Journal of Life Sciences
Volume 7, Number 7,July 2013 (Serial Number 63)
David Publishing Company www.davidpublishing.com
Publication Information
Journal of Life Sciences is published monthly in hard copy (ISSN 1934-7391) and online (ISSN 1934-7405) by David Publishing Company located at 3592 Rosemead Blvd #220, Rosemead, CA 91770, USA.
Aims and Scope
Journal of Life Sciences, a monthly professional academic journal, covers all sorts of researches on molecular biology, microbiology, botany, zoology, genetics, bioengineering, ecology, cytology, biochemistry, and biophysics, as well as other issues related to life sciences.
Editorial Board Members
Dr. Stefan Hershberger (USA), Dr. Suiyun Chen (China), Dr. Farzana Perveen (Pakistan), Dr. Francisco Torrens (Spain), Dr. Filipa João (Portugal), Dr. Masahiro Yoshida (Japan), Dr. Reyhan Erdogan (Turkey), Dr. Grzegorz Żurek (Poland), Dr. Ali Izadpanah (Canada), Dr. Barbara Wiewióra (Poland), Dr. Valery Lyubimov (Russia), Dr. Amanda de Moraes Narcizo (Brasil), Dr. Marinus Frederik Willem te Pas (The Netherlands), Dr. Anthony Luke Byrne (Australia), Dr. Xingjun Li (China), Dr. Stefania Staibano (Italy), Dr. Wenle Xia (USA), Hamed Khalilvandi-Behroozyar (Iran).
Manuscripts and correspondence are invited for publication. You can submit your papers via Web Submission, or E-mail to [email protected] or [email protected]. Submission guidelines and Web Submission system are available at http://www.davidpublishing.org.
Editorial Office
3592 Rosemead Blvd #220, Rosemead, CA 91770, USA Tel: 1-323-9847526, 1-302-5977046; Fax: 1-323-9847374
E-mail:[email protected], [email protected]
Copyright©2011 by David Publishing Company and individual contributors. All rights reserved. David Publishing Company holds the exclusive copyright of all the contents of this journal. In accordance with the international convention, no part of this journal may be reproduced or transmitted by any media or publishing organs (including various websites) without the written permission of the copyright holder. Otherwise, any conduct would be considered as the violation of the copyright. The contents of this journal are available for any citation. However, all the citations should be clearly indicated with the title of this journal, serial number and the name of the author.
Abstracted / Indexed in
Database of EBSCO, Massachusetts, USA Chemical Abstracts Service (CAS), USA
Database of Cambridge Science Abstracts (CSA), USA Database of Hein Online, New York, USA
Ulrich’s Periodicals Directory, USA Universe Digital Library S/B, Proquest
Chinese Database of CEPS, American Federal Computer Library center (OCLC), USA China National Knowledge Infrastructure, CNKI, China
Chinese Scientific Journals Database, VIP Corporation, Chongqing, China Index Copernicus, Index Copernicus International S.A., Poland
Google Scholar (scholar.google.com)
Subscription Information
Price (per year): Print $420, Online $300, Print and Online $560.
David Publishing Company
3592 Rosemead Blvd #220, Rosemead, CA 91770, USA Tel: 1-323-9847526, 1-302-5977046; Fax: 1-323-9847374 E-mail: [email protected]
David Publishing Company www.davidpublishing.org
J LS
Journal of Life Sciences
Volume 7, Number 7, July 2013 (Serial Number 63)
Contents
Biochemistry and Biotechnology
677 Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
Joseck Akoyi, Allan J. Mgutu, Jesse Machuka, Mieke van Lijsebettens, Catherine Taracha and Sylvester
E. Anami
690 Comparative Study of the Static Magnetic Field Effects on Growth Rate with Relative Antibiotic Susceptibility in Escherichia coli
Fouad Houssein Kamel, Ashti M. Amin, Khonaw Kader Salih and Saleem S. Qader
695 Use of Wood Fibre Compost for the Cultivation of Trichoderma sp. (Isolate Td22)
Yan Ramona and Martin A. Line
700 Evaluation of the Insecticidal Activity of the Aerial Part of Pseudocytisus integrifolius (Salisb) Rehder on Grain Borer, Rhyzopertha dominica Fab. (Bostrychidae) and Wheat Weevil, Sitophilus granarius Linn. (Curculionidae)
Kassemi Naima, Khelil Mohamed Anouar and Bendimerad Nassima
705 Structure - Antimicrobial Activity Relationship Investigation of Some Butadiene and Chalcone Derivatives
Hanoy AL-Amood, Hadeel T. AL-Hadithi and Ghazwan F. Fadhil
Plant Sciences
712 Influence of Agricultural Inputs on Growth and Yield of Jatropha curcas (L.) in Cameroon
Tchobsala, Mégueni Clautilde, Njintang Yanou Nicolas, Nenwôla Kona Bilele, Patrick Prudent, Joseph Wey, Lyana Jean and Djonbada Pouimo
