*Corresponding author. Tel.:#45-35-37-67-77; fax:#45-35-30-60-40.
HPLC analysis of the seasonal and diurnal
variation of iridoids in cultivars of
Antirrhinum
majus
Birgitte Dr
+
hse H
+
gedal, Per M
+
lgaard
*
Department of Medicinal Chemistry, Pharmacognosy Group, The Royal Danish School of Pharmacy, Univ er-sitetsparken 2, DK-2100 Copenhagen, Denmark
Received 17 June 1999; accepted 6 March 2000
Abstract
In this paper we show the seasonal and diurnal variation in the content of the four iridoids found in cultivars of Antirrhinum majus, antirrhinoside, antirrhide, 5-glucosyl-antirrhinoside and linarioside. The seasonal variation in total iridoid content showed a marked bimodal distribution with high total values (around 100 mg/g dry matter) early and late in the season and a very low content of all iridoids coinciding with the onset of#owering at the beginning of August. The relative contribution of antirrhinoside was signi"cantly higher before#owering than after bud break. The relative decrease in antirrhinoside was counteracted by an increase of antirrhide, which was signi"cantly higher after the onset of#owering than before. This pattern indicates a change in biosynthesis, although no explanation can be given to the phenomenon. The diurnal variation showed a variation between 20 and 60 mg/g dry weight, but there was no relation to light/darkness conditions, temperature patterns or water content. The analyses were performed by HPLC. The applied method has not previously been used in the quanti"cation of iridoids, but was developed speci"cally for the analyses of cultivars ofAntirrhinum majus. We have fully validated the method during its development. The limit of detection was calculated to 0.004 mg/ml and the limit of quanti"cation was 0.01 mg/ml. ( 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Antirrhinum majus; Scrophulariaceae; Iridoid glucoside; Antirrhinoside; Seasonal variation; Diurnal variation
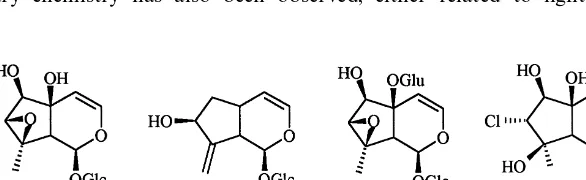
Fig. 1. Chemical structures of the four iridoids fromAntirrhinum majus: antirrhinoside (1), antirrhide (2), 5-glucosyl-antirrhinoside (3), and linarioside (4).
1. Introduction
Iridoids are secondary compounds con"ned to a restricted number of sympetalous plant families. They are found in Corni#orae, Loasi#orae, Lamii#orae and Gentianif-lorae and are part of the complex set of characters that bind these superorders together in the classi"cation of Dahlgren (1989). The value of chemical characters, including iridoids, in the classi"cation of the Asteridae s.l. has recently been discussed by Grayer et al. (1999).
There are two di!erent pathways in the biosynthesis of iridoids of which route II leads to the aucubin-type iridoids common in Lamii#orae (Jensen, 1992).Antirrhinum majus in the Scrophulariaceae is a member of Lamii#orae and thus produces aucubin-type iridoids. The cultivars&White Wonder'and&Yellow Monarch'contain the four major iridoid compounds antirrhinoside, antirrhide, 5-glucosyl-antirrhino-side and linario5-glucosyl-antirrhino-side (Fig. 1). Antirrhino5-glucosyl-antirrhino-side inAntirrhinumis biosynthetically derived from 8-epi-iridodial via route II as shown by Breinholt et al. (1992) and Damtoft et al. (1993, 1995).
The variation in the content of secondary compounds is important for the interac-tion of plants with pathogens and herbivores as highlighted in general by Coleman and Jones (1991). In several studies iridoids have been shown to a!ect the feeding behaviour of herbivores. By a turnover from production of aucubin to catalpol the seasonal variation could be related to a shift from generalist to specialist herbivores onPlantagospecies during the season (Bowers et al., 1992; Bowers and Stamp, 1992; Bowers, 1996). In general, iridoids act as a defence mechanism against herbivores, although they may also attract adapted herbivores at certain times according to composition and concentration (Adler et al., 1995; Bowers and Puttick, 1986; Bowers, 1984; Pereyra and Bowers, 1988).
