Antitumor
Effects
of
Eubacterium
Lentum
Fractions and
Its
Correlation
with
the Macrophages,
Natural
Killer
Activities in Mice
Mochammad Hatta
Abstrak
Dilakukan penelitianterhadap pengaruhfraksi sel dari Eubacteriu,n Ienturn, terutatna pengaruh dinding sel danfraksi 2 (ttetrbran sitoplasrna) terhadap "Ehrlich ascites tutnor bearing uice". Senuafraksi kecuali dinding sel danfraksi 2
Uai
eyetctyialatn tnenghatttbat pertutnbuhan "Ehrlich ascites twnor" pada nencit ICR. Inieksi intratunor dinding sel Eubacteriun lenturn dengan dosissikurang-kurangnya lOO 1tg secaraberntakna menghanbat pertunrbuhan seltunor pada harike
2I
setelahinokulasi selturnoràan mernperpanjang "survival time" mencit. lnieksi intravena dinding sel dengan dosis sekurang-kurangnya 20O Fg efektif dalanr rtenperpanjang ',surttival tinte"dari
"tumor bearing nùce". Selanjutnya diperiksa pula pengaruh penberian dinding sel dan fra6si 2 secara intraperitoniunt terhadap aktivitas makrofag dari cairan peritonewn. Hasil yang didapatkan menunjukkan bahwa baikdinling
sel naupun fraksi 2 uentputryai ketnanpuan untuk neningkatkan aktivitas tnakrofag dari cairan peritoneum pad0 nencit Balb/c. Disantping itu diteliti ,,celIrttediated cytotoxicity" pada level sel tunggal terhadap YAC-1 lyrnphona sebagai sel target dengan penberian clin4ing sel ataufraksi 2 dan kinetik dari aktivitas sel "natural killer" Iinpa. Hasil tersebut nenunjukkan bahwa baik dinding sel nnupunfraksi 2 nerupakan aktivator yang kuat dari sel "natural killer" Iitttpa padc ntencit
eH/He.
Abstract
The present stttdy wtts undertaken to eratnine the effects of cell.fraction.s 0f Eubacteriuttr lenturtt, especially cell wall and fraction 2 (cytoplasuic rnernbrane) on Ehrlich asciîes tuntor- bearing nice. AII fraction.s etcept cell wall and
fraction 2 4kl not effectively inhibit
the Ehrlich ascites tunror Srowth
in
ICR tnice. Inlratunoral iniectiort of cell wall of Eubacteriuril lentu,,t at a lose ofat
least 100 pg sisnificantly inhibited the twnor Slrowth 21 days afler tunor inoculating and prolonged the survival tirne of nice. Intravenous injection of cell wall at a dose of at least 2OO pS was effective in prolonging the survival titrte of tutttor-bearing trtice. Moreover, the efîectsof
intraperitoneal treatntent by cell wall orfractiorr 2 of Eubacteriun lentun on uacrophage activities ofperitoneal eru4ate cavity 1nfC1 cells was eranùned. The results obtained showed that both ceII wall attdfractiotr 2 have the ability to etiltance the peritoneal tnacrophage acfiviry in Balb/c nùce. Furthertnore, cell ntediated cytotoxicity at single cell levels against YAC-l lytnphotna as target cell when treoted with cell wall orfraction 2 and the kinetic.s of natural kiUer (NK) activity of spleen cells were observe1. These results s1,ggest that both cell wall andfraction 2 are potent activators of natural kilter (NK) of spleen cells in CtH/He rnice.Key words : Antitunor, Eubacteriun lentunfractiotts, Macrophage, Natural Killer
INTRODUCTION
Eubacterium lentum
is
an
anaerobic
gram
positive
short
rod
bacteria and
a
natural component
of
thehuman
intestinal flora.
Theantitumor
effe cts ofEubac-terium lentum
have been
studied continously
in
ex-perimental animals.l
Some basic
effects of Eubacterium
lentum on theimmune
system and its effects on various experimentaltumor cell lines
have
beenobserved.
Also,
Eubacte-rium
lentum has been shown to haveindirect
cytotoxic
effects againts
Ehrlich
ascites
tumor.2 Recently,
avariety
ofimmunomodulatory
effects have beenobser-ved
in tumor
bearing
hosts,following
treatment
with
bacteria
or bacterial
products as
biological
responsemodifiers, but
the mechanism
by which
these agentswork
isstill
unclear. Commonly,
grampositive
bacte-ria cell walls
such as thatof Bacillus
cereus,Lactoba-cillus
casei
and Nocardia rubra
have peptidoglycan
compounds3'4'5'6 und antitumor effects inexperimental
animal
systems.Macrophages have
multiple
functions
such asan-tineoplastic
activity
and regulation of immunerespon-ses. Some bacteria,
such
as
Listeria
monocytogenes86 Hatta
and
Mycobacterium tuberculosis
have beenshown
to increase macrophageactivity by in vivo
treatment.T'8Amongst
the
known
cellullar
effector
mecha-nisms,
natural
killer
(NK)
cells mediated
by large
granular lymphocytes
arethought to
represent thefirst
line
of
defence against
"un".r.'
