BIODIESEL PRODUCTION FROM PALM OIL IN A BUBBLE
COLUMN REACTOR BY NON-CATALYTIC PROCESS
(PRODUKSI BIODIESEL DARI MINYAK SAWIT DALAM REAKTOR
KOLOM GELEMBUNG SECARA NON-KATALITIK)
JOELIANINGSIH
A Dissertation
Submitted in partial fulfillment of the requirements for the Degree of Doctor in Agricultural Engineering Sciences
THE GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY (IPB)
BOGOR
i
STATEMENT OF RESEARCH ORIGINALITY
Hereby, I state that the dissertation entitled “Biodiesel Production from Palm Oil in a Bubble Column Reactor by Non-Catalytic Process” is my own work,
which has never previously been published in any university. All of incorporated originated from other published as well as unpublished papers are stated clearly in the text as well as in the references.
Bogor, April 2008
ii
ABSTRACT
JOELIANINGSIH. Biodiesel Production from Palm Oil in a Bubble Column Reactor by Non-Catalytic Process. Under direction of KAMARUDDIN ABDULLAH, YASUYUKI SAGARA, ARMANSYAH H TAMBUNAN, and TATANG H SOERAWIDJAYA.
Biodiesel has been defined as the monoalkyl esters of long-chain fatty acids derived from renewable feedstocks, such as vegetable oils and animal fats, for use in compression-ignition (diesel) engines. Biodiesel, consisting of fatty acid methyl esters (FAME) can be produced by transesterification of triglycerides (TG) and/or esterification of free fatty acids (FFA) in vegetable oils with short-chain alcohol, mainly methanol (MeOH). Alkaline-catalyzed transeseterification of TG in vegetable oils, with the addition of an acid-catalyzed reaction to esterify FFA are the technologies presently in use for industrial-scale biodiesel production. However, the desire to reduce or remove catalyst cost, waste output and to obtain the simpler process has stimulated the investigation of alternate methods of biodiesel synthesis.
A catalyst-free biodiesel production method has been developed using a bubble column reactor (BCR) at atmospheric pressure. Transesterification of TG and methyl esterification of fatty acids experiments have been conducted in a semi-batch mode reactor in which bubbles of superheated methanol vapor were blown into the oil/fatty acids containing reactor.The results show that methyl esterification of FFA and transesterification of TG can be conducted in a bubble column reactor. Comparing the obtained results of the transesterification of TG and the methyl esterification of FFA at the same reaction temperature (250 oC), a higher reaction rate constant can be obtained with the latter, but the quality of the product (the methyl ester content in the product) was better in the former.
A laboratory-scale continuous-flow BCR system has been developed and tested for biodiesel production from refined palm oil by non-catalytic transesterification. Preliminary results showed that the optimum MeOH feed flow rate was about 2.5-3.0 mL/min at 290 oC reaction temperature for a BCR with a fixed liquid volume in the reactor of 200 ml. The optimum value was based on the productivity of FAME and GL and the ME content in the product. Productivity at the 2.5 mL/min (= 0.593 kg/L/h) MeOH feed flow rate was 0.006 kg GL/L/h and 0.058 kg FAME/L/h with the ME content was 96.7% w/w after 300 min reaction time and the mass flow rate of oil was 0.06 kg/L/h. While at the 3.0 mL/min (= 0.711 kg/L/h) MeOH feed flow rate, productivity was 0.014 kg GL/L/h and 0.128 kg FAME/ L/h with the ME content was 95.1% w/w after 270 min reaction time and the mass flow rate of oil was 0.13 kg/L/h. The ratio of output to input energy excluding energy for electricity of this experiment was 26.5 and the specific energy consumption was 1.5 MJ/kg biodiesel at 3.0 mL/min MeOH feed flow rate and 290 oC (the molar ratio of MeOH to oil was 148).
iii
SUMMARY
JOELIANINGSIH. Biodiesel Production from Palm Oil in a Bubble Column Reactor by Non-Catalytic Process. Under direction of KAMARUDDIN ABDULLAH, YASUYUKI SAGARA, ARMANSYAH H TAMBUNAN, and TATANG H SOERAWIDJAYA.
Biodiesel has become more attractive recently because of its environmental benefits and the fact that it is made from renewable resources. Transesterification of vegetable oils with short-chain alcohol has long been a preferred method for producing biodiesel fuel. At present, most of the methods on transesterification reaction are in the employing of alkali catalyst. This method has, however, drawbacks such as difficulties in the recovery of GL, the need for removal of the residual catalyst and the saponified product (soaps) to obtain biodiesel product by neutralization, washing and drying. Furthermore, oils containing free fatty acids and/or water are incompletely transesterified using alkaline catalyst. It prevents a maximum utilization of low-quality feed stocks such as waste frying oil and waste industrial oil. The disadvantages resulted from the use of a catalyst and its removal from the products can be eliminated if the non-catalytic transesterification reaction of vegetable oils with alcohol can be realized.
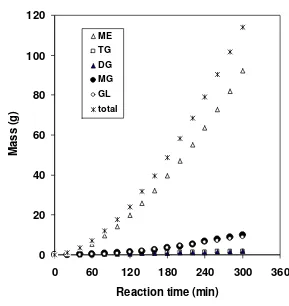
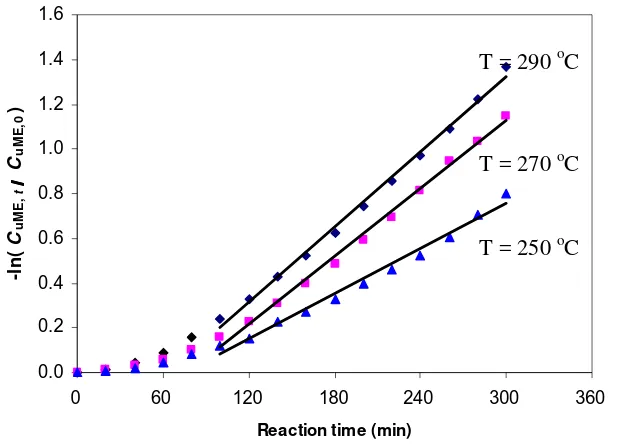
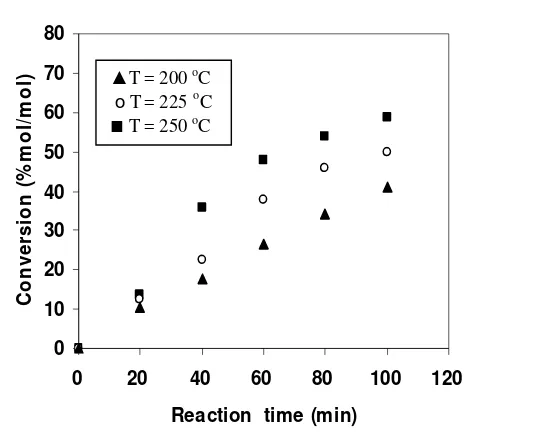
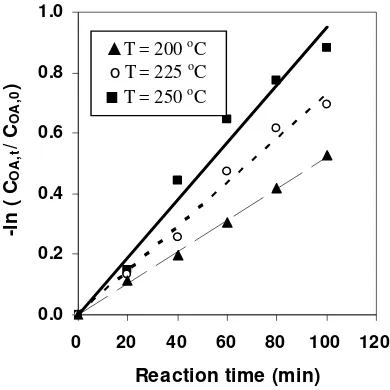
A Bubble Column Reactor (BCR) was developed to produce FAME by blowing bubbles of superheated MeOH vapour continuously into vegetable oil without using any catalysts. A kinetic study on the non-catalytic transesterification of palm oil was made in a BCR at atmospheric pressure. The BCR was a 500-mL four-necked flask (the diameter of flask is about 9.85 cm) equipped with a condenser, a pipe for MeOH vapour feed and a temperature controller. The effects of reaction temperatures (250, 270, and 290 oC) on the rate constant, conversion, yield of ME and composition of the reaction product under semi-batch mode operation are investigated. The mass of initial oil in the reactor was 200 g with the MeOH fixed flow rate of 4 g/min. Evaluation on the reaction kinetic based on the overall reaction and pseudo first order reaction assumption shows that reaction rate constant at 250, 270 and 290 oC was 0.0022, 0.0032 and 0.0039 min-1, respectively, activation energy was 35 kJ/mol, and frequency factor was 7.3. The rate constant, conversion and yield of ME showed an increase trend with the reaction temperature, but the ME content in the reaction product decreased as the reaction temperature was increased. The optimum reaction temperature which gives the highest ME content (95.17% w/w) was 250 oC, while the rate constant of the total system increased as the reaction temperature was increased. Experimental results indicated that in order to increase process efficiency showed by the value of rate constant, conversion and yield of ME, the interfacial area of gas-liquid phase should be enlarged. The principle of a BCR for transesterification is same with a reactive distillation where the reaction products in the gas phase (GL and ME) continuously removed from the reactive zone, while TG as the reactans were obtained in the reactive zone (liquid phase).