(Dickson, 1987; Sporer et al., 1993), to water de"cit (Itenov et al., 1999) or to more complex interactions (Rosa et al., 1994). Besides, seasonal#uctuations and variations in secondary plant metabolites may be governed by genotype and in#uenced by nutrient availability as shown for iridoids by Bowers et al. (1992) and Fajer et al. (1992), respectively.
This project is part of an ongoing investigation of secondary plant compounds for their potential use as starting material in the chemical and pharmaceutical industry. The speci"c interest in iridoids stems from their structure with an inherent chiral centre, which makes the compounds potential precursors for the semi-synthesis of drugs with activity against HIV and cancer (Franzyk et al., 1997, 1998a,b).
AlthoughAntirrhinum majushas a long reputation as an ornamental garden plant with detailed knowledge on the genetics of#ower colour patterns (e.g. Stubbe, 1974), very little is known on the variation in the content of iridoids. The content of iridoids may be of importance for the interaction with pathogens and herbivores onA. majus, and in relation to chemotaxonomy at the generic level. However, not only from a pure scienti"c point of view but also in relation to A. majus as a potential producer of starting material for"ne chemicals it is important to"nd out if there is a seasonal or diurnal variation and turnover of the iridoids. There may be an inherent di!erence between cultivars, and we designed this project to determine at which state the plants contain the largest amount of iridoids or the best combination of individual com-pounds, so that harvesting of raw material for"ne chemicals can be planned accord-ingly.
Previously, quantitative analyses of iridoids have been based on either gas chromatography (Bowers, 1996; Bowers and Stamp, 1992), or high-performance liquid chromatography, HPLC (Baghdikian et al., 1997; Dallenbach-Toelke et al., 1987; Lenherr et al., 1984; Meier and Sticher, 1977; Miyagoshi et al., 1986). The present analyses are made by HPLC using a newly developed method. We have developed this method speci"cally to quantify the iridoids in these cultivars of A. majus. The method has been fully validated alongside the development.
2. Materials and methods
2.1. Plant material
TwoAntirrhinum majus cultivars, White wonder and Yellow monarch, were"eld grown at the Danish Institute of Agricultural Science, Research Center Flakkebjerg. The seeds were obtained from Impecta Handels, 640 25 Julita, Sweden. The plants were grown in open"elds on silty clay soil, with a distance of 45 cm between the rows. Nitrogen was applied on June 2 at the rate of 50 kg N/ha.
2.1.1. Weather conditions
2.1.2. Seasonalvariation
Plant samples for chemical analyses were taken weekly between June 17, 1998 and September 30, 1998, and twice a week during the #owering period (July 19 until September 4). Seven shoots from separate plants were cut each time, washed, weighed and pressed. The shoots were dried at 403C for 24 h. After drying the shoots were weighed and the water content determined in percent. Five of these shoots (the two extremes were discarded) were selected each sample day, and the leaves were pow-dered. During the drying process on July 22 and July 29 some of the plants were damaged and therefore excluded from the study. After September 9 fungi attacked the plants, and the experiment was terminated. The development of the plants was observed during the whole period.
2.1.3. Diurnalvariation
Samples were taken at the onset of#owering (August 5}6, sunrise 5:22, sunset 21:09, Danish summer time 2 h ahead of GMT) and in the middle of the#owering period (August 18}19, sunrise 5:48, sunset 20:38, Danish summertime 2 h ahead of GMT). Each harvest started at 15:00 h, and"ve plants were sampled every hour over a 24 h period. No plants were used twice. The lower side branch and the pair of leaves above were collected. The lower side branch was placed in a plastic bag and frozen on solid CO
2 to stop instantly any enzymatic activity. The leaves were washed, dried and placed in sample vials, weighed and frozen on solid CO
2. After 1 h on solid CO2both samples were moved to a freezer at !203C. The samples were lyophilised on
Drywinner 6-85, Heto Holm & Halby, at!953C under vacuum for 48 h.
