Brain Research 884 (2000) 1–12
www.elsevier.com / locate / bres
Research report
Localization of
b
and
g
subunits of ENaC in sensory nerve endings in
the rat foot pad
*
H.A. Drummond, F.M. Abboud, M.J. Welsh
Howard Hughes Medical Institute, University of Iowa College of Medicine, 500 EMRB, Iowa City, IA 52242, USA
Accepted 8 August 2000
Abstract
The molecular mechanisms underlying mechanoelectrical transduction and the receptors that detect light touch remain uncertain. Studies in Caenorhabditis elegans suggest that members of the DEG / ENaC cation channel family may be mechanoreceptors. Therefore,
1
we tested the hypothesis that subunits of the mammalian epithelial Na channel (ENaC) family are expressed in touch receptors in rat hairless skin. We detected bENaC and gENaC, but not aENaC transcripts in cervical and lumbar dorsal root ganglia (DRG). Using immunofluorescence, we foundbENaC andgENaC expressed in medium to large lumbar DRG neurons. Moreover, we detected these two subunits in Merkel cell–neurite complexes, Meissner-like corpuscles, and small lamellated corpuscles, specialized mechanosensory structures of the skin. Within these structures,bENaC and gENaC were localized in the nerve fibers believed to contain the sensors responsive to mechanical stress. Thusb and gENaC subunits are good candidates as components of the molecular sensor that detects touch. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Somatic and visceral afferents
Keywords: Mechanoreceptor; Mechanosensation; DEG / ENaC; Meissner corpuscle; Merkel cell–neurite complex; Pacinian corpuscle
1. Introduction UNC-105, and UNC-8 [6,12,18,28,44]. These proteins are
part of the DEG / ENaC protein family that includes the 1
The sensation of light touch is an essential part of the epithelial Na channel (ENaC) [4,5,30]. The family also physical perception of the environment. It is required for includes other proteins that serve as sensors, including the detection of objects and discrimination of shape and acid-sensing ion channels and the FMRFamide-activated
1
texture. Many studies have described the mechanosensitive Na channel, FaNaCh [7,16,26,39,46]. DEG / ENaC pro-structures that are responsible for light touch sensation; in teins form non-voltage gated cation channels that in the skin these include Merkel cells, Meissner corpuscles, several cases are inhibited by amiloride Pacinian corpuscles, Ruffini receptors, and free nerve [4,5,8,15,27,30,43]. These channels are composed of endings [3,10,29,35]. The stimuli that activate these struc- multiple subunits, as homo- or hetero-multimers. They tures and their neuronal output have also been reported. each have intracellular amino- and carboxy-termini, two However, the molecular mechanisms for mechanoelectrical membrane spanning domains, and a large, cysteine-rich, signal transduction and the receptors that detect mechani- extracellular domain that is thought to serve as a sensor or cal stress have not been identified. receptor for extracellular stimuli.
Genetic studies in Caenorhabditis elegans have iden- Evidence that DEG / ENaC cation channels play an tified genes for several proteins that may function as important role in mechanosensation first came from the mechanosensors; these include DEG-1, MEC-4, MEC-10, finding that mutation of several C. elegans family mem-bers disrupts touch sensation and / or coordinated activity in the worm [6,12,18,28,44]. This result suggested DEG / *Corresponding author. Tel.: 11-319-335-7619; fax: 1
1-319-335-ENaC channels may serve as mechanosensors. The finding 7623.
E-mail address: [email protected] (M.J. Welsh). that another DEG / ENaC channel, Pickpocket, was
stricted to multiple dendritic mechanosensory neurons in (named hgN-1), a glutathione S-transferase (GST) fusion the Drosophila embryo also suggested a central role in protein containing the last 34 amino acids of the mechanosensation [1,9]. We recently showed that the g C-terminus of rgENaC (residues 616–649, subunit of ENaC was located in baroreceptor nerve termi- GSTVPGTPPPRYNTLRLDRAFSSQLTDTQLTNEL) (na-nals that innervate the aortic arch and carotid sinus [13]. med rgC-2), and a GST fusion protein containing the Moreover, amiloride and its analog benzamil attenuated N-terminus of hbENaC (HVKKYLLKGLHRLQKGPGY-baroreceptor function. These data suggest that the g TYKELLVWYCDNTNTGHPKRIICEGPKK) (named subunit of ENaC, and possibly other subunits, may form hbN-1). The antibodies were generated in sheep by Elmira part of the mechanosensory complex. This led us to Biologicals (Iowa City, IA). Immunoreactive sera was consider the possibility that ENaC subunits may also purified using cyanogen bromide-activated sepharose 4B contribute to peripheral mechanosensation in the skin. To (Pharmacia, Piscataway, NJ) linked to the appropriate begin to evaluate this hypothesis, we asked if ENaC antigen (gN-terminus peptide, GST-bN-terminus or GST-subunits are located in touch receptors in the hairless skin gC-terminus). Detection of ENaC subunits and antibody of the rat paw. specificity were evaluated in COS-7 cells transfected with both human and rata,b, andg ENaC [30]. Note that the N-terminus of rat and human gENaC are 90% identical, the C-terminus of rat and humangENaC are 76% identical, 2. Materials and methods
and the N-terminus of rat and human bENaC are 94% identical, in the regions used as immunogens.