iv found at high concentration in vegetable oil was chosen as a substrate. The effects of reaction temperatures on the composition of the gaseous product, conversion of the reaction and the rate constant are investigated. Then, reactivity of palm fatty acids (myristic, palmititic, stearic, oleic and linoleic acid) were studied at the same reaction temperature (250 oC). Evaluation on the reaction kinetic based on changes in the oleic acid concentration shows that the reaction rate constants at 200, 225, and 250 oC were 0.0052, 0.0073, 0.0095 min-1, respectively, activation energy was 24.8 kJ/mol, and the frequency factor was 2.9. The reaction rate of methyl esterification was faster than that of methyl transesterification, but the ME content in the gaseous product was lower. Therefore, FFA content in vegetable oil can give effect on the reaction rate and the quality of the product if the oil which contains significant amounts of FFA is used as a feedstock for biodiesel production via the non-catalytic process in a bubble column reactor. In methyl esterification of fatty acids, reactivity and FAME purity of saturated fatty acids (myristic, palmititic, and stearic acids) are lower than unsaturated fatty acids (oleic and linoleic acids). In the saturated fatty acids, reactivity increased with the length of fatty acids alkyl chains.
A laboratory-scale continuous-flow BCR system has been developed and tested for biodiesel production from refined palm oil by non-catalytic transesterification. The effects of the MeOH feed flow rate (1.5, 2.5, 3.0, 4.0 and 6.0 mL/min) and reaction temperatures (250, 270, and 290 oC) on the productivity and ME content in the product were investigated. Preliminary results showed that the optimum MeOH feed flow rate was about 2.5-3.0 mL/min at 290 oC reaction temperature for a BCR with a fixed liquid volume in the reactor of 200 ml. The optimum value was based on the productivity of FAME and GL and the ME content in the product. Productivity at the 2.5 mL/min (= 0.593 kg/L/h) MeOH feed flow rate was 0.006 kg GL/L/h and 0.058 kg FAME/L/h with the ME content was 96.7% w/w after 300 min reaction time and the mass flow rate of oil was 0.06 kg/L/h. While at the 3.0 mL/min (= 0.711 kg/L/h) MeOH feed flow rate, productivity was 0.014 kg GL/L/h and 0.128 kg FAME/ L/h with the ME content was 95.1% w/w after 270 min reaction time and the mass flow rate of oil was 0.13 kg/L/h. The ratio of output to input energy excluding energy for electricity of this experiment was 26.5 and the specific energy consumption was 1.5 MJ/kg biodiesel at 3.0 mL/min MeOH feed flow rate and 290 oC.
v @ Copyright 2008 by IPB
All rights reserved
1. No part or all of this dissertation may be excerpted without inclusion or mentioning the sources
a. excerption only for research and education use, writing for scientific papers, reporting, critical writing or reviewing of a problem.
vi
BIODIESEL PRODUCTION FROM PALM OIL IN A BUBBLE
COLUMN REACTOR BY NON-CATALYTIC PROCESS
(PRODUKSI BIODIESEL DARI MINYAK SAWIT DALAM REAKTOR
KOLOM GELEMBUNG SECARA NON-KATALITIK)
by
JOELIANINGSIH
A Dissertation
Submitted in partial fulfillment of the requirements for the Degree of Doctor in Agricultural Engineering Sciences
THE GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
vii Title of Dissertation : Biodiesel Production from Palm Oil in a Bubble Column
Reactor by Non-Catalytic Process
Name : Joelianingsih
NIM : F161040062
Approved by, Advisory Committee
Prof. Dr. Ir. Kamaruddin Abdullah, MSA Prof. Dr. Yasuyuki Sagara
Chairman Member
Prof. Dr. Ir. Armansyah H. Tambunan Dr. Ir. Tatang H. Soerawidjaya Member Member
Acknowledged by,
Head of Study Program in Dean of the Graduate School Agricultural Engineering Science
(Prof. Dr. Ir. Armansyah H Tambunan.) (Prof. Dr. Ir. Khairil A. Notodiputro )
viii
ACKNOWLEDGEMENT
I would like to thank and express my gratitude to:
Prof. Dr. Kamaruddin Abdullah MSA as the chairman of the advisory committee, without whom no dissertation has completed. His never-ending support, knowledge, idea, and recommendation to obtain scholarship for PhD sandwich program to the University of Tokyo Japan. Prof. Dr. Armansyah H Tambunan as the member of advisory committee for providing direction in conducting research, critical reading and valuable discussion on the dissertation.
Dr. Ir. Tatang Hernas Soerawidjaya as the member of advisory committee for useful advice and valuable discussion on the dissertation.
Prof. Dr. Yasuyuki Sagara as the member of advisory committee, who has provided an opportunity to study at the University of Tokyo, Japan. Also, Associate Prof. Dr. Tetsuya Araki has supported with valuable advice and
assistance from the beginning of study in Japan. Dr. Hiroshi Nabetani has provided me an opportunity to conduct research and experiments at National Food
Research Institute (NFRI), Tsukuba Japan and for useful advice on publication of our paper to International Journal. Dr. Shoji Hagiwara, who has supported to prepare the research experiments. Also, Hitoshi Maeda, who has taught me in the experiment and analysis (GC, HPLC and TLC/FID) at NFRI, Tsukuba Japan.
The Indonesian Government for financial support (scholarship) provided through Technological and Professional Skills Development Sector Project (TPSDP) Batch III, the Ministry of National Education of Indonesia, ADB Loan No.: 1792-INO.
My husband, Alex Andreas and our children (Berliana Nugraheni and Satria Adinugraha) for their supports, prays and love. Then, all of my friends in
Chemical Engineering Study Program, Institut Teknologi Indonesia and in Agricultural Engineering Science Study Program, The Graduate School, Bogor Agricultural University (IPB).
Bogor, February 2008
ix
BIOGRAPHY
Joelianingsih (author) was born in Semarang July 10th, 1964 as the first child of Hartono and Kustuni. In 1983, she was graduated from SMA Sedes Sapientiae Semarang and continued her study in Chemical Engineering Study
Program, Diponegoro University Semarang. Furthermore, a scholarship was received from “Konsorsium Pendidikan Pertamina-Kontraktor Production Sharing (KPP-KPS)” with contract No.951/K II/KPP-KPS/86 and she was graduated in 1989. She continued her study in Master Degree Program in Chemical Engineering Department, Institut Teknologi Bandung (ITB) in 1991 with scholarship from “STAID-BPPT” (Contract No. SP/434/STAID-BPPT/IX/1991)
and was graduated in 1994. In 2004 she got scholarship for PhD sandwich program in Agricultural Engineering Science, the Graduate School, Bogor Agricultural University (IPB) and the University of Tokyo Japan. This program was funded by the Directorate General of Higher Education, Ministry of National Education through the Technological and Professional Skill Development Sector
Project / TPSDP (ADB Loan. No: 1792 – INO) Batch III.
Author has been working as a lecturer in Chemical Engineering Study Program, Institut Teknologi Indonesia (ITI), Serpong-Tangerang, Banten since 1989 and now she is interested in field of energy conversion technology.