2.2. Sample preparation
50 mg of powdered leaf material was weighed out and extracted for 1 h in an ultra-sonic bath with 10 ml of a 20% methanol solution, containing 0.05 mg/ml of the internal standard,p-hydroxybenzaldehyde. The extract was"ltered through a Minis-art'"lter type RC-0.45lm and analysed directly on HPLC.
2.3. HPLC analysis
The HPLC analyses were performed on a Shimadzu SCL-6A System controller with Shimadzu SIL-6A Auto-injector with an injection volume of 10ll, Shimadzu
CTO-6A Column oven at 403C, Shimadzu SPD-6AV Spectrophotometric detector at 205 nm and two Shimadzu LC-6A pumps with a#ow of 1 ml/min. The data processing was carried out by a Class-LC10 version 1.41. The column was a Macherey-Nagel Nucleosil C18-5l, 125]4.6 mm2. The system was an isocratic 3% acetonitrile in
Fig. 2. Chromatogram of a standard solution containing the four iridoids and the internal standard: antirrhinoside (1), antirrhide (2), 5-glc-antirrhinoside (3), linarioside (4) and p-hydroxybenzaldehyde (5).
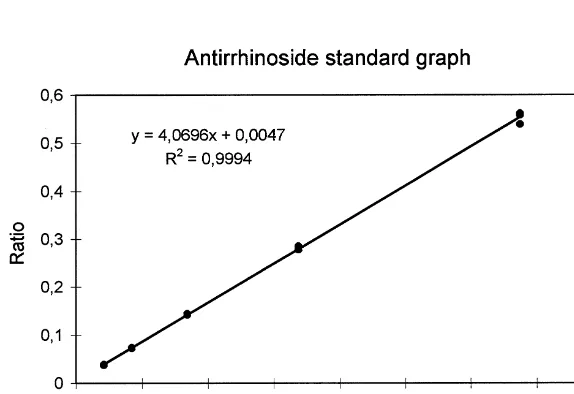
Fig. 3. Standard curve for antirrhinoside, usingp-hydroxybenzaldehyde as an internal standard.
standard solution with the four iridoids (Fig. 1) and the internal standard p-hy-droxybenzaldehyde is shown in Fig. 2.
and 0.99977 for antirrhinoside, antirrhide, 5-glucosylantirrhinosid and linarioside, respectively.
2.4. Assayvalidation and recovery
The extraction procedure was decided upon after a range of extractions had been carried out using a variation in methanol concentrations (0, 10, 20, 40, 60, 80 and 100%) and extraction times (10 and 30 min, 1, 2 and 4 h) in an ultra-sonic bath, prior to the analyses by HPLC. Based on the most stable results, we decided on extraction for 1 h with 20% methanol.
The HPLC method was carefully validated. Accuracy was measured on standard solutions with concentrations lower than those we measured during the season. For the highest concentration the accuracy for all compounds was between 86 and 100%. The relative standard deviation was between 0.48 and 2.55% in three replicates of three standard solutions, which gives a very good repeatability. There was no di!erence in the result of the standard solutions on di!erent chromatographs or di!erent analysts, and therefore the method has a good reproducibility. The diode-array scan showed no interference from other compounds in the plant extract, which gives a good selectivity. The method is very robust to variations in#ow and to change of column with the same material. The linearity of the calibration curve is shown in Section 2.3. The limit of detection for antirrhinoside is 0.004 mg/ml, the limit of quanti"cation 0.01 mg/ml.