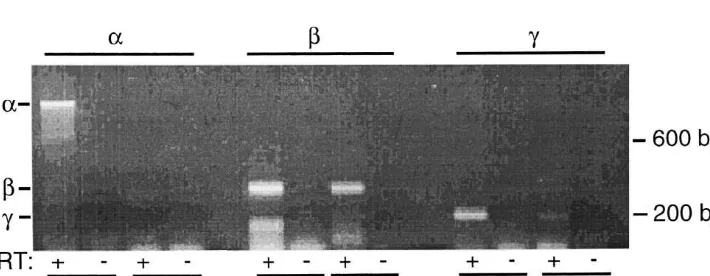
2.1. Reverse transcriptase–polymerase chain reaction
To localize expression ofbENaC and gENaC in touch receptors, we used immunofluorescence in rat DRG and To determine if ENaC family members are expressed in
paw pad. Rats were anesthetized with halothane, perfused dorsal root ganglia (DRG), we used reverse transcriptase–
transcardially with 60 ml ice cold PBS, followed by 60 ml polymerase chain reaction (RT–PCR) to detect a, b and
ice cold 4% paraformaldehyde. The lumbar and cervical gENaC transcripts. Spraque–Dawley rats, (Harlan,
In-DRG and hind paws were removed and fixed for 20 min to dianapolis, IN) were anesthetized with halothane, placed
2 h in 4% paraformaldehyde. The tissue samples were on ice, and cervical and lumbar DRG were removed. The
rinsed in PBS then stored overnight in 30% sucrose in ganglia were combined and flash frozen in EtOH and dry
PBS. Tissue samples were embedded in O.C.T. compound ice, then stored at2708C. Total RNA was extracted using
(Sakura Finetek, Torrance, CA) media then 10–50 mm RNA STAT-60 (TEL TEST ‘B’, Friendswood, TX) and
frozen sections were obtained. The sections were incubated reverse transcribed using a random hexamer primer and
with the primary antibody solution for 72 h at 48C with 5% RNase H-murine leukemia virus reverse transcriptase
normal donkey serum and 0.1% Triton X-100. DRG tissue (Superscript Preamplification System, Gibco BRL,
sections were double-labeled with a 1:100 dilution of the Gaithersburg, MD). Partial cDNA’s from DRG tissue
sheep antibodies hbN-1 or hgN-1 and a 1:100 dilution of were amplified using the following primer pairs:
mouse anti-NF200 (Neurofilament 200 kDa, Sigma Chemi-aENaC, 59-AGTACCTCAGCTACCCAGTGAGC-39,
cal Co., St Louis, MO). The hgN-1 antibody produced a 59-TTGACCGGGACATCGCTACCAT-39; bENaC, 59
-stronger signal-to-noise ratio only in DRG sections. Hin-CTACACCTACAAGGAGCTGCTAG-39, 59
-AAGTGC-dpaw tissue sections were double- or triple-labeled with TTGACCTTGGAGTACTG-39; gENaC, 59
-CCTCTGCT-various combinations of the following antibodies: (1) hb N-GTGGATCGCGTTCAC-39, 59
-CACAGCACTGTACTT-1 (-CACAGCACTGTACTT-1:-CACAGCACTGTACTT-100); (2) rgC-2 (1:100); (3) rabbit anti-S-100 which GTAAGGGTTGAT-39. Samples were preheated to 948C
stains Schwann cells in the peripheral nervous system [23] for 3 min then cycled 40 times at 948C for 20 s, 628C for
(1:100, Sigma Chemical Co); (4) mouse anti-cytokeratin 20 s and 728C for 20 s forb andgENaC; 948C for 45 s,
20 (CK20, 1:100, Research Diagnostics Inc., Flanders, 638C for 45 s and 728C for 2 min for aENaC. After
NJ); (5) rabbit anti-protein gene product 9.5 (PGP 9.5, cycling, all samples were held at 728C for 7 min. PCR
1:2,500, Chemicon, Temecula, CA); or (6) a cocktail of reactions in which reverse transcriptase was not added to
mouse anti-neuron antibodies that included anti-neurofila-RNA served as a negative control and lung anti-neurofila-RNA was a
ment 68 (1:3, Roche, Indianapolis, IN), anti-neurofilament positive control. The products of PCR reactions were run
160 (1:3, Roche), NF200 (1:100, Sigma), and anti-on a 1.5% agarose gel and visualized with ethidium
peripherin (1:100, Chemicon). The samples were rinsed in bromide.