During her study in PhD program, one of her papers has been presented in the seminar “Development in Biofuel Production and Biomass Technology” in
Jakarta, February 21-22, 2006. Some of her papers have been published in national and international journal. A paper with titled “Development of Biodiesel Production Process as a Biofuel” has been published in “Jurnal Keteknikan Pertanian” in December 2006. One of her papers has published in “Journal of Chemical Engineering of Japan Vol. 40, No.9 (2007). Another paper was available online in ScienceDirect, Renewable Energy Journal, Elsevier, in October
x
LIST OF CONTENTS
LIST OF TABLES ………... LIST OF FIGURES ………. LIST OF APPENDICES ………. LIST OF NOMENCLATURE ………. LIST OF ABBREVIATIONS ……….. 1. INTRODUCTION
Background of the Research ……… Objective of the Research ……… Benefits of the Research ………. 2. CURRENT STATUS OF BIODIESEL PRODUCTION PROCESS Introduction ……….
Vegetable Oils as Alternative Diesel Fuels ………. Biodiesel Production by Catalytic Process ……….
Development of the Catalytic Process for Biodiesel Production ……. Biodiesel Production by Non-Catalytic Process ……….. Conclusion ………... 3. NON-CATALYTIC TRANSESTERIFICATION OF PALM OIL IN A BUBBLE COLUMN REACTOR BY SEMI-BATCH PROCESS Introduction ……….. Materials and methods ………. Results and Discussion ………
Conclusion ……….. 4. NON-CATALYTIC ESTERIFICATION OF FATTY ACIDS IN
A BUBBLE COLUMN REACTOR BY SEMI-BATCH PROCESS Introduction ………..
Materials and methods ………. Results and Discussion ……… Conclusion ………..
Page
xii xiii xiv xv xviii
1 4 4
5
6 10
16 19 21
22 23 27
36
38
xi 5. CONTINUOUS PRODUCTION OF PALM METHYL ESTERS IN
A BUBBLE COLUMN REACTOR
Introduction ……….. Materials and methods ………. Results and Discussion ……… Conclusion ……….. 6. FINAL EVALUATION AND DISCUSSIONS ………..
7. CONCLUSION ……….. REFERENCES ……… APPENDICES ……….
57 57 60 69 70
73 75 82
xii
LIST OF TABLES
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
Chemical structure of common fatty acids ... Fatty acid compositions of vegetable oil samples ...
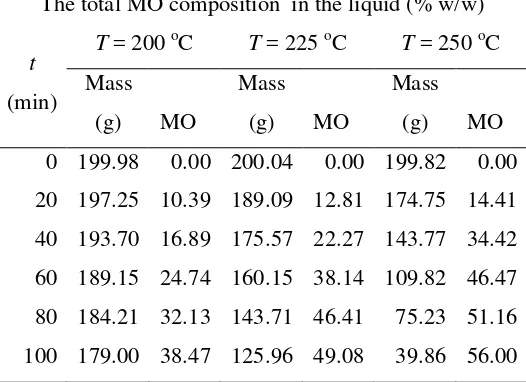
Fuel-related properties of vegetable oils ... The rate constant of palm oil transesterification ... The effects of reaction temperature on the uSG , dband Av ... Summary of transesterification experimental results ... The boiling point of components ... The composition of total MO in the samples B (gaseous product) ...
The composition of total MO in liquid in the reactor ... The concentration ofOA and MO in the total system ... The rate constant of OA methyl esterification ... Comparison of methyl esterification and tranesterification results ... The total mass of sample B and FAME composition ...
The total mass of liquid in the reactor and FAME composition ... The concentration of fatty acids and ME in the total system ... The rate constant of fatty acids methyl esterification ... The normal boiling points of pure fatty acid and FAME ... Effect of the operating condition on the dband Av...
xiii
LIST OF FIGURES
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
Structure of a typical TG molecule ... The reaction mechanism of alkaline-catalyzed transesterification ... The reaction mech. of transesterification with H2SO4 as a catalyst
Schematic flow diagram of reactor used in non catalytic
transesterification ... Change of component mass of the reaction product at 290°C ... Change of component mass of liquid in the reactor at 290°C ... Change of component concentration in the total system during the transesterification of palm oil at 290°C ...
The concentration of uME in palm oil during trans. Reaction ... First order reaction rate constant in Arrhenius plot during the
transesterification of palm oil ... Schematic flow diagram of the reactor used in non-catalytic
esterification ... Conversion of the reaction at various temperatures ...
First order reaction model of OA esterification at various temperatures First order reaction rate constant in the Arrhenius plot during OA methyl esterification ... Conversion of the reaction of various fatty acids at 250°C ... First order reaction model of MA esterification at 250°C ...
First order reaction model of PA esterification at 250°C ... First order reaction model of SA esterification at 250°C ... First order reaction model of LA esterification at 250°C ... Schematic flow diagram 0f the continuous-flow BCR system ... Mass of the product at the various MeOH feed flow rate at 290°C ... Effect of MeOH feed flow rate on the ME content in the product ...
Effect ofMeOH feed flow rate on the MG content in the product ... Mass of the product at the various reaction temperature ... Effect of reaction temperatur on the MG content in the product ... Biodiesel production process flow diagram ...
xiv
LIST OF APPENDICES
1
2 3
4 5 6
7
8 9
Biodiesel development roadmap ...
Characteristic comparisons of fossil diesel fuel (FDF) and biodiesel.. The Photograph of the equipment for transesterification and
esterification by semi-batch process ... The photograph of GC analyzer ... The photograph of HPLC analyzer ... The photograph of TLC/FID analyzer ...
The photograph of equipment for continuous transesterification Process ... Prediction of the bubble diameter and interfacial area ... Material and Heat Balances Calculation ...
Page 83
84
85 86 87 88
xv
LIST OF NOMENCLATURE
A Av
CFA,0
CFA,t
CM
COA, 0
COA, t
CP
CTG,0
CTG,t
CuME,0
CuME,t
D DAL
db dCOA
dCTG
dt Ea
g HR
HP
∆H1
Frequency factor
Gas-liquid interfacial area per unit gas+liquid volume
Fatty acids concentration at initial point of the reaction
Fatty acids concentration at t reaction time Methanol concentration
Oleic acid concentration at initial point of the reaction
Oleic acid concentration at t reaction time Heat capacity
Tryglycerides concentration at initial point of the reaction
Tryglycerides concentration at t reaction time Unmethyl esterified compounds concentration at initial point of the reaction
Unmethyl esterified compounds concentration at t
reaction time
Diameter of the reactor
Diffusivity of gas (methanol) in liquid (palm oil)
Bubble diameter
Change in oleic acid concentration Change in triglycerides concentration Change in reaction time
Activation energy Acceleration of gravity
Enthalpy of reactant Enthalpy of product
Latent heat of vaporization at T1
[-] [m2m-3]
[% mol/mol]
[% mol/mol] [% mol/mol] [% mol/mol]
[% mol/mol]
[kJ kg -1K-1]
[% mol/mol] [% mol/mol] [% mol/mol] [% mol/mol] [m] [m2s-1] [m] [% mol/mol]
[% mol/mol] [min] [kJ mol-1]
[ms-2] [kJ] [kJ]
xvi
∆H2
∆Hnb
∆Hf o
∆HR o
k1 k2 k3 k k' kL L ML P PC PME R R2 rOA rTG T TC Tnb Tr
Tr n
t uSG
Y
Latent heat of vaporization at T2
Latent heat of vaporization at Tnb
Standard heat of formation at 25°C Standard heat of reaction at 25 °C
Reaction rate constant defined by Eq. (3) Reaction rate constant defined by Eq. (4) Reaction rate constant defined by Eq. (5) Reaction rate constant defined by Eqs.(2) & (7) Reaction rate constant defined by Eqs.(11) & (16) Mass transfer coefficient for the liquid phase
Height of the reactor
Molecular weight of liquid (palm oil) Pressure
Critical pressure
Productivity of methyl ester
Universal gas constant defined by Eg. (6) Coefficient of determination
Reaction rate of oleic acid Reaction rate of triglycerides Temperature
Critical temperature
Normal boiling point Reduced temperature
Reduced temperature at normal boiling point Reaction time
Superficial gas velocity
Yield of methyl ester
[kJ/kg, kJ/kmol] [kJ/kg, kJ/kmol] [kJ/kmol] [kJ/kmol]
Depend on the reaction order
[min(% mol/mol)]-1 [min-1] [m s-1]
[m] [kg/kmol] [Atm, bar, Pa] [bar] [kg m-3s-1]
[J mol-1K-1] [-] [(%mol/mol)min-1] [(%mol/mol)min-1] [oC or K]
[oC or K]
[oC or K] [-] [-] [min] [m s-1]
xvii α
εG
µL σL
ρL
ρME
A
υ λ
Conversion of the reaction Gas hold up
Viscosity of liquid
Surface tension of liquid Density of liquid (palm oil) Density of methyl ester Molar volume of methanol gas Constant in Eq. (27)
[% mol/mol] [m3 m-3] [kg m-1 s-1]
xviii
LIST OF ABBREVIATIONS
BCR CN CP CR DG DT FAME FDF FFA FP GC GL HV HPLC KV LA MA ME MeOH MG MO OA PA PP RD SA SC TC TG
Bubble Column Reactor
Cetane Number Cloud Point Carbon Residu Diglycerides Density
Fatty Acid Methyl Esters Fossil Diesel Fuel Free Fatty Acids Flash Point
Gas Chromatography Glycerol
Heating Value
xix THF
TLC/FID uME
Tetrahydrofuran
CHAPTER I
INTRODUCTION
Background of the Research
Biodiesel has become more attractive recently because it has been shown to
be the best supplement to fossil-based fuels due to environmental advantages, renewable resource availability, and the ability to lessen dependence on imported oils. Additionally, it is well suited for immediate substitution of petro-diesel in system utilizing existing diesel engines and in fuel distribution infrastructure.