The ability
of some
bacteria
such as Streptococcuspyogenes,
lnctobacil-Ius casei, Mycobacterium tuberculosis, Bordetella
pertusk, Nocardia rubra, Corynebacterium
parvum
and
Propionebacterium
acnes
to
augment
NK
cells,
and
induce
various
cytokines
both in
human and
ex-perimental
animals has been reported, to'l I'12'r3'14'r5't6MATERIALS
AND METHODS
Animals.Inbred
maleICR mice,
C3H/Hemice, Balb/c
mice,5
to 8 weeksold
were purchasedfrom
Japan SLCInc.,
Hamamatsu, Japan,Tumor
cell
lines. The
Ehrlich
ascitestumor
syn-geneic to
ICR mice
were usedfor
in
vivo
experiments.YAC-1
lymphoma, P-815
mastocytoma,
EL-4
lym-phoma used
astarget
cells
were maintained
on
con-tinous
in vitro
culture
in
RPMI-1640
supplementedwith
10%
FCS,
100
U
pencillin/ml,
100g/ml
srrep-tomycin
and 2mM
L-glutamine.
Preparation
of
Eubacterium
lentum
fractions.
Eubacterium lentum
(TYH-I1)
was
obtained
in
our
laboratory
from
normal human intestinal
flora.
Thebacteria
were grown
in
GAM broth medium
(Nissui
Co., Ltd.) overnight at
37oCunder anaerobic
condi-tion.
The cellscollected by centrifugation
at 8000 gfor
30minutes
at 4oC were washed three timeswith
sterile
distilled
water.
The cells
were
suspendedin
distilled
water
andlyophilized.
Two
g
lyophilized Eubacterium lentum
whole
bodies were
suspendedin 100
ml distilled
water,
dis-persed
in
asonicator
for
15minutes
anddisrupted
in
Homogenizer
cells (SiberKikai K.K.,
Japan) at 900 bar pressure.The
process
was judged complete when
morethan 80%
of
non
fragmented
bacteria were
seen
in
smearsstained
by the
Gram method.
The
disruption
product was
centrifuged
at 3000 g
for
30 minutes
toprovide a
supernatant.
The
sediment
was
suspendedwith distilled
water
andlyophilized
toprovide
fraction
I
containing Eubacterium lentum whole body
im-purities, Then
the
supernatant
was centrifuged
at 20000 gfor I
hour
to remove a sedimentof
"crudecell
wall"
and
the
was
fraction
2,
containing
cytoplasmic
m
Crudecell
wall"
was treatedby
themethod
I.r7
Briefly,
3 gof
"crudecell
wall"
was suspendedin
400ml
0.07
M
phosphatebuffer
(pH. 7.8) containing
lO%
eachoftrypsin
andchymotrypsin.
The suspensionMed
J
Univ Indonwas
gently stirred
at room temperaturefor
24 hours and thencentrifuged
at 20000 gfor I
hour. Thetrypsin
andchymotrypsin
treatment was
repeatedand the
super-natantsprovided fraction 3
and
4
containing
the
cell
wall
impurities and teichoic acid, respectively.
The sediment was then suspendedin
0.01M Tris
HCI-buff-er (p,H 7.2)
containing lO%
pronase.
After
gentleagitation
for
24 hours at room temperature, thesuspen-sion was centrifuged
at
20000
g for
t
hour.
This
pronase treatment was repeated and supernatants were
fraction
5 and 6containing free
lipids.
The
sedimentswere
washed
with Tris HCI
buffer,
O.85%NaCl in
water,
distilled
water respectively and
lyophilized
to
provide "pure
cell wall" (cell wall
fraction).
Antitumor activity
in vivo. Ehrlich
ascitestumor
cells were
suspended
in
RPMI-1640
supplementedwith
lO% FCS
andinoculated
at
lOscells/animal
in-traperitoneal (i.p.)
or
106cells/animal
subcutaneous (s.c.). Eachfraction
wasinjected intraperitoneally,
in-tratumorally
or intravenously
for
7 days.Survival
mice were
followed for
42
daysfor
theintraperitoneal inoculation
(ascitesform)
and 100 daysfor
the
subcutaneous
inoculation (solid
form) of
Ehrlich
ascitestumor.
Mean
survival time was
calculated
using
following
formula
:MST
(%
T/C)
= meansurvival
daysof
treatedgroup/
control group
x
100.Tumor weight
was calculated usingfollowing
formula:
Tumor
weight
(mg)
= {major
axis
x (minor
axis|2112.Preparation
of
macrophages
cytostasls
assay.Balb/c
mice
were injected
intraperitoneally with
purecell
wall
orfraction
2for
7 days andperitoneal
exudatecavity
IPEC) cells were obtained
onday
14. The PECcells were collected
by
washing the peritoneal cavity
of
mice
with
2.5
ml
Hank's balance salt solution
(HBSS)
andcentrifuged at 800
g forl0
minutes.
ThePEC
suspensionwas incubated
in
a
plastic
dishes
at37"C
for
90
minutes and
plastic
adherent
cells
were used aseffector
cells. Suspensionofeffector
cells were added totriplicate
wells to give effector
targetratio
10: I
or 5
:
l.