3. Results
3.1. Plant analyses
3.1.1. Seasonalvariation
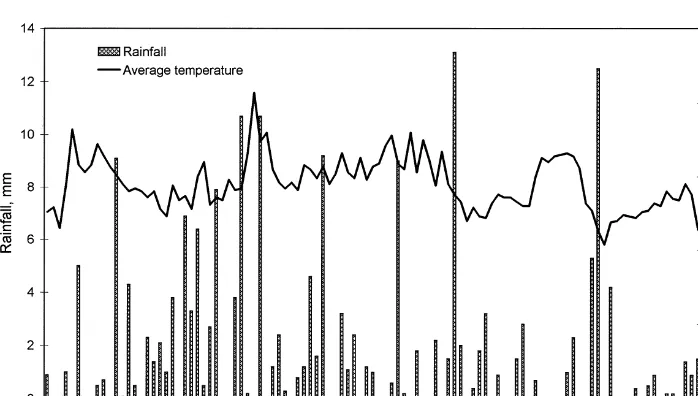
The weather was more or less stable during the sampling period. There was no event of especially warm or cold weather and no period was particularly dry or wet. Fig. 4 shows the average rainfall and temperature during the period of sampling. Compared to an average Danish summer this one was a bit cool and wet (Statistisk Asrbog, 1999). The plants developed normally during the season. After September 9 most of the plants were attacked by fungi, and the experiment terminated.
Fig. 4. Weather conditions during the sample period: daily rainfall and average temperature (3C).
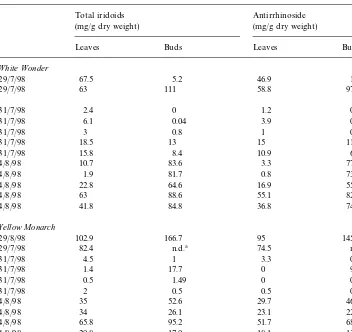
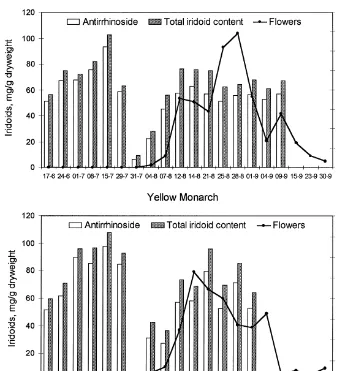
There is a pronounced bimodal variation in total iridoids during summer, and the two cultivars show a remarkable uniformity. Coinciding with the onset of#owering, the content of iridiods in leaves was very low in both cultivars. Some test samples were made of the spikes and#owers just around the time of#owering. Table 2 shows that some of the buds and#owers had a higher content of iridoids than the leaves sampled on the same day.
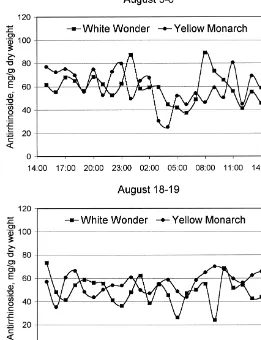
3.1.2. Diurnalvariation
Figs. 5 and 6 show the diurnal variation of antirrhinoside at the beginning of the
#owering and in the middle of the #owering season, respectively. The content of antirrhinoside varies substantially (between 20 and 60 mg/g) during the day and night, but no general pattern in relation to day length could be seen, neither for the two cultivars nor for the two sample days. However, the variation between individual samples is unquestionable, but presumably not related to light conditions, temper-ature patterns or humidity, which vary continuously in a symmetric pattern around midday (cf. Itenov et al., 2000).