Technically, biodiesel is a better fuel than petro-diesel in terms of engine performance, emissions reduction, lubricity, and biodegradability [1, 2]. In Indonesia, research and development activities on biodiesel research, production and utilization have advanced to such a stage that its application as diesel supplement. Answering the need, the Indonesian government has shown its seriousness in developing bio-fuel. Various policies which are supporting the
development of this energy have been made. Among them are the Presidential Regulation No. 5/2006 regarding the National Energy Policy [3], and Presidential Instruction No. 1/2006 regarding the utilization of bio-fuel which was released formally on 25 January 2006 [4].
Referring to the National Energy Policy, the Minister of Energy and
Mineral Resources has issued the National Energy Management Blueprint [5]. Blueprint covers the national strategy in managing and utilizing various energy resources including the roadmap of biodiesel. Detail of biodiesel road map is presented in Appendix 1. The estimated target of biodiesel utilization is set by 1.5 million kilo litre in 2010 (10% of diesel oil consumption in the transportation sector) and will be increased up to 6.4 million kilo litre in 2025 (20% of diesel oil
Biodiesel can be made from transesterification of vegetable oils or animal fats with short-chain alcohols, mainly methanol (MeOH). The major component of vegetable oils and animal fats are triacyglycerols or triglycerides (TG). In the
transesterification process, TG are first reduced to diglycerides (DG), and then to monoglycerides (MG). Lastly, the monoglycerides are reduced to fatty acid methyl esters (FAME; also called biodiesel) and glycerol (GL) [6, 7, 8].
Most of the currently known methods for biodiesel production use an alkaline and/or acid catalyst. However, there are at least two problems associated with this process. The first problem due to the two phase nature of vegetable oil/MeOH mixture requires vigorous stirring to proceed in the transesterification
reaction. The second problem is products purification because the reaction product contains residual catalyst, unreacted MeOH and saponified products [9]. The disadvantages resulting from the use of a catalyst could be eliminated if the non-catalytic transesterification can be realized.
The non-catalytic transesterification has several advantages. The removal of
free fatty acids (FFA) from oil by refining or preesterification is not required. In the non-catalytic process, two types of reaction exist in this method for methyl esters formation, transesterification of TG and methyl esterification of fatty acids. Thus, a higher yield can be obtained than that produced by the alkaline-catalyzed method [10, 11]. In addition, because of a catalyst free process, separation and
purification become much simpler and environmentally friendly. The disadvantages of the non-catalytic process are the necessity of the large molar excess of MeOH (the molar ratio of MeOH/oil was 24 – 42) and higher operating temperature (240-350 oC) than the catalytic process [9, 12, 13]. The optimum temperature of the catalytic transesterification was 60 oC when molar ratio of MeOH/oil was 6 [8]. Additionally, most of the non-catalytic transesterification
were conducted under pressurized conditions, i.e., supercritical or sub-crtical of MeOH, which are not viable in practice in industry.
Yamazaki et al., [14] studied the non-catalytic alcoholysis of sunflower oil for biodiesel fuel production in a bubble column reactor (BCR). Effects of
maximum out flow rate of FAME, the optimum condition is 290 oC and 0.1 MPa. Increase in MeOH feed flow rate and initial oil volume, and decrease in stirring rate all increased the outflow rate of FAME. Increase in outflow rate of FAME
with MeOH feed flow rate indicates the large effect of product inhibition in this reaction. Decrease in outflow rate and total production rate of FAME with increase in stirring rate shows the effect of MeOH bubble residence time in the liquid phase. Effect of initial oil volume on reaction also indicates the same effect. From these results, not the interface between top surface of liquid phase and bottom of gas phase but the interface between MeOH bubbles and the surrounding liquid phase seemed to affect the reaction. It is predicted that increasing this
interface area and prolonging the residence time of MeOH bubble in the liquid phase leads to an improvement in reaction rate. However, kinetic study of transesteerification reaction, the bubble interface, performance of BCR for methyl esterification of FFA, and the continuous-flow BCR have not yet been investigated.
The dissertation begins with giving background of the research by reviewing available biodiesel production technologies (Chapter I). Current status of biodiesel production process was described in Chapter II. The advantages and disadvantages of each production technology were described in this chapter. In the Chapter III, The kinetics study of the non-catalytic transesterification of palm oil
was studied in the BCR by semi-batch process. Performance of the BCR for the non-catalytic of methyl esterification of fatty acids was described in Chapter IV. This chapter presented the comparison of methyl esterification and tranesterification results. In addition, reactivity of palm fatty acids (myristic, palmitic, stearic, oleic and linoleic acids) was investigated in this chapter. Chapter V described the continuous flow BCR for non-catalytic transesterification of palm
Objective of the Research
a. To study the kinetics of the non-catalytic transestrification of palm oil at atmospheric pressure by semi-batch process. The effects of reaction temperature on the rate constant, conversion, yield of ME and composition of the reaction product in the transestrification are investigated.
b. To study the performance of a bubble column reactor for the non-catalytic methyl esterification of FFA at atmospheric pressure by semi-batch
process. Five fatty acids which are commonly found in palm oil were chosen as substrates.
c. To develop a process for the continuous non-catalytic transesterification of palm oil in a BCR. This study is focused on determining the optimal reaction time for maximum production of biodiesel from palm oil with the
best quality.
Benefits of the Research
a. To give contribution in the development of science and technology, specifically for bio-diesel production by non-catalytic process in a BCR. b. To provide basic information of biodiesel production by non-catalytic
process in a BCR such as the reaction temperatures for transesterification and esterification, effect of MeOH feed flow rate on the productivity and quality of bio-diesel.
c. The ultimate benefit of this research is to provide essential data for developing scale up model of biodiesel production system by non-catalytic
CHAPTER II
CURRENT STATUS OF BIODIESEL PRODUCTION
PROCESS
Introduction
As energy demands increase and fossil fuel reserves are limited, many
research efforts are directed towards alternative renewable fuels. Biomass is considered as one of the potential renewable energy resources for the future owing to its large potential, economic viability and various social and environmental benefits. Oils and fats, both being biomass resources, are considered as the best candidate for diesel fuel substitute in diesel engines. More than 100 years ago, a brilliant inventor named Rudolph Diesel designed the original diesel engine to run on vegetable oil. Rudolph Diesel used peanut oil to fuel one of his engines at the
Paris Exhibition in 1900 [15].