The
macrophages cytostasis
assay wasperformed
in
96well microtiter
using
slCr
prelabeledtarget.
The
assayplates
were
incubated
20 hours
at37"C
in
a
humidified
COz
incubation. After
incuba-tion,
the percentageof specific
5lCr
release wascalcu-lated
using
a gamma counter.with KAC-2
to remove
monocytes/macrophages. Themononuclear
cells
were obtained
after
centrifugation
on Ficoll-Hypaque gradients
(density
= L007) at 3000
rpm
for 30
minutes,
andcells were collected,
washed and used aseffector
cells.Cytotoxicity
was
measuredin
standard
4h slCr
releasemicrocytotoxicity assay using
96-well
round
bottome
Cambride,
MA).
YAC-L (2
x
I
t
cells were
labeled by
incubati
tt
CrOo
for
I
hour
at37oC
in
a shaking water bath.
After
incubation, the
cells
were
washedthree times
with
HBSS
to remove
unbound radio label.
Suspensionof
effector cells
was added totriplicate
wells to give
aneffector
targetratio
of
50
:
I
or
25:
1. Afteran
additional incubation at
37oC for4 hours,
each well wascounted
in a gamma
counter to determineexperimental
release(ER).
Spon-taneousrelease
(SR)
was obtained
from wells
recei-ving target cells
and
medium only,
and
total release
(TR) was obtained
from
wells receiving
l%
^lriton
x-100.
The
percentageof cytotoxicity
was calculated
by
fol-lowing formula
:% of cytotoxicity
= {(ER)
-(sR)l/{(TR)
-(sR)l
x 100.
RESULTS
Effects
of
intraperitoneal
(i.p.)
injection
of
Eubac-terium
lentum
fractions
on ascites
fornt
of
Ehrlich
ascites tumor.To
determine
antitumor effects
of
Eubacterium
lentum fractions,
ICR
mice were inoculated
intra-peritoneally
with
lO5 cells/mice
of
ascites
form
of
Ehtlich
ascites
tumor
and
injected
i.p.
with
Eubac-terium
lentum
or
Eubacterium lentum
fractions
for
7 days(table
1).All
control
animals died of progressive
tumor growth in the
peritoneal
cavity
on
day 17
after
tumor inoculation.
Mean
survival time
of
theEubac-terium
lentum group,
the fraction 2group
and thecell
wall fraction
group
(26,22 and
22
daysrespectively),
were
all
longer than
that
of
the control group.
No
significant
antitumor effects were shown
by the
other
fraction groups.
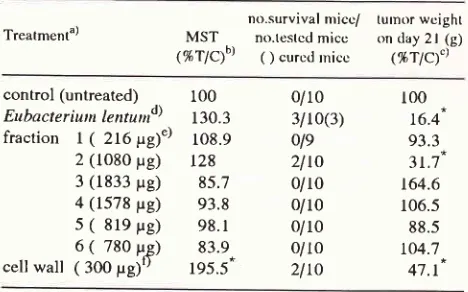
Effects
of intratumoral (i.t). injectiott
of Eubacterium
Ientum
fractions
on
solid
form of
Ehrlich
ascites tumor.The
i.t.
treatment
with
Eubacterium
lenturn,fraction2
andcell wall fraction slightly
prolonged the survival
time
and significantly suppressed thetumor weight
onday
2l
as compared to thecontrol group
(table 2). The meantumor
weight
(%T lC)of
these three groups were31.2%,25.2%
and 27.67orespectively.
In
contrast
noantitumor activities
were observed in the otherfraction
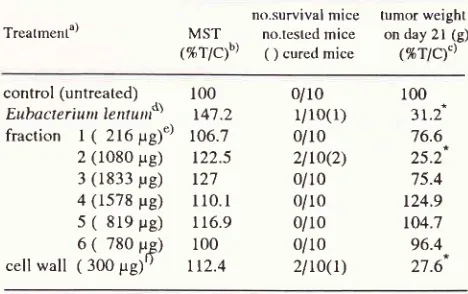
groups.Table
l.
Effectsof intraperitoneal
injection of Eubacterium Ierûutn fracttons on Ehrlich ascites tumorTreatment") MSTb) % survival
control (untreated) Eubacteriwn lentutn")
fraction
I
(
216;rg)d)2 (1080 pe) 3 (1833 pg) 4 (1578 pg) 5
(
819 prg) 6(
780 prg) cell wall ( 300 F,g)")17.3 + 0.8
*
26.7 + 2.8 15.8 + 3.1
** 22.2 + O.9
18.5 + 1.8
20.8 + 2.8 18.6 + 1.5
20.6 + 2.8 22.0 + 1.7
100
154
9l
128 128t2l
108 119t25
a) Ehrlich ascites tumor cells (105 cells/mouse) inoculated into ICR mice (10 mice/group) intraperitoneally (i.p.) on day O. b) mean survival time (days) indicates the mean + standard
deviation.
c)
Eubacteriutn lentun (107 cells/mouse) injected i.p. for 7 days. d) Each fraction injected i.p. for 7 days.e) pure cell wall injected i.p. for 7 days.
Statistical significance of difference from untreated control
*p.0.oI
""p.0.05.
Table
2.