4. Discussion
Table 1
The seasonal variation in iridoid content, absolute (mg/g dry weight) and relative (%), in the leaves of cultivars ofAntirrhinum majusduring the summer of 1998. The season is divided in two periods before #owering (incl. July 29) and after the onset of#owering (from August 7), respectively. The means of each period are calculated and listed in the table
Total iridoids Antirrhinoside Antirrhide 5-Glc-antirrhinoside Linarioside n (mg/g dry weight) relative relative relative relative White Wonder
Pre-#owering
17/06/98 56.32 90.63 7.03 1.99 0.36 5
24/06/98 74.60 90.00 7.24 2.09 0.67 5
01/07/98 72.04 93.98 3.14 2.89 0.00 5
08/07/98 81.78 92.32 2.57 4.26 0.86 5
15/07/98 102.84 90.68 4.53 3.83 0.93 5
29/07/98 63.00 93.33 3.49 2.22 0.95 2
Mean 91.82 4.67 2.88 0.63
s.d. 1.63 2.02 0.97 0.38
Onset of#owering
31/07/98 9.16 69.87 15.94 14.19 0.00 5
During#owering
04/08/98 28.04 80.53 12.84 5.42 1.21 5
07/08/98 55.98 80.71 12.86 5.72 0.71 5
12/08/98 76.36 75.22 9.35 13.88 1.55 5
14/08/98 75.38 83.26 8.74 6.90 1.14 5
21/08/98 74.60 76.19 11.98 11.42 0.40 5
25/08/98 62.50 82.21 11.55 5.82 0.42 5
28/08/98 64.44 86.56 8.32 4.69 0.43 5
01/09/98 67.53 83.56 8.88 6.66 0.89 4
04/09/98 60.75 86.88 9.27 3.29 0.58 4
09/09/98 66.85 85.12 8.68 5.68 0.52 2
Mean 82.02 10.25 6.95 0.79
s.d. 3.97 1.84 3.22 0.40
Yellow Monarch Pre-#owering
17/06/98 59.54 86.29 10.82 2.65 0.24 5
24/06/98 71.00 86.79 8.62 3.97 0.62 5
01/07/98 96.13 93.26 2.18 4.40 0.16 4
08/07/98 96.82 88.25 3.68 6.90 1.18 5
15/07/98 107.98 90.48 3.67 5.56 0.30 5
29/07/98 92.65 91.47 4.96 2.97 0.59 2
Mean 89.42 5.66 4.41 0.52
s.d. 2.76 3.34 1.61 0.38
Onset of#owering
31/07/98 2.10 45.24 11.90 42.86 0.00 4
During#owering
04/08/98 42.88 73.46 16.65 8.21 1.68 5
07/08/98 36.70 75.15 19.84 4.31 0.76 4
12/08/98 73.35 77.91 13.61 5.52 2.97 4
14/08/98 68.74 84.49 10.53 3.96 1.02 5
21/08/98 96.08 82.54 9.05 7.99 0.42 4
25/08/98 69.60 76.77 17.00 6.28 1.39 3
28/08/98 85.30 83.55 12.46 3.79 0.20 3
01/09/98 64.35 82.05 9.95 7.38 0.62 2
Mean 79.49 13.64 5.93 1.13
accumulate secondary compounds during the season as shown for alkaloids (Williams and Ellis, 1989) and terpenoids (Gershenzon et al., 1993). With the development of#owering intensity Kite and Smith (1997) showed an increase in the emission of monoterpenes with day}night variations, and for #avonoids Letchamo (1996) similarly showed an increase with increasing opening of the#owerheads in camomile. However, in our experiments, #owering at the main stem of A. majus is followed by regrowth of lower sidebranches, and as these make up part of the plant material, the samples subsequently contain more newly developed leaves. It is well documented that new leaves may have higher content of secondary metab-olites, e.g. monoterpenes (Goralka and Langenheim, 1996) and iridoids (Bowers et al., 1992).
We have not been able to relate this variation to changes in the abiotic conditions such as temperature, insolation, humidity (cf. Fig. 2), or to nutrient availability, which could explain the drop in iridoid content in the middle of the season. After September 9 a severe fungal attack to the leaves coincided with the disappearance of iridoids; however, this gives no explanation to the bimodal seasonal variation as the plants were attacked only at the end of the season, obviously coinciding with a drop in the content of iridoids.