However, several obstacles had to be overcome. Vegetable oils typically have viscosities ten to twenty times higher than the viscosity of fossil diesel fuel. This quality leads to poor fuel atomization and results incomplete combustion, which was already attested in the 1920s [15]. The high flash point attributes to its
lower volatility characteristics. This leads to more deposit formation, carbonization of injector tips, ring sticking and, a gradual dilution and degradation of the lubricating oil. The combination of high viscosity and low volatility of vegetable oils causes poor cold engine start up, misfire and ignition delay. Oxidative and thermal polymerisation of vegetable oil cause a deposition on the injectors forming a film that will continue to trap fuel and interfere with
combustion. As a consequence, long-term operation on neat vegetable oils or on mixtures of vegetable oils and fossil diesel fuel inevitably results in engine breakdown [16]. These problems can be solved by either adapting the engine to the fuel or by adapting the fuel to the engine. The first strategy led to the development of vegetable oil engines, but this strategy is hard to realize because such engines would be more difficult to be built since it would be larger, heavier
of fossil diesel. The four most widely used technologies in this context are pyrolysis, microemulsification, dilution, and transesterification [16], but only transesterification reaction can lead to the products commonly known as biodiesel, i.e., alkyl esters of oil and fats [17].
Vegetable Oils as Alternative Diesel Fuels
Nowadays, various of vegetable oils, such as palm, soybean, sunflower, peanut and olive oils have been used as alternative fuels for diesel engines. Due
to the rapid decline in crude oil reserves, the use of vegetable oils as diesel fuels is again promoted in many countries. Depending upon climate and soil conditions, different nations or regions have different vegetable oil sources. For example, soybeans oil in the United States, rapeseed and sunflower oils in Europe, palm oil in South East Asia (mainly Malaysia and Indonesia), and coconut oil in the Philippines.
Composition of Vegetable Oils
The basic constituent of vegetable oils is TG. Figure 1 shows a typical TG molecule, where R1, R2, R3 are long chains of carbons and hydrogen atoms,
sometimes called fatty acid chains. Vegetable oils comprise 90 to 98% TG and
O ║
H2 C – O – C - R1
O ║ HC – O – C - R2
O ║ H2 C – O – C - R3
Fig.1 Structure of a typical TG molecule.
carbon chain length and in the number of double bonds. The structures of common fatty acids are given in Table 1 [18].
Table 1 Chemical structure of common fatty acids
Fatty acid Systematic name Structure a Formula
Lauric Dodecanoic 12:0 C12H24O2
Myristic Tetradecanoic 14:0 C14H28O2
Palmitic Hexadekanoic 16:0 C16H32O2
Stearic Octadekanoic 18:0 C18H36O2
Arachidic Eicosanoic 20:0 C20H40O2
Behenic Docosanoic 22:0 C22H44O2
Lignoceric Tetracosanoic 24:0 C24H48O2
Oleic cis-9-Octadecenoic 18:1 C18H34O2
Linoleic cis-9, cis-12-Octadecadienoic 18:2 C18H32O2
Linolenic cis-9, cis -12,cis-15-Octadecatrienoic
18:3 C18H30O2
Erucic cis-13-Docosenoic 22:1 C22H42O2
a
xx:y indicates xx carbon in the fatty acid chain with y double bonds
Table 2 Fatty acid compositions of vegetable oil samples
Fatty acid composition in wt. % Sample
16:0 16:1 18:0 18:1 18:2 18:3 Others
Cotton seed 28,7 0 0,9 13 57,4 0 0
Poppy seed 12,6 0,1 4,0 22,3 60,2 0,5 0
Rapeseed 3,5 0 0,9 64,1 22,3 8,2 0
Safflower
seed
7,3 0 1,9 13,6 77,2 0 0
Sunflower seed
6,4 0,1 2,9 17,7 72,9 0 0
Sesame seed 13,1 0 3,9 52,8 30,2 0 0
Linseed 5,1 0,3 2,5 18,9 18,1 55,1 0
Wheat grain 20,6 1,0 1,1 16,6 56,0 2,9 1,8
Palm 42,6 0,3 4,4 40,5 10,1 0,2 1,1
Corn marrow
11,8 0 2,0 24,8 61,3 0 0,3
Castor a 1,1 0 3,1 4,9 1,3 0 89,6
Soybean 13,9 0,3 2,1 23,2 56,2 4,3 0
Peanut kernel
11,4 0 2,4 48,3 32,0 0,9 4,0
Hazelnut kernel
4,9 0,2 2,6 83,6 8,5 0,2 0
Walnut
kernel
7,2 0,2 1,9 18,5 56,0 16,2 0
Almond kernel
6,5 0,5 1,4 70,7 20,0 0 0,9
Olive kernel 5,0 0,3 1,6 74,7 17,6 0 0,8
a
Fuel-Related Properties of Vegetable Oils
The fuel-related properties of vegetable oils are presented in Table 3 [16, 19]..
Table 3 Fuel-related properties of vegetable oils
Oils KV CN HV CP PP FP DT CR SC
Corn a 34,9 37,6 39,5 -1,1 -40,0 277 0,9095 0,24 0,01 Cotton
seed a
33,5 41,8 39,5 1,7 -15,0 234 0,9148 0,24 0,01
Crambea 53,6 44,6 40,5 10,0 -12,2 274 0,9044 0,23 0,01
Linseed a 27,2 34,6 39,3 1,7 -15,0 241 0,9236 0,22 0,01 Peanut a 39,6 41,8 39,8 12,8 -6,7 271 0,9026 0,24 0,01 Rapeseed a 37 37,6 39,7 -3,9 -31,7 246 0,9115 0,30 0,01 Safflower a 31,3 41,3 39,5 18,3 -6.7 260 0,9144 0,25 0,01 Sesamea 35,5 40,2 39,3 -3,9 -9,4 260 0,9133 0,25 0,01
Soybean a 32,6 37,9 39,6 -3,9 -12,2 254 0,9138 0,27 0,01 Sunflowera 33,9 37,1 39,6 7,2 -15 274 0,9161 0,23 0,01
Palm a 39,6 42,0 - 31,0 - 267 0,9180 - 0,01
Babasu a 30,3 38,0 - 20,0 - 150 0,9460 - 0,01
Castor b 297 42,3 37,4 - - - - 0,21 0,01
Poppyseedb 42,4 36,7 39,6 - - - - 0,25 0,01
Wheat grainb
32,6 35,2 39,3 - - - - 0,23 0,02
Hazelnut b 24,0 52,9 39,8 - - - - 0,21 0,02
Walnut b 36,8 33,6 39,6 - - - - 0,24 0,02
Almond b 34,2 34,5 39,8 - - - - 0,22 0,02
Oliveb 29,4 49,3 39,7 - - - - 0,23 0,02
a
Source: [16] b Source: [19]
Biodiesel Production by Catalytic Process
Biodiesel has been defined as the monoalkyl esters of long-chain fatty acids derived from renewable feedstocks, such as vegetable oils and animal fats, for use
in compression-ignition (diesel) engines [20]. Biodiesel, consisting of FAME can be produced by transesterification of triglycerides and/or esterification of fatty acids with short-chain alcohol, mainly MeOH.
Chemistry of Transesterification Process
Transesterification [21], also called alcoholysis, is the displacement of alcohol from an ester by another alcohol in a process similar to hydrolysis, except than an alcohol is used in instead of water. This process has been widely used to reduce the viscosity of TG. The transesterification reaction is represented by general equation:
RCOOR1 + R2OH ↔ RCOOR2 + R1OH (1)
Ester Alcohol Ester Alcohol
If MeOH is used in the above reaction ( Eq.(1)), it is termed methanolysis. The overall transesterification reaction is given by Eq. (2). However, three consecutive
and reversible reactions are believed to occur. These reactions are given by Eq. (3), (4) and (5).