Effects of intratumoral injection of Eubacteriumlen-ltlrt
fractions on Ehrlich ascites tumor.Treatment")
no.survival
mice
tumor weightMST
no.testcdmice
on day 2l (g)(%TÆb)
( ) curetlmice
(%TlC')"\control (untreated) Eubacteriwn lentunf\
fraction
l(
216pg)')2 (1080 pg) 3 (1833 yg) 4 (1578 pg) 5
(
8le
pg) 6(
780 pg) cellwall
( 300 pg)')100 r4't.2 106.7
t22.5 t27
ll0.l
116.9
100
r12.4
0/10
l/ro(r)
0/102lto(z)
0/10 0/10 0/10 0/10 2l
ro(t)
100
3r.ù
76.6 25.2 75.4 t24.9 t04.7 96.4 27.6a) Ehrlich ascites tumor cells (106 cells/mouse) inoculated into ICR mice (10 mice/group) subcutaneously (s.c.) on day O. b) survival time (%T lC) indicates the mean tested/control group
x
100.c)
tumor weighT (%"îlC) indicates the mean tested/control groupx
1,00.d) Eubacteriuttt lentuttt (107 cells/mouse) injected intratumoral-ly (i.t) for 7 days.
e) Each fraction injected i.t. for 7 days.
f)
pure cell wall injected i.t. for 7 days.Statistical significance of difference from untreated control
[image:3.595.308.539.166.298.2] [image:3.595.305.539.454.601.2]88 Hatta
Effects
of intravenous
(i.v.) injection of Eubacterium
lentum
fractions on
solid
form of
Ehrlich
ascites
tumor.
After
multiple treatments
with
Eubacterium
lentum,fraction
2
andcell wall,
thetumor weights on day
2l
were
significantly
lower
than thatof
thecontrol group
(table 3). The mean
tumor weight (% TIC)
of
thesethree
groups were 16.4%,3I.7%
and47.1%
respec-tively. The survival times
of
mice receiving
Eubac-terium lentum,
fraction
2 and cell wall
were
slightly
prolonged.
In the other fractions
no antitumor effects
were seen.
Table
3.
Effects of intravenous injection of Eubacteriumlen-turt fractions on Ehrlich ascites tumor.
Med
J Univ Indon
Table
4.
Effects of intratumoral injection of various doses ofpure cell wall on Ehrlich ascites tumor
Treatmenta)
no.survival
mice/
tumor weightMST
no.testedmice
on day 2l (g) (%fPf\
( ) curedmice
(%r1c1')-control (untreated) 5o pe)d) 100 pg) 200 pg) 300 pg) 500 pg) 800 pg) t0O0 pg)
r00 130.6 162 212.5 t69.4 t97.2 2t3.9 222.2 0/10 0/r0
l/10
3/8(l) 0/102lto(t)
0/10 2le(2) 100 45.3 34.0 32.5 35.8 18.4 19.8 15.9 Treatment')control (untreated) Eubacter iunt le ntu t nd)
fraction
l(
216pg)")2 (1080 pg) 3 (1833 prg) 4 (1578 pg) 5
(
819 pg) 6(
780 prg) cell wall ( 300 Udt)100 130.3 108.9 r28 85.7 93.8 98.
l
83.9 195.5-0/10 3/10(3) ole2lto
0/10 0/10 0/10 0/r02lto
100 16.4 93.3 3t.7 t64.6 106.5 88.5 to4.7 47.1a) Ehrlich ascites tumor cells (106 cells/mouse) inoculated into ICR mice subcutaneously (s.c.) on day O.
b) survival time (%TlC) indicates the mean tested/control group
x
100.c) tumor weight (%T lC) indicates the mean tested/control group
x
100.d) Eubacteriutn lentutn (107 cells/mouse) injected
intravenous-ly (i.v) for 7 days.
e) Each fraction injected i.v. for 7 days.
f)
pure cell wall injected i.v. for 7 days.Statistical significance of difference from untreated control
*
P
'
o.ol.Effects of local injection of various
dosesof cell wall
on
tumor growth
Various
doses (50 pgto
1000pg) of cell wall fractions
were examined
for the
effects of theirintratumoral
(i.t.)
injection on tumor growth (table 4).
Dosesof
100pg
to
1000 pgsignificantly
inhibited
rhe tumor growth on day2L
Mice
treatedwith a
doseof
1000pg cell
wall
caused clearinhibition
of tumor growth
and prolongedthe
survival time of tumor-bearing
mice (two fold in
comparison with
thecontrol group);
there was acom-plete
curein
2of
9 tested mice.cell wall
a) Ehrlich ascites tumor cells (106 cells/mouse) inoculated into ICR mice subcutaneously (s.c.) on day O.
b) survival rime (%oTlC) indicates the mean tested/control group
x
100.c) tumor weighT (%TlC) indicates the mean tested/control group
x 100.
d) various doses of pure cell wall injected intratumorally (i.t.) for 7 days.