722 Restoration of Posidonia oceanica (L.) Delile Meadows: Is There An Effective Methodology?
727 The Strategies of Grassland Management in Farms of Northeastern Part of Poland
Jankowski Kazimierz, Kolczarek Roman, Jankowska Jolanta and Sosnowski Jacek
732 Improving the Size and Market Value of an Underutilised Yam (Dioscorea esculenta) in Ghana: Implications for Crop Breeding and Production Choices
Kwamina Banson and Kenneth Danso
Public Health
742 Adverse Events Clustering with NAT2 Slow Metabolisers following Deparasitization in Children in Bangolan, NWR Cameroon
Olivia Afa Achonduh, Babara Atogho-Tiedeu, Innocent Ali Mbulli, Jean Paul Chedjou, Mercy Achu, Akindeh Mbu Nji, Fokou Elie, Eric Kamgue, Vera Veyee, Orise Karana, Delphine Sahfe and Wilfred Fon Mbacham
749 Diagnosis Traps in Polyarteritis Nodosa — Original Case Report
Stoicescu Manuela
754 Body Weight Models in a Population-Based Study of Albanian School Adolescents
Enkelejda Shkurti, Diamant Shtiza and Diederik Aarendonk
760 Meeting the ADA Guidelines of Diabetic Care at King Fahd Hospital of University, Khobar, Eastern Province, Saudi Arabia in 2012
Waleed AlBaker, Fatemah AlFaraj, Reem AlArgan and Ammar Khamis
Biogeography
766 Comparative Data of Millipedes’ Distribution in Southern Region of Albania
Hajdar Kiçaj and Mihallaq Qirjo
773 Estimation of Artificial Plantings of Pinus sylvestris in Kazakhstan according to Their Geographical Origin
Nadezhda Konstantinovna Chebotko, Vitaliy Yurevich Kirillov and Bolat Mazhitovich Mukanov
781 An Analysis of Wetland Degradation in the Dimoria Region of Assam, India
July 2013, Vol. 7, No. 7, pp. 677-689
Journal of Life Sciences, ISSN 1934-7391, USA
Dicamba Growth Regulator Promotes Genotype
Independent Somatic Embryogenesis from Immature
Zygotic Embryos of Tropical Maize Inbred Lines
Joseck Akoyi1, Allan J. Mgutu1, Jesse Machuka1, Mieke van Lijsebettens2, 3, Catherine Taracha4 and Sylvester E. Anami5
1. Department of Biochemistry and Biotechnology, Plant Transformation Laboratory, Kenyatta University, Nairobi, Kenya 2. Department of Plant Systems Biology, VIB, Technologiepark 927, B-9052 Gent, Belgium
3. Department of Biotechnology and Bio-informatics, Ghent University, Technologiepark 927, B-9052 Gent, Belgium
4. Kenya Agricultural Research Institute, Nairobi, Kenya
5. Laboratory of Plant Genetics and Systems Biology, Department of Pure and Applied Sciences, Technical University of Mombasa,
Mombasa, Kenya
Received: December 13, 2012 / Accepted: March 03, 2013 / Published: July 30, 2013.
Abstract: Maize is one of the most important cereal crops in Sub-Saharan Africa and an important source of energy for humans. However, the difference in the dedifferentiation frequency of immature embryos among various genotypes indicates that callus induction and genetic transformation is dependent on the genotype. This phenomenon is an impediment in the fundamental process of improving tropical maize germplasm especially through genetic engineering. Here, five tropical maize (Zea mays L.) genotypes,
CML 216, CML 144, A 04, E 04 and TL 21, were evaluated for callus induction on MS medium supplemented with the growth regulator dicamba.Embryogenic and non embryogenic callus induction was independent of genotype when young immature embryos, 12 days after pollination (DAP) were used for tissue culture in combination with dicamba. The optimal concentration of dicamba for induction of embryogenic callus in all the genotypes was 3 mg/L, which was also the concentration at which non embryogenic callus formation was lowest. The frequency of embryogenic callus induction ranged from 35% to 79% among the five genotypes and somatic embryos regenerated R0 shoots that produced normal R1 progenies. This regeneration method is expected to facilitate the
development of a more efficient genotype independent Agrobacterium- mediated transformation system for tropical inbred lines.
Key words: Tropical maize, genotype independent, dicamba, somatic embryogenesis.
1. Introduction
Tropical maize is a major commodity in sub-Saharan Africa and Latin America agriculture and a major source of income for the poor resource populations [1]. Though protocols are available for embryogenic calli-mediated tropical maize regeneration, they are mostly variety-dependent [2-5], a phenomenon that has been an impediment to the
Corresponding author: Sylvester Anami, Ph.D., senior research fellow, research fields: molecular biology, biotechnology. E-mail: [email protected].
elemental process of efficient regeneration and breeding of tropical maize lines for agronomic traits through genetic engineering.
During dedifferentiation of the maize immature embryos into callus tissue, the cells acquire high energy charge due to enrichment of pyruvate, glycolysis and gluconeogenesis metabolic pathways [6]. The improvement in embryo cell number and quality as a result of ectopic expression of Brassica napus
Shoot Meristemless (STM) (BnSTM) was linked to the increased pyrimidine and purine salvage activity DAVID PUBLISHING
Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
678
during the early phases of embryogenesis and the enlargement of the adenylate pool (ATP + ADP) required for the active growth of the embryos. This was as a result of an increase in transcriptional and enzymatic activity of several salvage enzymes, including adenine phosphoribosyltransferase (APRT) and adenosine kinase (ADK) [7]. A number of genes have been found to be expressed during embryogenesis. Genes involved in amino acid and carbohydrate transport and metabolism, cell wall and cell membrane biogenesis and signal transduction mechanism were significantly changed during the dedifferentiation of maize immature embryos [6]. A Somatic Embryogenesis Receptor Kinase (SERK) [8], isoperoxidase, esterase and malate dehydrogenase isoenzymes [9] and higher levels of the primary amine, ethylamine gene [10] have been linked to somatic embryogenesis in many plant species. Allelic variation in some of the above-mentioned genes might explain the difference in dedifferentiation frequency of maize embryos among various genotypes that may cause genetic transformation and callus induction to be genotype dependent [6]. In particular, some tropical maize inbred genotypes fail to induce embryogenic calli and their recalcitrance can only be overcome by a single cross hybrid with better responding genotypes [3].
Induction of somatic embryos is usually promoted by auxins. Exogenously supplied auxins are involved in establishing auxin gradients within plant cells during the induction phase of somatic embryogenesis, essential for initiating dedifferentiation and cell division of already differentiated cells before they can express embryogenic competence [11]. Synthetic auxins normally used are categorized into different classes based on the position of their carboxylic acid moieties on their aromatic rings. The classes include phenoxyalkanoic acids (e.g. 2,4-D), benzoic acids (e.g. dicamba), and the pyridine-carboxylic acids (e.g. picloram) [12]. Among the different auxins, 2,4-D has been most commonly used for somatic embryo
induction in tropical maize genotypes [2-4, 13-15], where the response is genotype dependent and often accompanied with negative somaclonal variations. The optimal concentrations for embryogenic callus induction on media containing 2,4-D has been 2 mg/L [3, 5, 16]. Induction of somatic embryos on medium containing dicamba has been reported in well adapted tropical Indian maize inbred lines and South American tropical and subtropical maize genotypes [17]. Media supplemented with dicamba gave a better callusing response in terms of both quality and frequency when compared to 2,4-D in Indian elite tropical maize genotypes [17]. Interestingly, three tropical maize genotypes gave a similar callusing response on MS medium supplemented with dicamba [17], giving an indication of genotype independent response of tropical maize in tissue culture. It is reported that dicamba was superior to 2,4-D in promoting callus induction and production of type II calli, respectively [18]. Indeed, the presence of dicamba in medium reduced the processing of globulin-1 (Gbl1)-encoded protein in maize tissue culture [19], pointing to a regulatory role for auxins in the processing of Glb1-encoded polypeptides. In other plant species, thidiazuron (TDZ) was found to have genotype independent effect on callus initiation in sugar beet and rice [20, 21].
Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
679
2. Materials and Methods
2.1 Plant Material
Five tropical maize inbred lines, CML 216, CML 144, E 04, A 04 and TL 21 were used in the study. All the genotypes were white seeded. Seeds of the tropical maize (Zea mays L.) inbred lines CML 216 and CML 144 were obtained from CIMMYT (Kenya). Local Kenyan tropical inbred lines A 04 and E 04 were supplied by Dr. G.A. Ombakho (Kenya Agricultural Research Institute (KARI), Nairobi, Kenya). TL 21 was supplied by Dr. Jane Ininda, a breeder at KARI. The tropical genotypes were grown under field conditions at Kenyatta University (Nairobi, Kenya) and at KARI in the months of October, November and December 2011. Plants were self pollinated and the whole ears collected 12, 14, 16 to 18 days following pollination as described previously [3].
2.2 Callus Induction and Media Composition
Maize cobs were harvested and washed with 70% (v/v) ethanol for 3 min then rinsed five times with sterilized distilled water. Subsequently, the immature kernels were surface sterilized for 20 min under aseptic conditions using sodium hypochlorite 2.5% (v/v) containing Tween 20 (1 to 2 drops). Immature embryos of 1.0 to 1.5 mm in size were aseptically excised from the surface sterilized kernels under laminar flow and placed with scutellum side up and the embryo axis side down on solid callus induction medium (CIM) (Table 1).
The CIM was based on Murashige Skoog (MS) basal salts and vitamins [22] supplemented with 1 to 5 mg/L dicamba, 0.7 g/L L-proline, 30 g/L sucrose, 0.5 g/L MES and 0.85 mg/L silver nitrate (AgNO3) (Table 1). Callus maintenance media included MS salts supplemented with 1 mg/L NAA, 60 mg/L sucrose and 0.7 g/L proline whereas shoot induction medium was based on MS basal salts supplemented with only 30 g/L sucrose. The pH of the media was adjusted to 5.8 prior to autoclaving at 121 °C
Table 1 Media composition for establishing tropical maize tissue culture.
Organic/inorganic supplements CIM CMM SIM
Dicamba (mg/L) 1-5 - -
CIM: callus induction medium; CMM: callus maturation medium; SIM: shoot induction medium.
(108 kPa) for 20 min. Three replicates per treatment were maintained and arranged in a completely randomized design.
2.3 Effect of Days after Pollination (DAP)
The effect of DAP on callus induction, precocious germination, necrosis, swelling rhizogenic and dead calli were visually quantified and recorded after immature embryos were incubated in the dark at 28 °C for 2 weeks on CIM. Embryogenic calli were transferred to callus maturation medium (CMM) with the same composition as CIM, but without silver nitrate and an increased sucrose concentration of 60 mg/L to allow for somatic embryo maturation before germination.
2.4 Effect of Genotype on Callus Induction
The effect of genotype on embryogenic and non embryogenic callus induction and multiple shoot induction from immature embryos was studied by culturing immature embryos 12 DAP of the five genotypes on MS medium supplemented with 3 mg/L dicamba. The number of immature embryos forming embryogenic, non embryogenic callus and shoot induction per callus were recorded and compared among all the genotypes.
2.5 Regeneration
Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
680
The number of shoots formed was recorded after 1 week. Plantlets were transferred to peat moss in 10 cm diameter plastic pots, and covered with a moistened plastic paper, for 3 to 5 days, for gradual acclimatization and hardening in the greenhouse. Hardened plantlets were transferred into 20 L pots containing sterile soil for further development to maturity.
3. Results and Analysis
3.1 Effect of Zygotic Embryo Age on Somatic Embryogenesis
The authors evaluated embryogenic callus induction in five tropical maize genotypes from immature embryos 12 to 18 days after pollination (DAP) ranging from 1 to 1.5 mm in size (Fig. 1A). All cultures were maintained under the same experimental conditions on Murashige and Skoog (MS) medium (Table 1) at 28 °C under white fluorescent light with 16 h photoperiod. Callus initiation from cultured embryos was observed within 1 week following culture with swelling and subsequent formation of mass at the embryo axis side (Fig. 1B). Within two weeks of inoculations, precocious germination was observed on most of the embryos harvested at 14, 16 and 18 DAP and was not observed on embryos harvested 12 DAP at 3 mg/L dicamba (Fig. 2). 12 DAP was determined as the developmental stage for immature zygotic embryos to induce the highest frequency of embryogenic callus at all the growth regulators concentrations (Fig. 2). One way ANOVA revealed that the zygotic embryo age had a significant effect on
precocious germination (P = 0.008) and somatic
embryogenesis (P = 0.001) but no effect on
non-embryogenesis (P = 0.395). In addition to
precocious germination, immature embryos collected 14, 16 and 18 DAP displayed other phenotypic characteristics observed in tissue culture including swelling of the immature embryo that becomes unresponsive, pronounced rhizogenecity (Fig. 1C) and in some the swollen mass developed into an irregular
callus which turned organogenic (Fig. 1D). The calli generated were maintained on CIM medium for at least for 4 weeks before transferring to callus maturation medium (CMM) (Table 1). During the transfer of calli to SIM, necrosis was observed in some calli, which eventually died. Somatic embryos on SIM (Fig. 1E) markedly prematured by turning green in color and eventually the leaves temporary coiled during shooting (Fig. 1F). Within three week on SIM, clonal plantlets germinated (Fig. 1G) and eventually transferred to the field (Fig. 1H) with no somaclonal variation observed.