O O ║ ║
H2C -O-C-R1 CH3 -O- C-R1
| O O CH2 -OH
| ║ ║ |
HC - O-C-R2 + 3 CH3OH → CH3 - O-C-R2 + CH - OH
| O O |
| ║ ║ CH2 -OH
H2C- O-C-R3 CH3 –O-C-R3
TG 3 MeOH 3 FAME (ME) GL (2)
TG + MeOH ↔ DG + ME (3) DG + MeOH ↔ MG + ME (4)
MG + MeOH ↔ GL + ME (5)
The first step is the conversion of TG to DG, followed by the conversion of DG to MG, and of MG to GL , yielding one FAME molecule from each glyceride at each step [6, 7, 8].
Alkaline-Catalyzed Transesterification
Alkaline catalysis is far the most commonly used in transesterification reaction for biodiesel production. The main advantage of this form of catalysis over acid-catalyzed transesterification is about 4000 times faster under the same temperature condition and amount of catalyst. Moreover, alkaline catalysts are
less corrosive to industrial equipment, so that they enable the use of less expensive carbon-steel reactor material. Finally, alkaline-catalyzed transesterification make do with far smaller alcohol volumes than are required for acid-catalyzed reaction, so that reactor sizes can be reduced [15].
Different technologies are currently available and used in the industrial production of biodiesel fuel, which is sold under different trademarks. For
example, there are the Italian processes Novemont, the French IFP, the German Henkel and ATT. Generally, the process is batchwise in the presence of alkaline catalyst such as potassium hydroxide (KOH) or sodium hydroxide (NaOH) under atmospheric pressure and at temperature of approximately 60 to 70 oC (close to the boiling point of MeOH) with an excess of MeOH. The FFA is neutralized with alkali to form soap during the reaction. After the reaction is finished, an acid is
same condition, 67 to 84% conversion of crude vegetable oils into methyl esters can be obtained, compared with 94 to 97% when using refined oils [22].
There are several variables affecting the yield of FAME (biodiesel) by transesterification of vegetable oil. It would be related with the quality of feedstock (free fatty acids and water content), reaction parameters (temperature,
pressure, molar ratio of MeOH to oil, mixing intensity), the length of alkyl chain of alcohol (a number of simple alcohols up to n-hexanol), and catalyst (type and concentration). Some types of catalyst have been used for the transestrification that can be categorized as homogeneous alkaline catalysis and heterogeneous alkaline catalysis. The reaction mechanism of alkaline-catalyzed
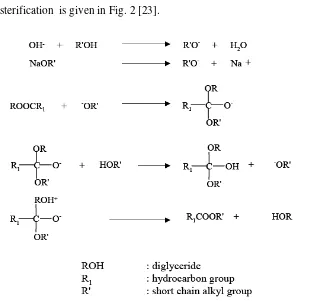
[image:32.612.160.481.284.585.2]transesterification is given in Fig. 2 [23].
Fig. 2 The reaction mechanism of alkaline-catalyzed transesterification.
R-COOH + KOH → R-COOK + H2O (6)
FFA Potassium hydroxide Soap Water
This means that alkaline-catalyzed transesterification optimally works with high-quality and low-acidic vegetable oils, which are however more expensive than waste oils. If low-cost materials, such as waste fats/cooking oils or unrefined vegetable oils with a high amount of FFA, are to be processed by alkaline catalysis, costly deacidification or pre-esterification steps are required.
Acid-Catalyzed Transesterification
Acid catalysis transesterification offers the advantage of also esterifying FFA contained in the fats and oils as presented in Eq.(7), and is therefore
especially suited for the transesterification of highly acidic fatty materials, such as palm oil or waste edible oil.
R-COOH + CH3OH → R-COOCH3 + H2O (7)
FFA MeOH FAME Water
Figure 3 shows the reaction mechanism with H2SO4 as a catalyst. The catalyst
(H2SO4) dissociates to 2H+and SO42-. In the first step, H+attaches to the oxygen
Fig. 3 The reaction mechanism of transesterification with H2SO4 as a catalyst.
However, acid-catalyzed tranesterification are usually far slower than alkali-catalyzed reaction and require higher temperatures and pressures as well as higher amounts of alcohol. The typical reaction conditions for homogeneous acid-catalyzed methanolysis are temperatures of up to 100 oC and pressures of up to 5 bars in order keep the alcohol liquid. A further disadvantage of acid catalysis -
probably prompted by the higher reaction temperatures – is an increased formation of unwanted secondary products, such as dialkylethers or glycerol ethers [17]. Finally, in contrast to alkaline reactions, the presence of water in the reaction mixture proves absolutely detrimental for acid catalysis. Canakci and Van Gerpen [25] reported that the addition of 0.5% water to a mixture comprising soybean oil, methanol and sulfuric acid reduced ester conversion from 95% to
Enzymatic Catalysis
Although biodiesel is at present successfully produced chemically, alkaline-catalyzed and acid-alkaline-catalyzed methods are both sensitive to the presence of water andfree fatty acids. In addition, the reaction has several drawbacks: it is energy
intensive, recovery of glycerol is difficult, the catalyst should be removed from the product and alkaline waste-water required treatment. The disadvantages of using chemical catalysts can be overcome by using lipases (enzymes) as the catalysts for ester synthesis [26]. As compared to other catalyst types, biocatalysts have several advantages. They enable conversion under mild temperature-, pressure- and pH-conditions. Neither the ester product nor the
glycerol phase has to be purified from alkaline catalyst residues or soap. That means that phase separation is easier, high-quality glycerol can be sold as a by-product, and environmental problems due to alkaline wastewater are eliminated [27]. Moreover, both the transesterification of triglycerides and the esterification of FFA occur in one step. As a consequence, also highly acidic fatty materials, such as palm oil or waste oil, can be used without pretreatment [28]. Finally,
many lipases show considerable activity in catalyzing transesterification with long or branched-chain alcohol, which can hardly be converted to fatty acid ester in the presence of conventional alkaline catalysts and catalysts can be reused.
Bottlenecks to use of enzymatic catalysts include the high cost of lipases compared with inorganic catalysts (in the absence effective schemes for multiple enzyme use), the reaction is slowly (8-70 h), inactivation of the lipase by contaminants in the feedstocks (phospholipids and water), and inactivation by
polar short-chain alcohols. An effort to reduce the production cost made by utilizing an immobilized enzyme was reported by Shimada et al. [29]. They researched the factors affecting methanolysis of vegetable oils by Candida antartica lipase for the continuous production of biodiesel. An important finding was that by a novel stepwise addition of methanol, serious degradation of lipase in
Development of the Catalytic Process for Biodiesel Production
Alkaline-catalyzed traneseterification of TG in vegetable oils, with the addition of an acid-catalyzed reaction to esterify FFA are the technologies
presently in use for industrial-scale biodiesel production. However, the desire to reduce or remove catalyst cost, waste output and to obtain the simpler process has stimulated the investigation of alternate methods of biodiesel synthesis. These methods, described here, are largely in the developmental stage, with little or no actual application in the biodiesel industry to date.
In-situ Transesterification
The term in-situ transesterification refers to process, in which the oil contained in vegetable seeds is extracted and transesterified in one step. That means that the lower alcohol serves both as an extracting agent for the oils and the
reagent for alcoholysis. This method may serve essentially to reduce substrate costs in biodiesel production. In-situ transesterification offers a series of advantages. First, hexane is no longer necessary as a solvent in oil recovery. Second, the whole oil seed is subjected to the transesterification process, so that losses due to incomplete oil production are minimized. Finally, the esterified oils tend to be easier to recover from the solid residue than native oils due to their
decreased viscosities [30].
Both alkaline catalysis [31, 32] and acid catalysis [30, 33, 34] have been applied. Unlike conventional alcoholysis reactions, ethanol and higher alcohols, such as 1-propanol or 1-butanol, are favored over MeOH for in-situ processes. This is due to the fact that MeOH is a very poor solvent for oils, so that the yields
of in-situ methanolysis tend to be low [34]. One exception is the acid-catalyzed in-situ methanolysis of high-acidity rice-bran oil, which was found to give higher conversion than the respective processes conducted with other alcohols [35]. The main limitation of in-situ ethanolysis reactions is the water content of the alcohol. It is suggested that only anhydrous ethanol will give satisfactory results, as otherwise the esters will be contaminated with sulphurous and phosphorous
longer-chain alcohols, in-situ transesterification is generally considered as uneconomic at the moment.