Statistical significance of difference from untreated control
*p.0.01
**p.0.05.
Effects
of systemic
injection
of various
dosesof cell
wall
ontumor growth
Cell
wall i.v.
injected
at
a dose
of
at least
2OO 1tgsignificantly inhibited the
tumor growth
on day2l
andprolonged
thesurvival time (table 5). Cell wall
at thedoses
of 200 pg
and500 pg
completely cured
I
of
II
tested
mice
and 3 of 7 testedmice respectively.
Table 5. Effects of intravenous injection
of various
doses ofpure cell wall on Ehrlich ascites tumor.
Trcatmcnta)
no.survival
mice/
tumor weightMST
no.tcstcdmice
on day2l
(g)('/,TÆb\
( ) curedmice
&îp1;l'
no.survival micc/MST
no.testcd micc(%T/Cb)
O curctl rniccturnor wcighl on day 2l (g) (%Tlc)")
control (untrcatcd) cell wall
(
50 pg)(l0O trg) (20o pg) (3oo pg) (50O trg)
t00 7f .8 87.3 t73.' 195.5-
229.2-0/r 0 0/10 0/10 li r
l(l)
2lto
317(3')
100 t28.7 t04.
l
77.4 47.1 38.8a) Ehrlich ascites tumor cells (106 cells/mouse) inoculated into ICR mice subcutaneously (s.c.) on day O.
b)
survival lime (%TlC) indicates the mean tested/control group x l0O.c)
tumorweight(tATlC) indicates the mean tesrcd/control groupx l0O. [image:4.595.52.286.293.439.2]Macrophages
activity of
peritoneal
cells
following
multiple
intraperitoneal
injection
of cellwallandfrac-tion
2of Eubacterium
lentum.The
ability
of
cell
wall
and fraction
2 to
augment macrophageactivity
was also assessed onday 14 after
the
initiation
ofcell wall and fraction
2 ofEubacterium
lentum
treatment,using
P-815mastocytoma and EL-4
lymphoma target cells by
)rCr
release assay(table
6).Percentage
of
cytotoxicity
of
PEC cells
from
mice
treated
with
cell wall
against P-815 and EL-4
targetcells as
E/T ratio
of
l0 : I
was l7.O% and 16.3%
respectively
(p < 0.01).
andwith fraction
2 treatment22.37o
and I5.8%.
Percentage cytotoxicity
of
PECcells from mice
treatedwith cell
wall
and fraction 2 atE/T ratio
of
10:
I
was
I'7 .O% and 15 .8% . Percentagecytotoxicity
of PEC cells from mice treated with cell
wall
and
fraction 2
at
EIT
ratio
of 5
: I
was
alsosignificantly increased both
against P-815 and EL-4
target cells
comparedwith
theuntrated control group
(p <
0.05).
These results demonstrate
that both
in-traperitoneal
injection
of
cell
wall
and fraction
2 of
Eubacterium lentum have the ability
to
augment
themacrophages
tumoricidal
activity
of
peritoneal
ex-udatecavity (PEC) cells in Balb/C mice.
Table 6. Macrophage
activity of peritoneal exudate cavity
(PEC) cells following treatmentwith
cellwall
and fraction 2 of Eubacteriwn lentunr.cytostasis activityb)
Group") treatment P-8 15") EL-4
l0: ld)
5l
reproducible
resultsin
thesurvival time of tumor
bear-ing
mice (about
two fold
as compared
with
control
group).
As shown
in
table 7, the
NK
activity
of
spleencells
againstYAC-l
lymphoma target cells
wasdeter-mined
by intravenous multiple injections of cell wall
or
fraction
2of Eubacterium
lentum.Cell wall
andfraction 2 treatments significantly
augmented the NK activity of spleen cells as compared
with
the
untreated
control group.
Percentage
cyto-toxicity of NK cell by cell wall treatment was 63.I%
(E/T 50 :
1) and49% (ElT
25 :
l)
respectively.
These results suggest that
both cell wall and
frac-tion
2 of Eubacterium lentum
havethe ability to
aug-ment
the
NK
activity
of
spleen
cells
in
an animal
experiment.
Table
7.
Effectsof
cellwall and
fraction2 of Eubacterium
lentwn on Natural Killer (NK) activity of spleen cells.cytostasis activity against YAC-lb)
Groupu) treatment
50: l")
25:L
I control
(untreated) 2 cell wall (300 g) 3 fraction 2 (1080 g)45.5d) 63.
l
62.O28.7 49.O 51.3
a) Balb/c mice (5 mice/group) injected intraperitoneally with cell wall or fraction 2 for 7 days.
b) percentage of cytostasis activity measured by the s I Cr release assay.
c) PEC cells collected on day 14 and cultured with target P-815 mastocytoma or EL-4 lymphoma for 20 hours.
d) effector : target ratio.
e) mean of triplicate culture.
Statistical significance of difference from untreated control
*p<0.01
**<0.05
Effects of
cell wall and
fraction
2 treatment onnatural
killer (NK)
activity
of spleen cells.Based on the results
shown in
table 5, doses of 300pg
cell wall fraction
used because at this dose weobtained
a) CrH/He (5 mice/group) injected intraperitoneally (i.v) three times with cell wall or fraction 2 on day -5, -3, and -1.
b) percentage of cytotoxic activity measured by the 5 I Cr release assay on day O.
c) effector
: target ratio.d) mean of triplicate culture.