3.2 Effect of Genotype on Callus Induction
Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
681
A
B
C
D
E
F
G
H
Fig. 1 Somatic embryogenesis from the surface of somatic embryos on MS medium with dicamba and plant regeneration of tropical maize CML 144 genotype. (A) Immature embryo size (1 mm) used in tissue culture (12 DAP). (B) Embryogenic type II calli of CML 144 genotype on CMM. (C) Rhizogenic calli derived from immature embryos. (D) Arrows show organogenic calli derived from immature embryos. (E) Proliferating embryogenic calli from tropical maize immature embryos, 2 weeks after culture on CMM. (F) Regenerating coiled plantlets one week on SIM without dicamba. (G) Plantlets on regenerating medium three weeks after culture. (H) R1, plants growing in the field.
Fig. 2 Effect of DAP on embryogenesis at 3 mg/L dicamba in CML 144. Color interpretation is indicated inthe legend at the top. The values are means ± standard error of three replicates per treatment. Values with the same letter are not significantly different by Tukey’s pair-wise comparison (P < 0.05). Each parameter was statistically analyzed independently from the other.
0) suggesting genotype independent callus induction response. Only 9% of the callus induction difference of the group mean was significantly different at the 0.05 level. Analysis by two-way ANOVA revealed a statistical lack of interaction between genotype and dicamba concentrations for type II embryogenic calli
Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
682
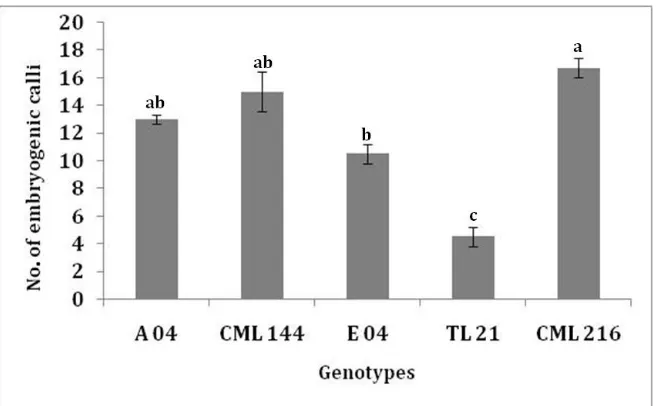
Fig. 3 Average number of type II embryogenic calli from three replicates per treatment induced from immature embryos harvested at 12 DAP in A 04, CML 144, E 04, TL 21 and CML 216 genotypes cultured at 3 mg/L dicamba concentration. Values with the same later are not significantly different by Tukey’s pairwise comparison (P < 0.05).
3.3 Effect of Dicamba on Induction of Regenerative
Calli
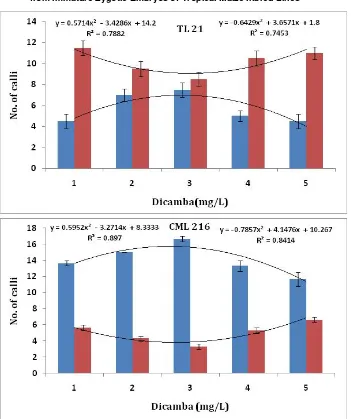
In this study, somatic embryogenesis from immature embryos harvested 12 DAP from all the five genotype seemed independent of genotype and this prompted us to test embryogenesis and non embryogenesis of tropical maize genotypes grown in Kenya on MS medium supplemented with dicamba in order to designate the optimal concentration of the growth regulator for embryogenic callus induction. Callus induction was investigated on callus induction medium (CIM) supplemented with dicamba concentrations ranging from 0, 1, 2, 3, 4 to 5 mg/L. After 5 to 7 days of tissue culture, the scutellum tissue increased in size, 2 weeks later, the coleoptile emerged from the embryo axis site in a number of the immature embryos (precocious germination). Thereafter, the embryo axis site becomes swollen and develops into an irregular callus mass (Fig. 1E) [3]. Finally, embryogenic and non embryogenic calli then begins to form in all the five genotypes. The formation of embryogenic calli increased as the level of dicamba increased in the medium up to 3 mg/L as shown in (Fig. 4). The number of immature embryos
forming embryogenic calli was highest at 3 mg/L dicamba. A significant correlation between dicamba concentration and embryogenic calli formation was observed from regression R2 values of 0.887 for CML 144, 0.8414 for CML 216, 0.9784 for A 04, 0.7453 for TL 21 and 0.7581 for E 04, indicating that the influence of dicamba on somatic embryogenesis process in tropical maize is independent of genotype (Fig. 4 R2values on the right).
Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
684
Fig. 4 Effect of dicamba on somatic embryogenesis and non-embryogenesis in five tropical maize genotypes CML 144, CML 216, TL 21, E 04 and A 04. Red bars: Genotype independent hyperbolic functions of the number of immature embryos forming non embryogenic calli; Blue bars: Genotype independent parabolic function of the number of immature embryos forming embryogenic calli. Shown on the left of each graph are the regression values for the non embryogenesis and to the right the regression values of the embryogenic calli formation. Thevalues are means ± standard error.
Table 2 Percentage embryogenic and non embryogenic callus induction in tropical maize genotypes on MS medium supplemented with dicamba (% callus induction ± standard error).
Genotypes
Dicamba, mg/L
1 2 3 4 5
E N.E E N.E E N.E E N.E E N.E
CML 144 45.94±0.49 45.94±1.49 60.97±1.49 36.58±1.49 73.17±0.9926.82±1.49 54.76±1.49 45.23±0.49 48.71±0.49 51.28±0.99 A 04 63.63±2.4 33.33±0.49 67.56±1.49 35.13±0.49 74.28±0.0 28.57±1.99 57.14±0.99 28.57±0.99 52.5±0.49 30±1.99
Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
685
values of 0.6473 for CML 144, 0.8970 for CML 216, 0.7882 for TL 21 and 0.8824 for E 04, indicating that the influence of dicamba on non embryogenesis in these tropical maize is independent of genotype (Fig. 4, R2 values on the right) except A 04 which was genotype dependent (Fig. 4, R2 = 0.033).
The number of immature embryos forming embryogenic calli was lower at concentrations of dicamba higher than the 3 mg/L compared to those forming non-embryogenic calli. Those forming non embryogenic calli reduced with increase in the concentrations of dicamba up to 3 mg/L, and then increased with further increase in the concentrations of the growth regulator (Fig. 4 and Table 2). Dicamba at 3 mg/L was therefore considered the optimal concentration for genotype independent embryogenic calli induction (or genotype independent inhibition of non embryogenic calli formation) (Fig. 4 and Table 2).