H.A. Drummond et al. / Brain Research 884 (2000) 1 –12 3
MRC1024 (Hercules, CA) laser scanning confocal micro- in small neurons was occasionally observed. Expression of
scope. bENaC and gENaC in some small neurons would not be
unexpected since subpopulations of type III and IV skeletal muscle afferents, whose cell bodies are also located in the
3. Results and discussion DRG, also respond to mechanical stimuli and may
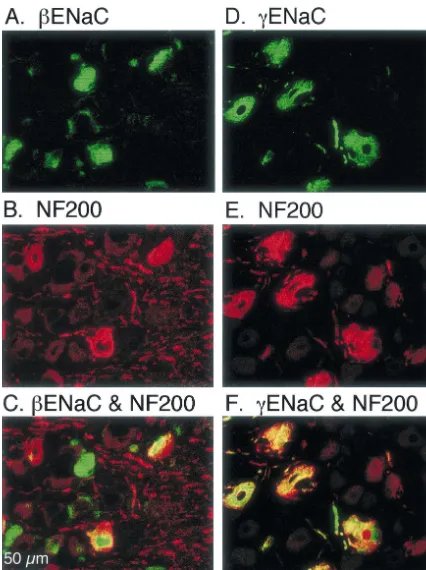
there-fore express mechanosensitive ion channels [24,38]. The As a first step towards determining if ENaC subunits sections were also co-labeled with an antibody directed might be involved in mechanosensation, we asked if their against NF200. NF200 is expressed in large DRG neurons transcripts are present in the DRG, which contains the cell and nerve terminals innervating Merkel cells, Meissner bodies of somatosensory neurons. We used RNA prepared corpuscles, Pacinian corpuscles, hair follicles, and free from DRG and performed RT–PCR for the three ENaC nerve endings [41] (Fig. 2B and 2E). Merging the images subunits. Fig. 1 shows that RT–PCR detected b and showed that bENaC and NF200 are coexpressed in some gENaC, but not a ENaC in cervical / lumbar DRG. These of the large and medium-sized neurons (Fig. 2C). How-primers were used previously to detect b and gENaC as ever, there were clearly some cells that expressedbENaC determined by sequence analysis [13]. Of interest, other but not NF200, and the converse. This result suggests that DEG / ENaC transcripts have also been reported in DRG bENaC is expressed in some, but not all, of the large [47]. We obtained similar results in the nodose ganglia mechanosensory neurons. It is possible that bENaC may [13]. be expressed in specific types of mechanosensory neurons.
1
Coexpression of a, b, and gENaC generates Na Alternatively, the bENaC antibody may not detect low channel currents in heterologous cells; channels formed by levels ofbENaC expression. Fig. 2F shows that most DRG these three subunits play an important role in epithelial cells expressing gENaC also express NF200, suggesting
1
Na absorption [5,30]. However, expression of b and g that gENaC is expressed in some but not all large subunits either alone or together does not generate current; mechanosensory neurons. Lack of expression of b and the a subunit is required to produce constitutively active gENaC in all DRG neurons suggests that these proteins are currents. The lack ofaENaC transcripts in DRG suggests not simply required for sensory nerve cell function. We that b and gENaC do not generate constitutively active have obtained similar results in nodose ganglia [13]. currents in these neurons. Thus, ifbandgare components Moreover, these proteins were not expressed in ventral of a neuronal mechanically-sensitive ion channel, then they horn neurons of the spinal cord or pancreas nerve endings must behave differently in DRG neurons than in epithelia (not shown).