The biosynthesis rate of the iridoids must change after the onset of#owering, as shown by the change in the relative content of antirrhinoside and antirrhide. Translo-cation of iridoids from the leaves to other parts of the plants, e.g. in#orescenses under development, may be an explanation to this pattern, with a resultant lack of iridoids in the leaves during#ower development. We have only a few analyses to justify this hypothesis (Table 2), and it is noticeable that the iridoid content in some of the plants from August 4 is substantially higher in the buds than in the leaves. This could be a result of transport of the iridoids at the onset of #owering, in order to secure protection of the young tissue. But more detailed analyses are needed to con"rm this transport to the buds. According to Gowan et al. (1995) antirrhinoside is biosyn-thesized in the leaf blade and subsequently transported through the petiole to other parts of the plant. Gupta (1991) states that an attack ofPeronospora plantaginiaeon Plantago psylliumappeared at the initiation of spikes. In comparison to our study, this fungal attack at the onset of#owering inP. psylliumcould be due to lack of iridoids in the leaves.
The diurnal observations show that the content of iridoids varies during the day (Figs. 5 and 6). Although the variation is substantial, from 20 to 60 mg/g dry matter, there is no pattern to be read from these graphs. Most of the published results on diurnal variation in secondary chemistry concerns alkaloids with a rapid change in concentration, e.g. morphine (Fairbairn and Wassel, 1964) and coniin (Fairbairn and Suwal, 1961). However, the extremely rapid turnover of alkaloids in the latex of Papaver somniferum has recently been shown to be an artefact as only the water
Table 2
The seasonal variation in total iridoid content and antirrhinoside in the leaves and #owersbuds of Antirrhinum majusin a short period at the onset of#owering. Note the substantial variation between individuals
Fig. 5. Seasonal variation of antirrhinoside and total iridoid content (mg/g leaf dry matter) in the two cultivars White Wonder (top) and Yellow Monarck ofAntirrhinum majus.The total number of#owers is shown with solid line. The values are means based on samples of"ve individual plants every week and an additional sample from July 31 when#owering started (compare Tables 1 and 2).
We recommend that cultivars ofAntirrhinum majusbe harvested 2}4 weeks before
Fig. 6. Diurnal variation in the content of antirrhinoside in two cultivars ofAntirrhinum majus,White Wonder and Yellow Monarch. The values are based on"ve samples sampled every hour during August 5}6 and August 18}19, with sunrise and sunset at 5:22 and 21:09, and 5:48 and 20:38, respectively.
Acknowledgements
This work was planned in relation to the project&Special chemicals and pharmaca from plants', which is a cooperation between Danish Institute of Agricultural Science, The Royal Danish School of Pharmacy and the Danish Technical University," nan-cially supported by a grant from the Danish Science Foundation, ref. No. 9501145.
References
Adams, R.P., 1987. Yields and seasonal variation of phytochemicals fromJuniperusspecies of the United States. Biomass 12, 129}139.
Adler, L.S., Schmitt, J., Bowers, M.D., 1995. Genetic variation in defensive chemistry inPlantago lanceolata (Plantaginaceae) and its e!ect on the specialist herbivoreJunonia coenia(Nymphalidae). Oecologia 101, 75}85.
Baghdikian, B., Lanhers, M.C., Fleurentin, J., Ollivier, E., Maillard, C., Balansard, G., Mortier, F., 1997. An analytical study, anti-in#ammatory and analgesic e!ect of harpagophytum procumbens and har-pagophytum zeeyheri. Planta Med. 63, 171}176.
Bowers, M.D., 1984. Iridoid glycosides and host-plant speci"city in larvae of the buckeye butter#yJunonia coenia(Nymphalidae). J. Chem. ecol. 10, 1567}1577.
Bowers, M.D., 1996. Variation in iridoid glycosides in a population ofPlantago patagonicaJacq. (Plan-taginaceae) in Colorado. Biochem. Systems Ecol. 24, 207}210.
Bowers, M.D., Collinge, S.K., Gamble, S.E., Schmitt, J., 1992. E!ects of genotype, habitat, seasonal variation on iridoid glycoside content ofPlantago lanceolata(Plantaginaceae) and the implications for insect herbivores. Oecologia 91, 201}207.