3.4 Quantitative Analysis of Regeneration
A higher number of shoots per callus were regenerated at the optimal dicamba concentration for all the genotypes except E 04. TL 21 genotype had the highest number of shoots that were produced via organogenesis and were not clonal at the optimal callus induction (Table 3). Regeneration started with the appearance of green coloration on mature embryos within 4-5 days on growth regulator free regeneration medium supplemented with 30% sucrose under continuous illumination. Green shoots formed within 1 week of culture (Fig. 1F). Mature embryos from
CMM produced shoots that coiled and eventually developed into normal shoots (Fig. 1F and G). The coiling could have been impacted by the growth regulator dicamba since this phenotype has not been observed in regenerants produced on media supplemented with 2,4-D growth regulator [3]. Indeed analysis of regeneration data by ANOVA showed that genotype significantly influenced regeneration more (P = 0.003) than dicamba (P = 0.012) though still
significantly. The in vitro rooted plants were
acclimatized by transfer to peat moss-containing pots covered with plastic sheets to maintain high moisture conditions for 3 days. The moisture was gradually reduced over a period of 15 days and plantlets were transferred to large soil-filled 20-liter pots in the open field.
4. Discussion
Green and Phillips [23] were the first to report plant regeneration from tissue culture of maize via somatic embryogenesis which was confirmed microscopically in Refs. [24, 25]. Since then, many published reports are available for tropical maize suggesting successful callus induction and regeneration from immature embryos [3-5, 13, 14, 26] as well as mature embryos [26]. However, callus induction and regeneration either on N6, MS or LS medium supplemented with 2,4-D growth regulator, has strongly been genotype dependent. It is reported that N6 medium supplemented with dicamba gave efficient callus induction and plant regeneration intropical and
Table 3 Regeneration of tropical maize genotypes calli after culture on MS medium supplemented with dicamba (Mean number of shoots per callus piece (% regeneration efficiency*)).
Genotypes Dicamba, mg/L
1 shoot/calli 2 shoots/calli 3 shoots/calli 4 shoots/calli 5 shoots/calli
CML 144 2/6 (33) 4/7 (60) 5/6 (84) 2/6 (33) 3/6 (50)
A 04 2/6 (33) 3/6 (50) 3/6 (50) 2/5 (40) 2/5 (40)
CML 216 2/6 (33) 5/6 (84) 5/6 (84) 3/6 (50) 3/6 (50)
E 04 2/6 (33) 3/8 (40) 1/8 (18) 1/8 (13) 1/7 (14)
TL 21 7/18 (39) 8/17 (50) 6/10 (60) 5/10 (50) 6/12 (50)
Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
686
subtropical maize germplasm [26]. It is also suggested that callus obtained from immature embryos in the presence of dicamba developed into somatic embryos at a higher frequency than the callus obtained with 2,4-D [27]. Furthermore, it is showed that three tropical maize genotypes produced nearly similar callus induction response when cultured on N6 medium with dicamba [17]. MS salts have recently been reported to improve Agrobacterium mediated transformation of temperate maize [16]. Therefore, efforts were made to promote callus induction in a genotype independent way in tropical maize genotypes adapted to Kenya using MS media supplemented with different concentrations of the dicamba growth regulator.
Between 8 and 20 days after pollination (DAP), immature embryos provide several discrete developmental stages of meristematic tissue that exhibit varied competence to callus induction and
Agrobacterium infectivity. Immature embryos at the right developmental stage and size (1 to 1.5 mm) are optimal for successful callus induction and transformation in temperate maize [28]. In tropical maize genotypes, calli have been induced from immature embryos harvested 11 to 21 days after pollination and this has been predominantly genotype dependent [3, 4, 14, 29]. Embryos harvested 14, 16 and 18 DAP germinated at the expense of forming embryogenic calli. This precocious germination is accompanied by reduction in maturation association gene expression during zygotic embryogenesis. The reduction is attributed to lack of endogenous ABA production or sensitivity to external ABA and absence of expression of Viviparous-1 (Vp1) gene [30]. Zygotic embryo maturation is associated with the accumulation of 7S globulin storage protein [31] and a variety of late embryogenesis abundant proteins (LEAs) [32]. Expression of Vp1
gene is required to prevent maize zygotic embryo germination and in vp1 mutants, the expression of a 7S globulin gene is blocked [30, 31]. Vp1 expression
declines in cultured embryos [33], which might potentiate precocious germination.
It is also likely that immature embryos collected 14, 16 and 18 DAP had elevated levels of gibberellins [34] and were insensitive to external growth regulator dicamba, which could further contribute to precocious germination. This factor and the likely lack of expression of Vp1 might have potentiated precocious germination. Precocious germination was not observed on embryos harvested 12 DAP because embryos at this stage have apparently not yet developed the competence to germinate. Immature embryos harvested 12 DAP induced type II embryogenic calli within 15 days following culture in all the genotypes suggesting embryos 12 days after pollination consisted the right developmental stage for embryogenic calli induction that is genotype independent. This data is in line with recent findings that the immature embryo at 12 days after pollination (DAP) showed the highest competence for embryogenesis [35]. Thus, the embryogenic genotype independent responses of immature embryos at 12 DAP may be a pointer to understanding the induction of somatic embryogenesis in tropical maize. Genes associated with the formation of embryonic calli may offer additional insights into the mechanism of somatic embryogenesis. Further research on these genes may determine their role in increasing the rate of induction of embryonic calli.
Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
687
controlled by different genetic elements or regulators [35]. Tropical maize genotypes have been reported to vary greatly in embryogenic capacity reflecting differences in their ability to activate key elements/genes of the embryogenic pathway. Our data suggest that at 3 mg/L dicamba, key elements of the embryogenic genetic program are activated and may have an inhibitory effect on key elements of the non embryogenic program or their expression is repressed [36] for instance by ZmSERK genes [35].