tissue. This would not be unexpected, because a mech- In hairless skin, Merkel cells and Meissner corpuscles anosensitive channel complex would be expected to remain play a central role in the detection of light touch [3,10,29]. closed until activated by a specific stimulus [17,34,40]. The Merkel cell–neurite complex is a slowly-adapting To determine ifbandgENaC protein is present in DRG mechanoreceptor located in the basal epidermal layer. The neurons, we used immunocytochemistry with antibodies Meissner corpuscle is a rapidly-adapting mechanoreceptor directed against these two subunits. In 10mm cryosections located in the apex of the dermal papillae [19,35]. Im-from the DRG, we detected both b and gENaC subunits portantly, both structures are abundant and readily identifi-using laser scanning confocal microscopy (Fig. 2A and 2D, able in the rat paw pad. To determine if b and gENaC respectively). Labeling was observed predominantly in the subunits are expressed in these structures, we prepared 50 large and medium-size cells which are thought to play an mm frozen horizontal sections of rat paw pad. To identify important role in mechanosensation [29], however labeling expression of b and gENaC subunits in Merkel cell–
Fig. 2. Immunocytochemical detection ofbandgENaC in medium and large DRG neurons. Cryosections (10mm) of rat lumbar DRG were exposed to antibodies directed againstbENaC (A),gENaC (D), or NF200 (B and E). Panels C and F show superimposition of panels A and B, and panels D and E, respectively; neurons with co-localization of the two labels appear yellow.
H
.A
.
Drummond
et
al
.
/
Brain
Research
884
(2000
)
1
–
12
5
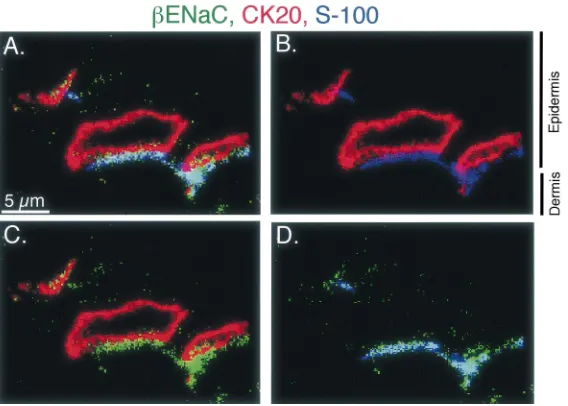
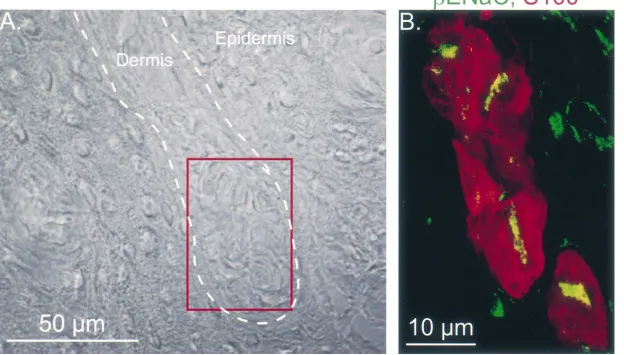
Fig. 4. A cluster of Merkel cells from the rat foot pad immunolabeled for
gENaC. These sections and their preparation are as described in the legend of Fig. 3 withgENaC staining in green, the Merkel cell staining (CK20) in red, and a nerve cell marker (PGP 9.5) in blue.
optical sections (0.5 mm) revealed that bENaC was concentrated adjacent to the Merkel cell where it colocal-ized with the neuronal marker. Limited staining was occasionally observed overlying the Merkel cell
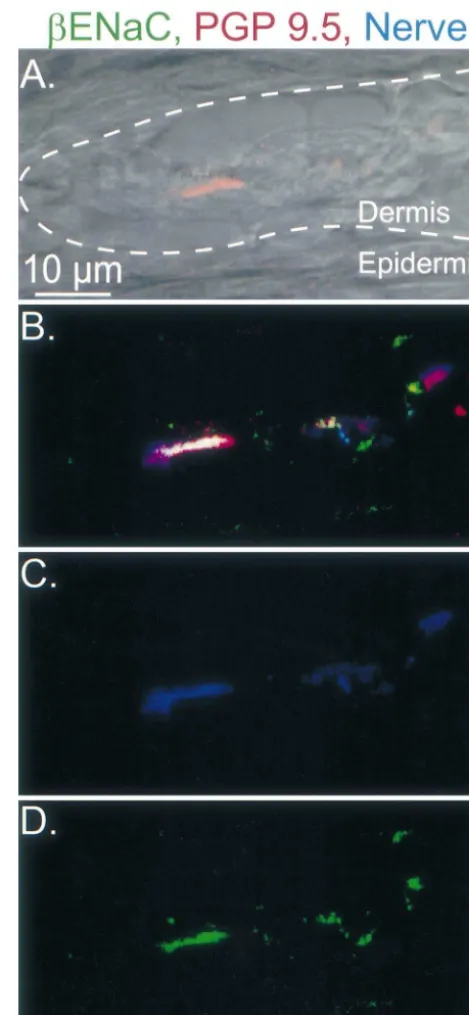
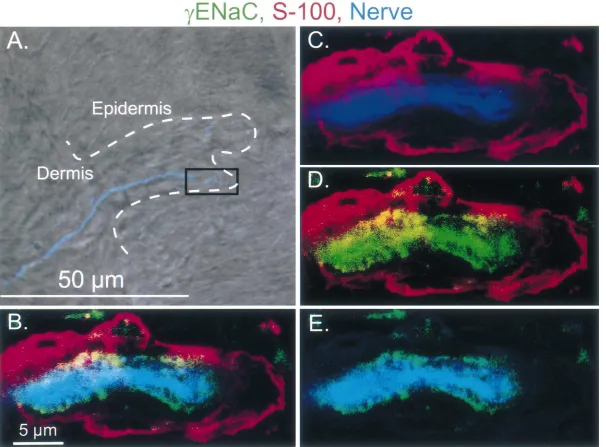
immedi-ately adjacent to the nerve. This was a variable and Fig. 5. Longitudinal section through a Meissner-like corpuscle labeled for
bENaC. Image represents a series of 18 optical sections taken at 0.5mm inconsistent observation; we are not able to determine
intervals by laser scanning confocal microscopy. Sections were stained whether in addition to its expression in the neuron,bENaC
with antibodies directed againstbENaC in green, PGP 9.5 in red, and may also be expressed in the Merkel cell. A similar
other neuronal markers in blue. Panel A shows the dermal–epidermal expression pattern was observed with antibodies togENaC junction as a dashed line.