Statistical significance of difference ftom untreated control
*
P
'
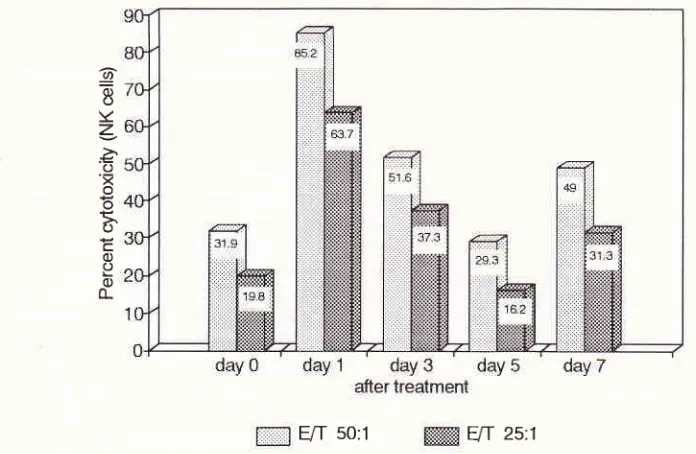
o.ol.Kinetics of systemic single injection
of cell wall and
fraction
2 on naturalkiller
(NK) activity
of spleen cells.The
cytotoxic
effectsof
intravenoussingle injectio_n
of
cell wall
and fraction
2
were
assessedby
the
5ICr
release method deseribedin "Materials
andMethods".
The
following
experiments were performed
to
ex-amine the kinetics
of cellular cytotoxicity
against theYAC-1 lymphoma cells. As shown in figure
l,
spleencell
treatedwith cell wall showed the same
pattern
in
both ratios of E/T 50 :
I
and25 :
I.
Spleencells from
untreated
control
mice showed percentagecytotoxicity
of
34.6% (E/T
50
: l)
and 25.2%
(ElT 25
:
1). In
addition,
cytotoxicity of
spleen cellsfrom
mice treatedwith cell wall were markedly
increasedon day
I
andthereafter
gradually
decreasedup
to
the
level lower
than that of untreated control. The treatment by
frac-5l
l0 l
I control
(untreated) 2 cell wall (300 prg) 3 fraction 2 (1080 pg)4.5"
*
9.g **t1.o
***
15.222.3
16.690
tion
2 (1080pg),
significantly
increased the percentagecytotoxicity
ascompared
to
the
control
group on
day 1 and 3(31.9 and
I9.8% for E/T
50
:
1 andEIT
25
:
I
respectively).
Med
J
Univ IndonIn
contrast
to
the
kinetics of cell wall
treatment,the cytotoxic
activity
of
spleen
cells
by
fraction
2 treatment was increased again on day 7 at ratios ofboth
50
:
i
and25
:
1(figure2).
@
b
oY
z
P. o'=
o oç.
o=
oo o o_
[image:6.595.141.449.150.372.2] [image:6.595.94.442.449.676.2]F/I
50:1 ffi
VT
zs:t
Figure 1. Kinetics of Natural Killer (NK) acitivity of spleen cells treated with cell wall of Eubacteriutn lentuttt
a) C jH/He
il
) injecîed intravenously (i.v.) with pure cell wall (30Oydnice)
on duy 0.b)
cytotoxici
gairct YAC-I lynphoua cells tletennirted in a 4-hr 5tCr release assay.c) cytotoxic activity indicated as the mean oftriplicate culture.
E/T
50:1E[f
25:1Figure 2. Kinetics of Natural Killer (N K) activity of spleen cells treated with fraction 2 of Eubacteriun lentutn a)
Cfl/He
nice (5 mice/group) injected intravenously (i.v.) withfraction 2 (1080 y,g/nice) on day O.b) cytotoxicity ofspleen cells against YAC-1 tyntphona cells detennined n a 4-hr
)tCr
release assay. c) cytotoxic activity indicated as the nean oftriplicate culture.9
oo
Y
z
>P,
O
'x
o o
5,
oc
o)
DISCUSSION
The
presentstudy
demonstratesthat
theantitumor
ef-fects
of
Eubacterium
lentum
fractions
in
Ehrlich
as-cites tumor-bearing mice are the
consequencesof
acombination
of
sequential
immunologic
factors,
lead-ing
to
subsequentinhibition of Ehrlich
ascitestumor
cell
growth and the long-term survival time
of
theanimals. Halpern,
et
all8
reported that
Corynebac-terium
parvum
has a potent
inhibition
of
Ehrlich
as-cites tumor growth
by
intraperitoneal injection,
but
intravenous treatment has
not influenced
significantly
the
mortality
rate
of mice
andalso that
it
is
useful
asan immunotherapeutic
agentfor
patients
with
ascitic
ovarian
tumors. le Bast, et al20 alsà reported thattreal-ment
with
Corynebacterium
parvum
hasinhibited
thegrowth
of
human ovarian carcinoma and that
it
mayprove useful
for
modulating the
activity
of
humaneffector
for
antibody-dependent
cell
mediated
cyto-toxicity.