Bowers, M.D., Puttick, G.M., 1986. Fate of ingested iridoid glycosides in lepidopteran herbivores. J. Chem. Ecol. 12, 169}178.
Bowers, M.D., Stamp, N.E., 1992. Chemical variation within and between individuals ofPlantago lanceolata (Plantaginaceae). J. Chem. Ecol. 18, 985}995.
Boyd, F.T., Aamodt, O.S., Bohnstedt, G., Truog, E., 1938. Sudan grass management for control of cyanide poisoning. J. Am. Soc. Agron. 30, 569}582.
Breinholt, J., Damtoft, S., Demuth, H., Jensen, S.R., Nielsen, B.J., 1992. Biosynthesis of antirrhinoside in Antirrhinum majus. Phytochemistry 31, 795}797.
Dahlgren, G., 1989. The last Dahlgrenogram, a system of classi"cation of the dicotyledons. In: Tan, K. (Ed.), Plant Taxonomy, Phytogeography and Related Subjects: The Davis and Hedge Festschrift. Edinburgh University Press, Edinburgh, pp. 237}260.
Dallenbach-Toelke, K., Nyiredy, Sz., Sticher, O., 1987. Application of various planar chromatographic techniques for the separation of iridoid glycosides fromVeronica ozcinalis. J. Chromatogr. 404, 365}371. Damtoft, S., Jensen, S.R., Jensen, C.U., 1993. Intermediates between 8-epi-deoxyloganic acid and
6,10-dideoxyaucubin in the biosynthesis of antirrhinoside. Phytochemistry 33, 1087}1088.
Damtoft, S., Jensen, S.R., Schacht, M., 1995. Last stages in the biosynthesis of antirrhinoside. Phytochemis-try 39, 549}551.
Dickson, R.E., 1987. Diurnal changes in leaf chemical constituents and14C partitioning in cottonwood. Tree Physiol. 3, 157}170.
El-Gengaihi, S.E., Wahba, H.E., 1995. Seasonal variation in the growth and chemical constituents of Ginger cultivated in Egypt. Acta Hortic. 390, 25}32.
Fairbairn, J.W., Suwal, P.N., 1961. The alkaloids of Hemlock (Conium maculatum). II. Evidence for a rapid turnover of major alkaloids. Phytochemistry 1, 38}46.
Fairbairn, J.W., Wassel, G., 1964. The alkaloids ofPapaver somniferumL. I. Evidence for a rapid turnover of major alkaloids. Phytochemistry 3, 253}258.
Fajer, E.D., Bowers, M.D., Bazzas, F.A., 1992. The e!ects of nutrients and enriched CO2 environments on production of carbon-based allelochemicals inPlantago: a test of the carbon/nutrient balance hypothe-sis. Am. Nat. 140, 707}723.
Franzyk, H., Rasmussen, J.H., Jensen, S.R., 1997. Ozonolysis of protected iridoid glucosides. Eur. J. Org. Chem. 1, 365}370.
Franzyk, H., Rasmussen, J.H., Jensen, S.R., 1998b. Synthesis of carbocyclic homo-N-nucleosides from iridoids. Eur. J. Org. Chem. 1, 2931}2935.
Gershenzon, J., Murtagh, G.J., Croteau, R., 1993. Absence of rapid terpene turnover in several diverse species of terpene-accumulating plants. Oecologia 96, 583}592.
Goralka, R.J.L., Langenheim, J.H., 1996. Implications of foliar monoterpenoid variation among on-togenetic stages of the Californian Bay Tree (Umbellularia californica) for Deer herbivory. Biochem. Systems Ecol. 24, 13}23.
Goralka, R.J.L., Schumaker, M.A., Langenheim, J.H., 1996. Variation in chemical and physical properties during leaf development in Californian Bay Tree (Umbellularia californica): predictions regarding palatability for Deer. Biochem. Systems Ecol. 24, 93}103.