(Fig. 4).
The question of whether the mechanosensor is present in
the Merkel cell or in the neurite has been discussed. In tained. Injury to Merkel cells has been reported to disrupt support of a role for the Merkel cell as the mechanosensor, Merkel cell function in isolated sinus hairs [42] and touch
21
H
.A
.
Drummond
et
al
.
/
Brain
Research
884
(2000
)
1
–
12
7
wild-type mice, this result suggests that the Merkel cell is in the central axis of the corpuscle, the expected location not required for mechanosensation [25]. Our findings place of the central axon. This region appears yellow because red bandgENaC in the nerve at the interface with the Merkel staining from lamellar cells in some optical sections are
cell. merged with green bENaC staining in other optical
We also localized b and gENaC subunits in lamellated sections.
receptors located in the apex of the dermal papilla. gENaC staining in a Pacinian-like corpuscle is shown in Because of their location and their appearance under light Fig. 8. Compared to Merkel cell and Meissner-like corpus-microscopy, we believe that these receptors are Meissner- cle staining, gENaC staining in Pacinian-like corpuscles like corpuscles. Sections from the rat foot pad were triple- was much weaker than bENaC signal, suggesting that labeled with antibodies directed against b or gENaC, a there may be lessgENaC in Pacinian-like corpuscles. In a cocktail of antibodies directed against peripherin and few specimens,gENaC staining was occasionally observed neurofilaments 68, 160, and 200, and the nerve marker overlying the lamellar cell processes. This was an inconsis-PGP 9.5 or S-100 which labels Meissner corpuscle lamel- tent observation; we are not able to determine whether in lar cells as well as peripheral Schwann cells [23]. Fig. 5 addition to its expression in the neuron,gENaC may also shows a longitudinal section of a Meissner-like corpuscle. be expressed in the lamellar cell. This expression pattern In Fig. 5A, a light field image, super-imposed with a was rarely observed with antibodies to bENaC.
fluorescent nerve marker PGP 9.5 (in red), shows innerva- Our data show that b andgENaC are expressed in the tion of a Meissner-like corpuscle. The dermal-epidermal DRG neurons and the nerve fibers innervating Merkel border is marked by the dashed white line. Fig. 5B shows cells, Meissner-like corpuscles and Pacinian-like corpus-the colocalization of bENaC (green), PGP 9.5 (red), and cles. Within these structures, the neuronal components may other neuronal markers (blue). Staining with the neuronal contain the sensors that respond to mechanical stress. The markers only and bENaC only are shown in Fig. 5C and location ofbandg subunits in the nerve terminals, at the D, respectively. These results suggest that bENaC is interface with Merkel cell, Meissner-like and Pacinian-like expressed in the nerve ending in the Meissner-like corpus- corpuscle lamellar cells places them at the location where cle. the response to a mechanical signal is thought to originate. Fig. 6 shows a longitudinal section through a Meissner- Thus,bandgENaC are good candidates as components of like corpuscle; lamellar cell process staining is shown in the molecular sensor involved in detecting touch.
red and neuronal cell staining in blue. A low power light If b and gENaC are part of a mechanosensitive ion field image, superimposed with neuronal cell staining channel in tactile sensory receptors, then that channel is (blue) is shown in Fig. 6A. The dermal–epidermal border not the same channel as ENaC in kidney, colon and lung is marked by the dashed white line. Fig. 6B shows a higher epithelia. In epithelia, aENaC, bENaC and gENaC power view of the boxed region in Fig. 6A; lamellar cell subunits combine to form a constitutively active channel
1
H
.A
.
