2012. Effect of twin-bearing ewe body condition score and late pregnancy nutrition on lamb performance. J. of Anim. Prod. Sci. 52(7):483-490.
Khotijah L, Setiadi MA, Astuti DA, Wiryawan KG. 2013. Reproductive performance, cholesterol, and progesterone status of garut ewes fed with ration containing different levels of sunflower seed oil. Proceeding of 4th International Conference on Sustainable Animal Agriculture for Developing Countries (SAADC2013): 235-237.
Khotijah L. 2014. Reproductive Performance And Endurance Lamb-Based Local Feed Prolific Source Of Linoleic Sunflower Oil. Ph. D. Thesis. Bogor Agricultural University. Bogor. Indonesia.
Khotijah L, Zulihar R, Setiadi MA, Wiryawan KG, Astuti DA. 2014. Effect of sun flower oil addition in diet on nutrient intake, growth performance, and characteristic of estrous of pre-mating Garut sheep. JITV 19(1):9-16.
Kia HD, Safdar AHA. 2015. Effect of calcium salts of fatty acids with different profiles during the flushing period on reproductive performance of Afshari ewes. J. of Small Rumint. Res. 126:1-8.
Kirbas A, Yildrim BA, Baydar E, Kandemir FM. 2014. Status of lipid peroxidation and some antioxidant in sheep with acute ruminal lactic acidosis. J. of Med. Weter. 70(6).
Kongsted AH, Tygesen MP, Husted SV, Oliver MH, Tolver A, Christensen VG, Nielsen JH, Nielsen MO. 2014. Programing of glucose-insulin homoeostasis: long term consequences of prenatal versus early postnatal; nutrition insults. Evidence from a sheep model. J. of Acta. Physiol. 210:84-98.
Kotsampasi B, Christodoulou V, Zotos A, Liakopoulou-Kyriakides M, Goulas P, Petrotos K, Natas P, Bampidis VA. 2014. Effects of dietary pomegranate byproduct silage supplementation on performance, carcass characteristics and meat quality of growing lambs. J of Anim. Feed Sci. Tech. 197: 92-102. Lourenco M, Ramos-Morales E, Wallace RJ. 2010. The role of microbes in rumen
lipolysis and biohydrogenation and their manipulation. J. of Anim. 4: 1008-1023.
Luczaj W, Skrzydlewska E. 2005. Antioxidative properties of black tea. Prev Med. 40(6):910-918.
Marai IFM, El-Darawany AA, Fadiel A, Abdel-Hafez MAM. 2007. Physiological traits as affected by heat stress in sheep- A review. J. small Rumin. Res. 71:1-12.
Matteri RL, Carrol JA, Dyer CJ. 2000. Neuroendocrine Responses to Stress. In Moberg GP, Mench JA (Eds), Chapter 3 in the biology of animal stress. CAB international.