Monophasic Transesterification
One major problem in alkaline-catalyzed transesterification of TG is the fact that the oil substrate is not miscible with the alcohol-catalyst phase. Reaction occurs at the interface between the two phases, resulting in a much lower rate than if the reaction mixture was a single phase. That means that transesterification does
not proceed properly, unless the reaction mixture is homogenized in some way. Vigorous stirring/mixing of components is one method of homogenization, which has been found successful for both batch processes [8] and continuous operation [36]. Also the application of low frequency ultrasonic irradiation to form emulsions of oil and alcohol has been reported [37]. Alternatively a common
solvent for both alcohol and oil may be added, including toluene [38], and tetrahydrofuran (THF) [39]. In addition to the use of solvent to promote the miscibility of methanol and oil, a high-MeOH:oil molar ratio (27:1) is employed, raising the polarity of the medium sufficiently to allow a one-phase system, thereby increasing the transesterification rate. The advantages of this approach are the use of a one-step transesterification process, methyl ester yields >98%,
reaction times of <10 min, and lower reaction temperatures. The disadvantages are necessity of recovering the THF and the large molar excess of unreacted MeOH, and inherent hazards associated with flammable solvent. Nonetheless, adoption of this technology for commercial biodiesel production was reported recently [40].
Using Reactive Distillation (RD) Technique
In reactive distillation both chemical conversion and the distillative separation of the product mixture are carried out simultaneously. Through this integrative strategy, chemical equilibrium limitations can be overcome, higher selectivities can be achieved and heat of reaction can be directly used for the process. Increased process efficiency and reduction of investments and
various aspects of it are being investigated worldwide at a tremendous pace. Some works exits in which the production of biodiesel by RD is reported, as the one developed by Omata et al [41, 42], He et al [43, 44]. A continuous-flow reactor using RD has been found feasible for biodiesel production from canola oil with
potassium hydroxide as the catalyst [44]. The operating parameters of 65 °C column temperature and 4:1 MeOH:oil molar ratio with a pre-reactor have yielded promising results. Preliminary results showed that the RD reactor was very effective in transesterifying canola oil to biodiesel. The use of excess alcohol in the feed was reduced by 66%. This implies that the downstream alcohol recovery effort would also be reduced by 66%. The short reaction time, which was 10 to 15
times shorter than those used in batch and existing continuous-flow reactors, led to a 6 to 10 times higher productivity. In summary, this RD reactor bears three major advantages over batch and traditional continuous-flow processes: (1) shorter reaction time and higher unit productivity, which is highly desirable in commercial production units; (2) much lower excess alcohol requirement, which
greatly reduces the effort of downstream alcohol recovery and operating costs; and (3) lower capital costs due to its smaller size and the reduced need for alcohol recovery equipment.
Generally speaking, the operation of an RD reactor is complicated because its performance is affected by several parameters, including the reaction kinetics,
size of the reaction and separation zones, reflux ratio, feed rate, and feeding tray location, etc. The optimum operating conditions are determined as the result of systematic investigations of all operating parameters. Since it is the combination of reaction, distillation and mixing, the design and control of such processes is extremely difficult and at least with the present knowledge and experience, one cannot just rely on thumb rules and gut feelings. Systematic design methods and
simulation strategies are being worked out to design a commercial reactive distillation unit for the given application. Complex interaction of reaction and phase equilibria may lead to non-linear dynamic effects such as multiple steady states, oscillation etc., which are important considerations while operating a
Biodiesel Production by Non-Catalytic Process
At present, as mentioned above, most of the methods on transesterification reaction are in the employing an alkali catalyst. This method has, however, drawbacks such as difficulties recovery of GL, the need for removal of the residual catalyst and the saponified product (soaps) to obtain biodiesel product by neutralization, washing and drying. Furthermore, oils containing free fatty acids and/or water are incompletely transesterified using alkaline catalyst. It prevents a
maximum utilization of low-quality feed stocks such waste frying oil and waste industrial oil. In fact, the use of acid catalyst results in long reaction time and this process is also sensitive to water and free fatty acids content. As a result, it may then affect the success of biodiesel application because of high-energy production cost. Nowadays, the high cost of biodiesel is the major obstacle to its commercialization. The high cost of biodiesel is mainly due to the cost of virgin
vegetable oil. Exploring ways to reduce the high cost of biodiesel is of much interest in recent biodiesel research, especially for those methods concentrating on minimizing the raw material cost and production cost as well. The disadvantages resulted from the use of a catalyst and its removal from the products can be eliminated if the non-catalytic transesterification reaction of vegetable oils with alcohol can be realized.
Non-Catalytic Transesterification in Supercritical MeOH
monomer. This condition also contributes to the change in ionic product in which methanol can be expected to act as acid catalyst. Or in another word, supercritical state is possible to substitute the function of catalysts usually used in the reaction. By applying such a high pressure, the solubility of MeOH can be improved. Therefore, many non-polar organic substances including vegetable oil could be
highly soluble in supercritical methanol so that restrictions in mass transfer due to the phase boundaries do not apply. As a result, a vigorous stirring which is normally applied for heterogeneous reaction such as those in the biodiesel fuel production can be eliminated and the reaction was found to be complete in a very short time (4-10 min).The disadvantages of the non-catalytic process in
supercritical MeOH are the necessity of the large molar excess of MeOH (the molar ratio of MeOH/oil was 24 – 42) and high operating temperature and Pressure (240-350 oC, 9-65 MPa), which are not viable in practice in industry. Therefore, non-catalytic process for biodiesel production need to be developed so that a feasible process and simpler can be realized.
Non-Catalytic Transesterification in a BCR
As mentioned above, using RD technique for biodiesel production is a newest catalyzed process that has many advantages as compared to conventional catalyzed process. If the advantages of the RD can be applied in the non-catalytic
process so a process that more and more advantage can be obtained. Thus, the advantages of non catalytic process are combined with the advantages of RD technique. In the catalytic process, reactants (oil and MeOH) react in the liquid phase so the reaction temperature is close to the boiling point of MeOH (± 65oC at atmospheric pressure). In the non-catalytic process reaction temperature must be increased to obtain the feasible reaction rate. Therefore, if the reaction
temperature is increased to be > 65 oC at atmospheric pressure, MeOH changes to be vapor and the transesterification is conducted in the heterogeneous (gas-liquid) reaction.
for biodiesel production. Due to their simple construction, low operating cost, high energy efficiency and good mass and heat transfer, bubble columns offer many advantages when used as gas-liquid contactors [45]. For the first time, research about biodiesel production in BCR by non-catalytic process was
conducted by Yamazaki et al [14] that studied the effects of reaction temperature, MeOH feed flow rate, operating pressure, stirring rate and initial oil (sunflower oil) volume on out flow rate of FAME. Based on the maximum out flow rate of FAME in the gas phase, the optimum condition is 290 oC and 0.1 MPa. This condition is same with normal boiling point of GL but is below normal boiling point of TG and FAME. This research need to be continued to discuss about
kinetics study of the transesterification and esterification reaction, the effect of FFA in the vegetable oil, etc.
Conclusion
Efforts are underway in many countries, including Indonesia, to search for suitable alternative diesel fuels that are environment friendly. Among the different possible sources, diesel fuels derived from TG (vegetable oils/animal fats) present a promising alternative to substitute diesel fuels. Although TG can fuel diesel engines, their high viscosities, low volatilities and poor cold flow properties have
led to the investigation of various derivatives. Fatty acid methyl esters, known as biodiesel derived from TG by transesterification with MeOH received the most attention. At present, the high cost of biodiesel is the major obstacle to its commercialization. The various process and research for biodiesel production have been developed to reduce the high cost of biodiesel, especially for those
CHAPTER III
NON-CATALYTIC TRANSESTERIFICATION OF PALM OIL
IN
A BUBBLE COLUMN REACTOR BY SEMI-BATCH
PROCESS
IntroductionTransesterification of vegetable oils with short-chain alcohol has long been
a preferred method for producing biodiesel fuel. A BCR has developed to produce fatty acid methyl esters (FAME) by blowing bubbles of superheated MeOH vapor continuously into vegetable oil without using any catalysts. Successful design and operation of chemical reactors requires understanding of chemical kinetics as well as such physical processes as mass and energy transfer. Chemical kinetics is study of the rate and mechanism by which one chemical species is converted to another. The rate is the mass, in moles, of a product produced or reactant consumed per unit time. The mechanism is the sequence of individual chemical events whose overall result produce the observed reaction [46].