Drummond
et
al
.
/
Brain
Research
884
(2000
)
1
–
12
9
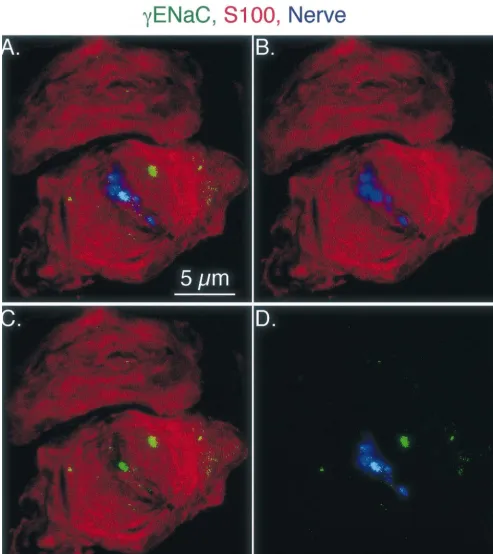
Fig. 8. gENaC staining of a small lamellated corpuscle. Image is a series of seven images taken at 0.15mm intervals. Labeling forgENaC is shown in green; lamellar cell process staining (anti S100 antibody) in red; and neuronal cell staining (nerve cocktail) in blue. Panel A shows labeling with all three antibodies and panels B–D show labeling with two of the three antibodies to aid in examining the spatial relationship between the labeled proteins.
H.A. Drummond et al. / Brain Research 884 (2000) 1 –12 11
[7] C.-C. Chen, S. England, A.N. Akopian, J.N. Wood, A sensory The results of this study also raise issues for future
neuron-specific, proton-gated ion channel, Proc. Natl. Acad. Sci. investigation. One critical issue will be to identify other
USA 95 (1998) 10240–10245.
subunits and associated proteins that may contribute to the [8] D.P. Corey, J. Garcıa-Anoveros, Mechanosensation and the DEG /´ ˜ mechanosensory complex. It will also be important to ENaC ion channels, Science 273 (1996) 323–324.
[9] I. Darboux, E. Lingueglia, D. Pauron, P. Barbry, M. Lazdunski, A identify the physiologic role for these subunits in mice
new member of the amiloride-sensitive sodium channel family in bearing targeted disruptions of ENaC genes. Unfortunately,
drosophila melanogaster peripheral nervous system, Biochem.
Bio-b and gENaC knockout mice die of severe metabolic phys. Res. Commun. 246 (1998) 210–216.
abnormalities within one or two days after birth, preclud- [10] I. Darian-Smith, The sense of touch: performance and peripheral neural processes, in: J.M. Brookhart, V.B. Mountcastle (Eds.), ing a physiologic analysis [2,31]. Tissue-specific disruption
Handbook of Physiology Section I: The Nervous System III, 1984, of these genes in mice may allow a detailed physiologic
pp. 739–788, Chapter 17.
assessment in the future. Interestingly, humans with pseu- [11] J. Diamond, L.R. Mills, K.M. Mearow, Evidence that the Merkel dohypoaldosteronism type I have mutations inborgENaC cell is not the transducer in the mechanosensory Merkel cell–neurite subunits, but they have not been reported to have defects in complex, Prog. Brain Res. 74 (1988) 51–56.
[12] M. Driscoll, M. Chalfie, The mec-4 gene is a member of a family of mechanosensation. However, there is likely to be
signifi-Caenorhabditis elegans genes that can mutate to induce neuronal cant redundancy in mechanosensors, person to person
degeneration, Nature 349 (1991) 588–593.
differences in sensation may be difficult to detect, and [13] H.A. Drummond, M.P. Price, M.J. Welsh, F.M. Abboud, A molecu-subtle defects might go undetected without a detailed lar component of the arterial baroreceptor mechanostransducer,
Neuron 21 (1998) 1435–1441. electrophysiologic analysis such as single nerve recordings
[14] H. Du, G. Gu, C.M. William, M. Chalfie, Extracellular proteins which are not possible in humans. In summary, by
needed for C. elegans mechanosensation, Neuron 16 (1996) 183– suggesting that the b and gENaC subunits may be com- 194.
ponents of a mechanosensory receptor for touch, this work [15] G.K. Fyfe, A. Quinn, C.M. Canessa, Structure and function of the Mec-ENaC family of ion channels, Semin. Nephrol. 18 (1998) helps lay the groundwork for understanding this elusive
138–151. sense.