Inthis study, we
injected Eubacterium
lentum orEubacterium
lentumfractions intraperitoneally (i.p)
into ICR mice
inoculated
with Ehrlich
ascitestumor
i.p. The
i.p.
injection
of
Ehrlich
ascites
tumor
cellsresulted
in
subsequentgrowth of
the
tumor cells
and themice
diedfrom
thetumor
with
increasedperitoneal
effusion (table
l).
This finding
suggesrsthat
this
ex-perimental model
is
suitable
for
studying
exudation
into
the peritoneal
cavity.
Intraperitoneal
injection
of
Eubacterium
lentum,fraction
2 andcellwall following
the
inoculation
of
Ehrlich
ascitestumor cells
into ICR
mice
hassignificantly
prolonged the
survival time of
mice
andintratumoral treatment
with
fraction
2or cell
wall
of
Eubacterium
lentum
following
subcutaneous(s,c.)
inoculation of
the
solid form of Ehrlich
ascitestumor cells
also
significantly
prolonged
the
survival
time
(table 2). Treatmentwith
cellwall was
aseffective
as
that
with
Eubacterium
lentum when the agents wereinjected
i.t.
Furthermore,
we
examined the effects
of
intravenous
injection of
Eubacterium
lentumfraction.
Both fraction
2 and cellwall significantly
inhibited
thetumor growth
as effective as Eubacterium
lentumwhole body.
We also observed a dose dependency of effects
of
cell
wall
fraction
by
local
or
systemic treatment
onErhlich
ascitestumor-bearing mice. Our
present studyindicates that
i.t.
injection
of
cell
wall with
doseshigher
than
100pg/mouse was
effective in inhibiting
the
tumor growth
(table 4) andi.v.
injection
ofcell
wall
with
doses
higher than 200
ug/mouse
significantly
prolonged
thesurvival
time
of
mice (table
5).It
has beenreported
that therole of
macrophagesin
vitro
as
effector cells
was responsible
for
killing
turnor cells and that this effect was
augmented by
Corynebacterium
parvum
treatment.2l
In
the
experi-ments
reported
here,effector
cells
which
appearedin
mouse
peritoneal
exudatecavity
after
intra
peritoneal
injection
of
cell
wall or
fraction
2
of
Eubacterium
lentum were examined
by
the
5lcr
release assay.We
observedthat i.p. treatment
of
cell
wall
andfraction
2 have theability
toinhibit
thegrowth of
Ehrlich
asertestumor in ICR
mice.
Although
themechanism
respon-sible
for
the inhibition
of
tumor
growth was not
clarified, we
considered
that
the activated peritoneal
macrophages inducedby i.p. injected cell
wall
orfrac-tion
2of
Eubacterium lentum
released somemediator
such as
cytotoxic factor (CTF), and that
mediators
activated theperitoneal
macrophages,resulting
macro-phagesof
Kupffer cells were
activated
by
treatment
with Lactobacillus casei
and Corynebacterium
par-uu*.22 We
determined
the
cytolytic activity
of
peri-toneal
macrophagesfrom
mice injected
with cell wall
or
fraction
2 of Eubacterium lentumi.p. and found
thatit
was augmentedby
treatment
with cell wall
or
frac-tion
2
of
Eubacterium lentum (table 6).
These results suggestthat
the ability
of
peritoneal
macrophages toexlude tumor cells
was augmentedby i.p.
administra-tion
of
cell
wall
or fraction 2, resulting
in
inhibited
tumor growth
in
theperitoneal cavity.
It
has beenreported
thatNK
cells
inducedby
thetreatment
with
bacteria such as
Streptococcus
pyo-genes play an
important role in
killing
tumor
cells bothin
animal experiments and
in
humun..23'24In
this
present
study, we
determined
NK
activity
of
spleencells
from mice systemically injected
with
cell wall
and
fraction
2
of
Eubacterium lentum.
NK activity of
spleen
cells
weresignificantly
augmentedby cell
wall
and
fraction
2of
Eubacterium lentum treatment
(table7).
These results suggest
that
NK
activity
of
spleencells play
a key rolein killing
tumor cells
in vitro.
We
also observed that thekinetics
of cytotoxic actil,ity
of
spleen
cells from
mice treated
with cell wall
aredif-ferent
from
mice
treatedwith
fraction
2.The
cytotoxic
activity
of
spleen cellsfrom
mice treatedwith
cell
wall
was
decreasedon day 5
to
day
7following
treatment(figure
l),
but
when treated
with
fraction
2
thecytotoxic activity
increased again onday
7after
treat-ment
(figure 2). In
conclusion,
NK
activity of
spleencells induced
by i.v.
administration
of
cell wall
or
fraction 2
of
Eubacterium lentum should also be
ex-pected
to play
an
important role
in
destroying tumor
cells.Acknowledgements
I
amextremely grateful
toDr.K. Kawai for
his
advice andmanuscript review
and toDr.T.Hayashi
for
92 Hatta
REFERENCES
l.
Sakamoto K, Konishi K. Antitumor effect of Normal Intes-tinal Micro flora on Ehrlich Ascites Tumor. Jpn J Cancer Res (Gann) 1988; 79: 109-16.2. Morinaga S, Sakamoto
K,
KonishiK.