It is not necessary to know the mechanism of a reaction in order to design a reactor. What is necessary is a satisfactory equation for the intrinsic rate. This means studying what variables are important and how they affect the rate. At
present, rates of chemical reaction cannot be predicted, so they must be measured. To do this, it is necessary to use a reactor, preferably a small-scale, laboratory unit. Rates cannot be measured directly but must be obtained by interpreting data measured in a reactor. Such data normally consist of concentration of reactants and products, and the specific result depends upon the type of reactor employed.
Various studies have reported kinetics of the non-catalytic transesterification for vegetable oil. Diasakou et al [47]studied the effects of reaction condition (220
o
C, 5.5 MPa and 235 oC, 6.2 MPa ) and molar ratios of MeOH to soybean oil (6, 12, 21) on rate constants and methyl ester content in the reaction mixture. Kusdiana and Saka [48] investigated the effects of molar ratio of MeOH to
MeOH at 120, 150, and 180 oC and reactivity of higher alcohols such as ethanol and isopropanol. Most of these studies were conducted under pressurized conditions, i.e., supercritical or subcritical conditions of methanol. However,
kinetic studies has not been implemented under atmospheric pressure conditions with the non-catalytic alcoholysis process in a bubble column reactor.
This chapter is aimed to describe the kinetics of non-catalytic transesterification reaction under atmospheric pressure. The feeding material is palm oil, since it is ready to be the main feedstock for biodiesel program and Indonesia has a great opportunity to expand its oil plantation [50]. Therefore, in this study, palm oil was chosen as a raw material of biodiesel fuel and kinetics of
the non-catalytic transesterification were studied at atmospheric pressures without using catalyst. Transesterification experiments were made in a semi-batch mode reactor in which bubbles of superheated MeOH vapor were blown into the reactor containing oil. The effects of reaction temperature on the rate constant, conversion, yield of ME and composition of the reaction product in the
transestrification reaction are investigated.
Materials and Methods
Materials
Refined palm oil was obtained from Spectrum Chemical Mfg. Corp.,
Gardena, New Brunswick, with the following characteristic: Iodine Value 50-55, free fatty acid (as oleic) 0.1% w/w, myristic acid, 0.5-5.9% w/w; palmitic acid, 32-47% w/w; stearic acid, 2-8% w/w; oleic acid, 34-44% w/w; linoleic acid, 7-12% w/w. High Performance Liquid Chromatography (HPLC) grade MeOH and molecular sieves 4A 1/16 for transesterification experiment were purchased from Wako Pure Chemical Industries, Ltd., Japan.
Benzene and hexane (all HPLC grade) used in the Thin Layer Chromatography/Flame Ionization Detector (TLC/FID) analysis were purchased from Wako Pure Chemical Industries, Ltd., Japan. Benzene was used as developing solution, and hexane as solvent. Squalane (C30H62) as internal standard
obtained from Sigma Chemical, St. Louis, MO. Acetonitrile (HPLC grade) as solvent and glycerol as standard compound used in the HPLC analysis were purchased from Wako Pure Chemical Industries, Ltd., Japan.
Reactor for Non-Catalytic Trasesterification
The experiments were conducted using a semi-batch mode reactor in which bubbles of superheated MeOH vapour were blown into the oil containing reactor. Schematic flow diagram of reactor used in non-catalytic transesterification
[image:44.612.142.506.291.464.2]experiment is shown in Fig. 4. The photograph of equipment is given in Appendix 3.
Fig. 4 Schematic flow diagram of reactor used in non catalytic transesterification experiment.
The BCR was a 500-mL four-necked flask (the diameter of flask is about 9.85 cm) equipped with a condenser, a pipe for methanol vapour feed and a
temperature controller (TC). The reactor was placed in a mantle heater. The glass dehydration column was filled with the molecular sieves. A pump with a variable speed motor (model NPD-461, Nihon Seimitsu Kagaku Co., Ltd., Japan) was used to control charging rate of MeOH. The tin bath was placed on an electric stove. Temperature of tin bath was monitored by a temperature indicator (TI).
Transesterification Reaction Procedure and Conditions
The reactor was initially charged with 200 g of the refined palm oil and heated to the desired temperature. Reactions were conducted at 250, 270, and 290
o
C under atmospheric pressure. Liquid MeOH was pumped out of the dehydration column to the tin bath for vaporization. The MeOH vapor was taken through a ribbon heater and the reaction started by blowing the bubbles of superheated MeOH (0.1 MPa, 230-260 oC) continuously into the reactor at fixed flow rate of 4 g/min. Reacted products in gas phase were condensed and collected using a glass container. The reaction products were taken from the glass container every 20 min and then weighed (samples A). During the reaction course (300 min), 15 samples
were collected. Liquid samples in the reactor were taken every 20 min to analyze TG, DG, MG and ME contents using TLC/FID.
Analysis
The fatty acid composition of palm oil was analyzed by gas chromatography using a GC-2010AF (SPL/FID) Series Gas Chromatograph System equipped with a split/splitless injection system, a flame-ionization detector (FID), an auto injector AOC-20i and a LabSolution GSsolution software from SHIMADZU CO. Japan. The column was a 30 m x 0.25 mm, 0.25 m DB-Wax capillary column
(J&W Scientific from Agilent Technologies USA) with He at 40 cm/s as the carrier gas and a split ratio of 5.0:1. Injector and detector temperatures were 300
o
C, oven temperature started at 50oC for 3 min, increased to 250 oC at a rate of 10
oC/min, and held at this temperature for 8 min. About 1 ml of prepared sample
was put into GC auto sampler vials and 1 L of sample was injected into the
column. The calculation of mass percentage for each fatty acid was based on ratio of peak area of each component to total peak area. The photograph of GC-2010AF is given in Appendix 4.
The GL contents in the samples A were analyzed by an HPLC (JASCO, Tokyo, Japan), equipped with a model 880-PU pump, a degasser DG-2080-53, a column oven CO-965, an intelligent sampler 855-AS, a recorder Borwin
in inner diameter, Shiseido, Tokyo, Japan) was used for separation. Samples A (0.5 mL) which had been filtered by using advantec filter (DISMIC-13 JP, PTFE 0.20 µm, Toto Roshi Kaisha, Ltd., Japan) were diluted with 1.5 mL solvent
(acetonitrile : water = 85 : 15 vol) and then were put into the intelligent sampler. Mass of the samples was measured before and after dilution. The HPLC mobile phase consisted of a 85:15 volumetric mixture of acetonitrile and water was used as a carrier solvent. The HPLC pump was operated at 1 mL/min solvent, and the column temperature was kept at 40oC. The sample injection volume was 10 L. Based on the results of the preliminary experiment, the glycerol contents in the liquid samples taken from the reactor were very small so it can be ignored. The
photograph of HPLC analyzer is given in Appendix 5.
TLC/FID was used to analyze contents of TG, DG, MG and ME in the samples of reaction products without MeOH and the liquid samples in the reactor [51]. MeOH contents in the samples A were evaporated using a rotary evaporator and then the reaction products without MeOH were obtained (samples B). Each of
samples B and the liquid samples in the reactor was weighed and its composition was analyzed using TLC/FID. Analyses were performed with an Iatroscan MK-5 Analyzer (IATRON LABORATORIES, INC., Japan). The flame ionization detector used hydrogen and air with flow rates of 160 mL and 2000 mL/min, respectively. Type SIII Chromarods were used as thin layer. Before being spotted,
rods were scanned as blank on the instrument to obtain the proper degree of hydration. The samples B and the