Gowan, E., Lewis, B.A., Turgeon, R., 1995. Phloem transport of antirrhinoside, an iridoid glycoside, in Asarina scandens(Scrophulariaceae). J. Chem. Ecol. 21, 1781}1788.
Grayer, R.J., Chase, M.W., Simmonds, M.S.J., 1999. A comparison between chemical and molecular characters for the determination of phylogenetic relationships among plant families: an appreciation of Hegnauer's&Chemotaxonomie der P#anzen'. Biochem. Systems Ecol. 27, 369}393.
Gupta, R., 1991. Agrotechnology of medicinal plants. In: Wijesekera, R.O.B. (Ed.), The Medicinal Plant Industry. CRC Press, London, pp. 43}57.
Heeger, E.F., 1956. Handbuch derArznei- und GewuKrzp#anzenbaus. Deutscher Bauernverlag, Berlin. Hendriks, H., Anderson-Wildeboer, Y., Engels, G., Bos, R., Woerdenbag, H.J., 1997. The content of
partheno-lide and its yield per plant during the growth ofTanacetum parthenium. Planta Med. 63, 356}359. Itenov, K., M+lgaard, P., Nyman, U., 1999. Diurnal#uctuations of the alkaloid concentration in latex of
poppy,Papaver somniferumis due to day}night#uctuations of the latex water content. Phytochemistry 52, 1229}1234.
Jensen, R.S., 1992. Systematic implications of the distribution of iridoids and other chemical compounds in the Loganiaceae and other families of the Asteridae. Ann. Mo. Bot. Gard. 77, 284}302.
Kite, G.C., Smith, S.A.L., 1997. In#orescence odour ofSenecio articulatus: temporal variation in isovaleric acid levels. Phytochemistry 45, 1135}1138.
Lenherr, A., Meier, B., Sticher, O., 1984. Modern HPLC as a tool for chemotaxonomical investigations: iridoid glycosides and acetylated#avonoids in the groupStachys recta.. Planta Med. 50, 403}409. Letchamo, W., 1996. Developmental and seasonal variations in#avonoids of diploid and tetraploid
Camomile#orets. J. Plant Physiol. 148, 645}651.
Miyagoshi, M., Amagaya, S., Ogihara, Y., 1986. Determination of gardenoside and related iridoid com-pounds by reversed-phase high-performance liquid chromatography. J. Chromatogr. 357, 293}300. Meier, B., Sticher, O., 1977. High-performance liquid chromatography of iridoid an secoiridoid glycosides.
J. Chromatogr. 138, 456}457.
Ndamba, J., Lemmich, E., M+lgaard, P., 1994. Investigation of the diurnal, ontogenetic and seasonal variation in the molluscicidal saponin content of Phytolacca dodecandra aqueous berry extracts. Phytochemistry 35, 95}99.
Pereyra, P.C., Bowers, M.D., 1988. Iridoid glycosides as oviposition stimulants for buckeye butter#y, Junonia coenia(Nymphalidae). J. Chem. Ecol. 14, 917}928.
Rosa, E.A.S., Heaney, R.K., Rego, F.C., Fenwick, G.R., 1994. The variation of glucosinolate concentration during a single day in young plants ofBrassoca oleraceavar.acephalaandcapitata. J. Sci. Food Agric. 66, 457}463. Scheer, R., Sche%er, A., Errenst, M., 1992. Two harvest times, summer and winter: are they essential for
preparing pharmaceutical from Mistletoe (Viscum album). Planta Med. 58 (Suppl. 1), A594.
Sporer, F., Sauerwein, M., Wink, M.,, 1993. Diurnal and developmental variation of alkaloid accumulation inAtropa belladonna. Acta Hortic. 331, 379}386.
William R, .D., Ellis, B.E., 1989. Age and tissue distribution of alkaloids inPapaver somniferum. Phytochem-istry 28, 2085}2088.