Antitumor Activity and Its Properties of Eubacteriwn lentwn. Jpn J Cancer Res (Gann) 1988; 79:ll7
-24.3. Araki
Y,
NakataniT,
NakayamaK,
Ito
E. Occurence ofN-Nonsubstituted Glucosamine Residues in Peptidoglyean
of Lysozyme-resistant Cell Walls ftom Bacilltts cereus. J Biol Chem L97 2; 247 : 63 12-22.
4. Knox KW, Brandsen J.
fie
isolation of Components from the Cell Wall of ktctobacillus casei. Biochem J 1962; 85:15-23.
5. Sato K, Saito H, Tomioka H, Yokokura T. Enhancement of
Host Resistance a gainst Listeria Infection by lttctobacillus casei : Efficacy of Cell Wall Preparation of htctobacillus ccsci. Microbiol Immunol 1988; 32: ll89-200.
6.
Azuma
I,
Taniyama
T.
Yamawaki
M,
Sugimura K,Yamamura Y. Adjuvant and antitumor activities of Nocardia cell-wall skeletons. Gann 1976; 7:733-6.
7. Beller
DI, Kielly
JM, Unanue ER. Regulationof
Macro-phage Populations.I
Preferential Inductionof
Ia-Rich Peritoneal Exudatesby
Immunologic Stimuli. J Immunol1980;124: 1426-32.
8. Nibbering
PH, Van Der
HeideGA,
Van FurthR.
Im-munocytochemical analysis of cellular responses to BCG. Clin exp Immunol 1989;75: 147-54.9. Whiteside
TL,
Herberman RB. Short Analytical Review. The Role of NaturalKiller
Cellsin
Human Disease. Clin Immunol Immunopathol 1989; 53: l-23.10. Bonavida
B,
JewettA.
Activationof
Human PeripheralBfood-Derived
Monocytesby
OK-432
(Streplococctts pyogenes) : Augmented Cytotoxicity and Secretion of TNF and Synergy with rIFN-r. Cell Immunol 1989; 123: 373-83.ll.
KatoI,
Yokokura T, Mutai M.Macrophage Activation by Ittctobacillus casei in Mice. Microbiol Immunol 1983;27:6l t-8.
12. Yasumoto K, Manabe H, Ueno M, et al. Immunotherapy of
Human Lung Cancer with BCG Cell-Wall Skeleton. Gann 1976;67:787-95.
13. Minagawa H, Kobayashi H, Yoshida H, et al. Intratumoral induction of tumor necrosis factor by systemic
administra-Med
J
Univ Indontion of Bordetella pertussis vaccine. Br J Cancer l99O;62:
372-5.
14. Ogura T, Namba N. Hirao F, et al. Association of Macro-phage Activation with Antitumor Effect on Rat Syngeneic Fibrosarcoma by Nocardia rubra Cell Wall Skeleton. Can-cer Research L979.
39
4706-12.15. Chapes
SK,
Haskill
S.,Synergistic
Effect
between Neutrophil and Corynebacteriutn parvwn in the Process ofMacrophage Activation. Cancer Research 1984;44:
3l-4.
16. Pringle
AT,
Cummins CS, Bishop BF, Viers VS. Fateof
Y accines of P rop io nibacte riun acnes After Phagocytosis by Murine Macrophages. Infection Immunity. 1982;38: 371-4. 17. Azuma I, Ribi EE, Meyer
TI,
et al. Biological active com-ponents ftom mycobacterial cell walls. I. Isolation and com-position of cell wall skleton and component P3. J Natl Cancer Inst 1974;52:95-101.18. Halpern BN, Biozzi G, Stiffel C, et
al.
Inhibition of Tumor Growth by administration of Killed Corynebocteriutil par-vrar. Nature 1966;212: 853-4.19. Mantovani
A,
Sessa C, Peri G, et al. Intraperitoneal ad-ministrationof
Corynebacteriun parvutnin
patient withascitic ovarian tumors
to
Chemotherapy:
Effects
on cytotoxicity of tumor-associated macrophages and NK cells. Int J Cancerl98l;27:437-46.
20. Bast RC Jr, Berek JS, Obrist R, et al. Intraperitoneal
Im-munotherapy
of
Human
Ovarium
Carcinoma with
Corynebacteriu,tt porvunt. Cancer Res 1983; 43:. 1395-401. 21. Reynolds CW, Brunda
MI,
HoldenHT,
Herberman RB.Role
of
Macrophagesin
Invitro
Augmentationof
Rat, Mouse, and Human Natural Killer Activities. J Natl Cancer Instl98l;66:837-42.
22. Hashimoto S, Seyama Y, Yokomura T, Mutai M. Cytotoxic factor production by Kupffer cells elicited with
Lttctobacil-lus casei and Corynebacterium parwu,t. Cancer Immunol Immunother 1985; 20: LI7 - 21.
23. Reynolds CW, Fukui H. Antitumor Activity of Streptococ-cus pyogenes Preparation (OK-432).
I.
Sequential Effector Mechanisms Following a Single OK-432Injection in F344 Rats Leadingto
the Rejectionof
SyngeneicMADB
106Tumor Cells. J Nat Cancer Res 1987; 79:, IOLL-7.