˜ ´
[16] J. Garcıa-Anoveros, B. Derfler, J. Neville-Golden, B.T. Hyman, D.P. Corey, BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels, Proc. Natl. Acad. Sci. USA 94 (1997) 1459–1464.
Acknowledgements
[17] O.P. Hamill, D.W.J. McBride, Rapid adaptation of single mech-anosensitive channels in Xenopus oocytes, Proc. Natl. Acad. Sci. This work was supported by HL14388 and the Howard USA 89 (1992) 7462–7466.
[18] M. Huang, M. Chalfie, Gene interactions affecting mechanosensory Hughes Medical Institute (HHMI), and received assistance
transduction in Caenorhabditis elegans, Nature 367 (1994) 467– from the University of Iowa Central Microscopy Research
470. Facility. H.A. Drummond was supported by HL07121.
[19] C. Ide, The fine structure of the digital corpuscle of the mouse toe M.J. Welsh is an investigator of the HHMI. pad, with special reference to nerve fibers, Am. J. Anat. 147 (1976)
329–355.
[20] C. Ide, Development of Meissner corpuscle of mouse toe pad, Anat. Rec. 188 (1977) 49–67.
References [21] C. Ide, S. Hayashi, Specializations of plasma membranes in Pacinian
corpuscles: implications for mechano-electric transduction, J. Neuro-[1] C.M. Adams, M.G. Anderson, D.G. Motto, M.P. Price, W.A. cytol. 16 (1987) 759–773.
Johnson, M.J. Welsh, Ripped pocket and pickpocket, novel Dro- [22] I. Ikeda, Y. Yamashita, T. Ono, H. Ogawa, Selective phototoxic sophila DEG / ENaC subunits expressed in early development and in destruction of rat Merkel cells abolishes responses of slowly mechanosensory neurons, J. Cell Biol. 140 (1998) 143–152. adapting type I mechanoreceptor units, J. Physiol. (Lond.) 479 [2] P.M. Barker, M.S. Nguyen, T. Gatzy, B. Grubb, H. Norman, E. (1994) 247–256.
Hummer, B. Rossier, R.C. Boucher, B. Koller, Role of gENaC [23] T. Iwanaga, T. Fujita, Y. Takahashi, T. Nakajima, Meissner’s and subunit in lung liquid clearance and electrolyte balance in newborn Pacinian corpuscles as studied by immunohistochemistry for S-100 mice. Insights into perinatal adaptation and pseudohypoaldosteron- protein, neuron-specific enolase and neurofilament protein, Neuro-ism, J. Clin. Invest. 102 (1998) 1634–1640. sci. Lett. 31 (1982) 117–121.
[3] P.R. Burgess, E.R. Perl, Cutaneous mechanoreceptors and nocicep- [24] M.P. Kaufman, K.J. Rybicki, Discharge properties of group III and tors, in: A. Iggo (Ed.), Handbook of Sensory Physiology II IV muscle afferents: their responses to mechanical and metabolic Somatosensory System, Vol. 2, Springer-Verlag, New York, 1973, stimuli, Circ. Res. 61 (1987) I60–5.
pp. 29–78, Chapter 2. [25] I. Kinkelin, C.L. Stucky, M. Koltzenburg, Postnatal loss of Merkel cells, but not of slowly adapting mechanoreceptors in mice lacking [4] C.M. Canessa, J.D. Horisberger, B.C. Rossier, Epithelial sodium
the neurotrophin receptor p75, Eur. J. Neurosci. 11 (1999) 3963– channel related to proteins involved in neurodegeneration, Nature
3969. 361 (1993) 467–470.
[26] E. Lingueglia, G. Champigny, M. Lazdunski, P. Barbry, Cloning of [5] C.M. Canessa, L. Schild, G. Buell, B. Thorens, I. Gautschi, J.D.
1
the amiloride-sensitive FMRFamide peptide-gated sodium channel, Horisberger, B.C. Rossier, Amiloride-sensitive epithelial Na
chan-Nature 378 (1995) 730–733. nel is made of three homologous subunits, Nature 367 (1994)
[27] E. Lingueglia, S. Renard, R. Waldmann, N. Voilley, G. Champigny, 463–467.
H. Plass, M. Lazdunski, P. Barbry, Different homologous subunits of [6] M. Chalfie, E. Wolinsky, The identification and suppression of
1
the amiloride-sensitive Na channel are differently regulated by inherited neurodegeneration in Caenorhabditis elegans, Nature 345
[28] J. Liu, B. Schrank, R.H. Waterston, Interaction between a putative [40] F. Sachs, Baroreceptor mechanisms at the cellular level, FASEB J. mechanosensory membrane channel and a collagen, Science 273 46 (1987) 12–16.