5. Conclusion
In this work, the authors report genotype independent somatic embryos formation on MS medium containing dicamba. In all the genotypes studied herein, dicamba effectively induced somatic embryos from immature zygotic embryos. The optimal concentration for somatic embryogenic callus induction was 3 mg/L in all the genotypes tested. These results indicate a new phenomenon in tropical maize tissue culture where somatic embryogenic, callus induction and non embryogenic callus inhibition seem to be genotype independent on medium containing the auxin dicamba contrary to those induced by 2,4-D. The underlying biochemical/signaling pathways seem to operate distinctly for the two pathways as already reported for 2,4-D [35]. The growth regulator dicamba, however, seems to allow for the rare dissection of the specific cellular events related to the overlapping phases of dedifferentiation, cell cycle reactivation and the acquisition of embryogenic competence in sub-Saharan tropical maize that could not be achieved with 2,4-D [2, 3, 5 ].
In some Indian elite tropical maize, it is noted a similar independent interaction between auxin treatment and genotypes when using both 2,4-D and dicamba as well, on N6 basal medium [37, 38]. The absolute requirement for exogenous auxin to maintain callus cultures in plant cells is complemented by production of substantial amounts of the native auxin
indole acetic acid (IAA) within the cells [36]. Therefore, the application of exogenous auxin and subsequent elevation (fluctuations) of endogenous auxin are both determining factors in the induction of callus tissue and somatic embryogenesis [35, 36]. The implication from this study is that the interaction of the endogenous auxin (IAA) in tropical maize tissues with the exogenous synthetic auxins such as 2,4-D and dicamba may be varying with the type of synthetic auxin, as genotype independent somatic embryogenesis occurred with dicamba yet it has been genotype dependent with 2,4-D.
Acknowledgments
This work was sponsored by NCST/5/003/3rd STI CALL/189 on food security and climate change and IFS grant C5174-20120319 on tropical maize for food and health. The authors declare that they have no conflict of interest.
References
[1] S. Anami, M. De Block, J. Machuka, M. Van
Lijesebettens, Molecular improvement of tropical maize for drought stress tolerance in sub-Saharan Africa, Critical Reviews in Plant Science 28 (2009) 16-35. [2] L.T. Bedada, M.S. Seth, S.M. Runo, W. Tefera, J.
Machuka, Regenerability of elite tropical maize (Zea mays
L.) inbred lines using immature zygotic embryo explants, African Journal of Biotechnology 11 (2012) 598-605. [3] S. Anami, A. Mgutu, C. Taracha, G. Coussens, M.
Karimi, P. Hilson, et al., Somatic embryogenesis and plant regeneration of tropical maize genotypes, Plant Cell, Tiss and Organ Cult 102 (2010) 285-295.
[4] R.O. Oduor, S. Ndung'u, E.N. Njagi, J. Machuka, In vitro
regeneration of dryland Kenyan maize genotypes through somatic embryogenesis, Int. J. Bot. 2 (2006) 146-151.
[5] O. Ombori, N.M. Gitonga, J. Machuka, Somatic
embryogenesis and plant regeneration from immature embryos of tropical maize (Zea mays L.) inbred lines, Biotechnol. 7 (2008) 224-232.
[6] Y. Shen, Z. Jiang, X. Yao, Z. Zhang, H. Lin, M. Zhao, et al., Genome expression profile analysis of the immature maize embryo during dedifferentiation, PLoS One 7 (2012) e32237.
Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
688
in the regulation of the shoot apical meristem, J Exp Bot 61 (2010) 4069-4085.
[8] S.Z. Zhang, X.G. Liu, Y.A. Lin, G.N. Xie, F.L. Fu, H.L. Liu, et al., Characterization of a ZmSERK gene and its relationship to somatic embryogenesis in a maize culture, Plant Cell Tiss Organ Cult 105 (2001) 29-37.
[9] K.V. Rao, P. Suprasanna, G.M. Reddy, Biochemical changes in embryogenic and non-embryogenic calli of
Zea mays L., Plant Sci 66 (1990) 127-130.
[10] M. Zacchini, A. Graverini, S. Grego, M. de Agazio, Ethylamine in maize callus: isolation, identification and first approach to the physiological role of its hyper-production, Plant Sci 150 (2000) 147-151.
[11] X. Yang, X. Zhang, D. Yuan, F. Jin, Y. Zhang J. Xu, Transcript profiling reveals complex auxin signalling pathway and transcription regulation involved in dedifferentiation and redifferentiation during somatic embryogenesis in cotton, BMC Plant Biology 12 (2012) 110. doi:10.1186/1471-2229-12-110.
[12] K.B. Kelley, D.E. Reichers, Recent developments in auxin biology and new opportunities for auxinic herbicide research, Pesticide Biochem Physiol 89 (2007)1-11.
[13] J.J. Binnot, J.M. Songa, J. Ininda, E.M. Njagi, J. Machuka, Plant regeneration from immature embryos of Kenyan maize inbred lines and their respective single cross hybrids through somatic embryogenesis, African. J. Biotechnol. 7 (2008) 981-987.
[14] J.W. Danson, M. Lagat, M. Mbogori, Screening tropical maize lines for the production and regeneration of friable and embryogenic type II Callus, African J. Biotechnol. 5 (2006) 2367-2370.
[15] L.M. Prioli, W.J. Silva, Somatic embryogenesis and plant regeneration capacity in tropical maize inbreds, Rev Bras Genet 12 (1989) 553-566.
[16] B.R. Frame, H. Shou, R.K. Chikwamba, Z. Zhang, C.
Xiang, T.M. Fonger, et al., Agrobacterium
tumefaciens-mediated transformation of maize embryos using a standard binary vector system, Plant Physiol 129 (2002) 13-22.
[17] S. Rakshit, Z. Rashid, J. Sekhar, T. Fatma, S. Dass, Callus induction and whole plant regeneration in elite Indian maize (Zea mays L.) inbreds, Plant Cell Tiss Organ Cult 100 (2010) 31-37.
[18] C.H.S. Carvalho, N. Bohorova, P.N. Bordallo, L.L. Abreu, F.H. Valicente, W. Bressan, et al., Type II callus production and plant regeneration in tropical maize genotypes, Plant Cell Rep. 17 (1997) 73-76.
[19] D.R. Duncan, A.L. Kriz, R. Paiva, J.M. Widholm, Globulin-1 gene expression in regenerable Zea mays
(maize) callus, Plant Cell Rep. 21 (2003) 684-689. [20] Golovko, Genetic variability of somatic embryogenesis in
tissue cultures of sugar beet breeding lines, Tsitologiia i
Genetika 35 (2001) 10-17.
[21] M. Dey, S. Bakshi, G. Galiba, L. Sahoo, S. K. Panda, Development of a genotype independent and transformation amenable regeneration system from shoot apex in rice (Oryza sativa spp. indica) using TDZ, Biotech 2 (2012) 233-240.
[22] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassays with tobacco tissue cultures, Physiol. Plant. 15 (1962) 473-497.
[23] C.E. Green, R.L. Phillips, Plant regeneration from tissue cultures of maize, Crop Science 15 (1975) 417-421. [24] V. Vasil, I.K. Vasil, C.Y. Lu, Somatic embryogenesis in
longterm callus cultures of Zea mays L. (Graminae), Ann J Bot 71 (1984) 158-161.
[25] J.W. McCain, K.K. Kamo, Hodges, Characterization of somatic embryo development and plant regeneration from friable maize callus cultures, Bot Gaz 149 (1998) 16-20. [26] N.E. Bohorova, B. Luna, R.M. Brito, L.D. Huerta, D.A.
Hoisington, Regeneration potential of tropical, subtropical, midaltitude and highland maize inbreds, Maydica 40 (1995) 275-281.
[27] A. Furini, D.C. Jewell, Somatic embryogenesis and plant regeneration from immature and mature embryos of tropical and subtropical Zea mays L. genotypes, Maydica 39 (1994) 155-164.
[28] Y. Ishida, Y. Hiei, T. Komari, Agrobacterium-mediated transformation of maize, Nature Protocols 2 (2007) 1614-1621.
[29] L.M. Prioli, W.J. Silva, Somatic embryogenesis and plant regeneration capacity in tropical maize inbreds, Rev Bras Genet 12 (1989) 553-566.
[30] D.R. McCarty, T. Hattori, C.B. Carson, V. Vasil, M. Lazar, I.K. Vasil, The Viviparous-1 developmental gene of maize encodes a novel transcriptional activator, Cell 66 (1991) 895-905.
[31] A.R. Kriz, M.S. Wallace, R. Paiva, Globulin gene expression in embryos of maize viviparous mutants, Plant Physiol. 92 (1990) 538-542.
[32] H.I.L. Dure, M. Crouch, J. Harada, T.H.D. Ho, J. Mundy, R.S. Quatrano, et al., Common amino acid sequence domains among the LEA proteins of higher plants, Plant Molecular Biology12 (1989) 475-486.
[33] X. Cao, L.M. Costa, C. Biderre-Petit, B. Kbhaya, N. Dey, P. Perez, et al., Abscisic acid and stress signals induce
Viviparous1 expression in seed and vegetative tissues of maize, Plant Physiol143(2007)720-731.
[34] C.N. White, W.M. Proebsting, P. Hedden, C.J. Rivin, Gibberellins and seed development in maize. I. Evidence that gibberellin/abscisic acid balance governs germination versus maturation pathways, Plant Physiol 122 (2000) 1081-1088.
Dicamba Growth Regulator Promotes Genotype Independent Somatic Embryogenesis from Immature Zygotic Embryos of Tropical Maize Inbred Lines
689
Characterization of a ZmSERK gene and its relationship to somatic embryogenesis in a maize culture, Plant Cell, Tissue and Organ Culture 105 (2011) 29-37.
[36] X. Yang, X. Zhang, D. Yuan, F. Jin, Y. Zhang, J. Xu, Transcript profiling reveals complex auxin signalling pathway and transcription regulation involved in dedifferentiation and redifferentiation during somatic embryogenesis in cotton, BMC Plant Biology 12 (2012)
110. doi:10.1186/1471-2229-12-110.
[37] A. Manivannan, J. Kaul, A. Singode, S. Dass, Callus induction and regeneration of elite Indian maize inbreds, Afr. J. Biotechnol 9 (2010) 7446-7452.
July 2013, Vol. 7, No. 7, pp. 690-694
Journal of Life Sciences, ISSN 1934-7391, USA
Comparative Study of the Static Magnetic Field Effects
on Growth Rate with Relative Antibiotic Susceptibility in
Escherichia coli
Fouad Houssein Kamel, Ashti M. Amin, Khonaw Kader Salih and Saleem S. Qader
Medical Technical Institute, University of Polytechnic, Erbil PO box 44001, Iraq
Received: April 07, 2013 / Accepted: June 14, 2013 / Published: July 30, 2013.
Abstract: We studied the biological effects of different magnetic fields. Identified bacterial strain Escherichia coli (type 1) has been exposed to the dipolar magnetic field force (400, 800, 1200 and 1600 Gausses) which prepared locally with incubation for different period times (24, 48 and 72 hrs) at 37 °C. The effects were evaluated by optical density (OD) at 600 nm determining their growth density incorporation with negative control and depending of McFarland turbidity standard (0.5), in addition to its susceptibility to various antibiotics. Results illustrate different forces of magnetic field decreased the growth rate of E. coli in particular at 24 hrs incubation comparing with unexposed or control samples. The magnetic field increased the logarithmic phase within 4-6 hrs of treatment but decreased after 16 to 18 hrs. Furthermore, changes in the antibiotic sensitivity were observed after exposure period of 6 hrs since E. coli cells became more sensitive to certain antibiotics. While after a 16 hrs exposure period, it became more resistant to the same antibiotics comparing with control groups.
Key words: Magnetic field, bacteria, optical density, Escherichia coli, antibiotic susceptibility.
1. Introduction
A magnetic field is the area of influence exerted by a magnetic force. This field is normally focused along two poles. These poles are usually designated as north and south. However these directions are not the only two that a magnetic field can have. Most magnetic objects are composed of many small fields called domains [1]. The search for a biological effect due to magnetic fields has a long history dating back a hundred years. The literature on biomagnetic effects on the growth and development of various organisms has been quite extensive showing both positive and negative findings. Among the positive findings attributed to strong magnetic fields are: altered growth rate, enzyme activities, cellular metabolism, DNA synthesis and animal orientation [2].
Corresponding author: Fouad Houssein Kamel, Ph.D., research field: medical technology. E-mail: [email protected].