(1996) 361–364. [41] H. Sann, P.W. McCarthy, G. Jancso, F.K. Pierau, RT97: a marker for [29] J.H. Martin, T.M. Jessell, Modality coding in the somatic sensory capsaicin-insensitive sensory endings in the rat skin, Cell Tissue
system, in: E.R. Kandel, J.H. Schwartz, T.M. Jessell (Eds.), Princi- Res. 282 (1995) 155–161.
ples of Neural Science, Elsevier, New York, 1991, pp. 341–352, [42] S.S. Senok, Z. Halata, K.I. Baumann, Chloroquine specifically Chapter 10. impairs Merkel cell mechanoreceptor function in isolated rat sinus [30] F.J. McDonald, M.P. Price, P.M. Snyder, M.J. Welsh, Cloning and hairs, Neurosci. Lett. 214 (1996) 167–170.
expression of the beta- and gamma-subunits of the human epithelial [43] N. Tavernarakis, M. Driscoll, Molecular modeling of mechanotran-sodium channel, Am. J. Physiol. 268 (1995) C1157–C1163. sduction in the nematode Caenorhabditis elegans, Annu. Rev. [31] F.M. McDonald, B. Yang, R.F. Hrstka, H.A. Drummond, D.E. Tarr, Physiol. 59 (1997) 659–689.
P.B. McCray, J.B. Stokes, M.J. Welsh, R.A. Williamson, Disruption [44] N. Tavernarakis, W. Shreffler, S. Wang, M. Driscoll, unc-8, a 1
of thebsubunit of the epithelial Na channel in mice: hyperkalemia DEG / ENaC family member, encodes a subunit of a candidate and neonatal death associated with a pseudohypoaldosteronism mechanically gated channel that modulates C. elegans locomotion, phenotype, Proc. Natl. Acad. Sci. USA 96 (1999) 1727–1731. Neuron 18 (1997) 107–119.
[32] A.K. McIntyre, Perception of vibration, Proc. Aust. Assoc. Neurol. 3 [45] M. Tazaki, T. Suzuki, Calcium inflow of hamster Merkel cells in (1965) 71–76. response to hyposmotic stimulation indicate a stretch activated ion [33] I. Moll, C. Kuhn, R. Moll, Cytokeratin 20 is a general marker of channel, Neurosci. Lett. 243 (1998) 69–72.
cutaneous Merkel cells while certain neuronal proteins are absent, J. [46] R. Waldmann, F. Bassilana, J. de Weille, G. Champigny, C. Invest. Derm. 104 (1995) 910–915. Heurteaux, M. Lazdunski, Molecular cloning of a non-inactivating
1
[34] C.E. Morris, Mechanosensitive ion channels, J. Membr. Biol. 113 proton-gated Na channel specific for sensory neurons, J. Biol.
(1990) 93–107. Chem. 272 (1997) 20975–20978.
1
[35] B.L. Munger, C. Ide, The structure and function of cutaneous [47] R. Waldmann, M. Lazdunski, H -gated cation channels: neuronal sensory receptors, Arch. Histol. Cytol. 51 (1988) 1–34. acid sensors in the ENaC / DEG family of ion channels, Curr. Opin. [36] P.B. Nava, R.C. Mathewson, Effect of age on the structure of Neurobiol. 8 (1998) 418–424.
Meissner corpuscles in murine digital pads, Microsc. Res. Tech. 34 [48] Y. Yoshida, T. Ushiki, M. Takashio, B.L. Munger, C. Ide, Membrane (1996) 376–389. relationships in murine Meissner corpuscles: cytology of freeze-[37] C. Nolte, M. Schachner, R. Martini, Immunocytochemical localiza- substituted tissue, Anat. Rec. 223 (1989) 437–445.
tion of the neural cell adhesion molecules L1, N-CAM, and J1 in [49] J. Zelena, The development of Pacinian corpuscles, J. Neurocytol. 7 Pacinian corpuscles of the mouse during development, in the adult (1978) 71–91.
and during regeneration, J. Neurocytol. 18 (1989) 795–808. [50] J. Zelena, I. Jirmanova, T. Nitatori, C. Ide, Effacement and [38] A.S. Paintal, Functional analysis of group III afferent fibres of regeneration of tactile lamellar corpuscles of rat after postnatal nerve
mammalian muscles, J. Physiol. (Lond.) 152 (1960) 250–270. crush, Neuroscience 39 (1990) 513–522. [39] M.P. Price, P.M. Snyder, M.J. Welsh, Cloning and expression of a
1