A wide variety of methods have been reported in the literatures which are directed to the use of magnetic energy as a diagnostic technique and also for the treatment of diseases in warm blooded animals including humans. For example, magnetic energy has been utilized quite successfully over the past several years to promote the formation of osteoblasts in conjunction with the healing of bone fractures. In many instances markedly improved results in healing times have been achieved by the application of magnetic energy to the site of bone fractures and other injuries. Magnetic enhancement or retardation of bacterial or cellular growth rates has been reported in the literatures e.g. Davis and Rawls presented numerous examples of enhancement to seeds and various types of cellular growth [3].
It was found that E. coli, Leclercia adecarboxylata
and Staphyloccus aureus viability was affected with the magnetic field (10 mT, f = 50 Hz) they also found DAVID PUBLISHING
Comparative Study of the Static Magnetic Field Effects on Growth Rate with Relative Antibiotic Susceptibility in Escherichia coli
691
that the decrease of the colony forming units (CFU) starts immediately after the magnetic field was switched on [4]. Inactivation of micro organisms by a pulsed magnetic field has been studied [5]. It was reported that the application of electromagnetic pulses evidently cause a lethal effect on E. coli cells suspended in a buffer solution.
The non-thermal sterilization by using the self designed generator of magnetic field has been investigated [6]. The results showed that the magnetic flux density, which had the greatest effect on E. coli, was 1 T.
The greatest destruction rate of E. coli was 78% under 8 hours of magnetic field (1T) treatment. Also, the exposure of the microorganism S. typhi to the magnetic field (10, 20 G for a period of 2 hrs) caused pronounced changes in the growth characteristics and the number of cells at the stationary phase increased has been reported [7].
The objectives of this research were to study the effects of different exposure periods to 400, 800, 1200 and 1600 G locally prepared static magnetic field on the cell activity. The effects of such magnetic fields on the growth rate and antibiotic sensitivity were explored, too.
The study has been approved by the Ethics and Scientific Committees of Medical Research Centre, Hawler Medical University, Erbil, Iraq.
2. Materials and Methods
2.1 Magnetic Field
Dipolar magnetic field was prepared locally with
different forces including 400, 800, 1200, 1600
Gausses and measured by Teslometer in Physical Department, College of Science, University of Salahddin, Erbil, Iraq.
2.2 Bacterial Growth
Culture of E. coli (Escherichia coli) was isolated from the urine of patient after culture onMacConkey agar at 37 °C for 24 hrs. The bacteria re-cultivated
overnight on nutrient broth at 37 °C.
Bacterial suspension of Escherichia coli will be inoculated in to five groups of tube containing nutrient broth media incubated for 24, 48 and 72 hrs at 37 °C as following: Group 1: subjected to 400 G; Group 2: subjected to 800 G; Group 3: subjected to 1200 G; Group 4: subjected to 1600 G; Group 5: control, without magnetic power.
The API kits were prepared by BioMerieux
Company and used due to process fixed by the
BioMerieux Company. Inoculation of API kit with bacteria from each group was doneseparately [8].
McFarland Turbidity Standards (0.5) process was used to evaluate the effects of different forces of magnetic fields on growth rateby measurement of the optical density [9].
2.3 Antibiotic Susceptibility Test
Muller-Hinton agar medium was prepared for culture antibiotic susceptibility test of E. coli
depending Kirby-Bauer Disk Diffusion technique. Antibiotic used are Azithromycin, Trimethoprim, Amikacin, Tobramycin, Clindamycin, Vancomycin, Cefixime, Rifampin, Bacitracin, Ciprofloxacin and Carbencillin [10, 11].
3. Results
E. coli were exposed to different forces of MFs (400, 800, 1200 and 1600 Gausses). The growth rate of E. coli cells were significantly affected by exposure to these magnetic forces in particular at 24 hrs incubation and the number of cells was significantly decreased in bacteria exposed to magnetic fields when compared with the control (Table 1). In addition to that the magnetic field increased the logarithmic phase within 4-6 hrs of treatment, but it was decreased after 16-18 hrs comparing with the control. The bacterial enzymes such as, ADH, CITand GEL are effected to magnetic field.
Comparative Study of the Static Magnetic Field Effects on Growth Rate with Relative Antibiotic Susceptibility in Escherichia coli
692
Table 1 The growth rate of E. coli for each group is determined by spectrophotometer.
Magnetic force OD 600 nm at
24 hrs
Bacterial cell count CFU ( 106/mL)
Control 1.192 715.2
400 G 1.103 661.8
800 G 1.120 672.0
1200 G 1.080 648.0
1600 G 1.112 667.2
study, the highest decrease of the viability by using
biggest magnetic field was observed on E. coli
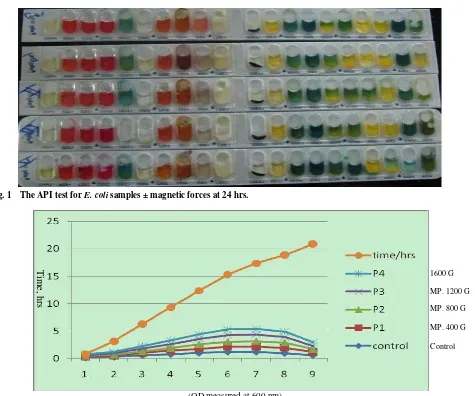
comparing with unexposed bacterial growth, immediately after the magnetic field was switched on. According to API 20E, the bacterial enzymes ADH, CIT and GEL are affected to magnetic field at 24 hrs incubation time but during 48 and 72 hrs incubation
times only ADH and CIT are affected to magnetic forces (Fig. 1).
Fig. 2 and Table 2 show the variation of the number of microorganisms in CFU/mL as function of the sample absorbance measured at 600 nm.
The isolated E. coli cells were tested for their in vitro susceptibility to various antibiotics such as Azithromycin, Trimethoprim, Amikacin, Tobramycin, Clindamycin, Vancomycin, Cefixime, Rifampin, Bacitracin, Ciprofloxacin and Carbencillin by disk diffusion test [10, 11]. The antibiotics were chosen to be with different modes of action. The diameters of the inhibition or stimulation zone of the difference magnetic forces were measured after 24 hrs from the
Fig. 1 The API test for E. coli samples ± magnetic forces at 24 hrs.
(OD measured at 600 nm)
Fig. 2 Absorbance at 600 nm of E. coli cells at a different exposure period and power.
Time, hrs
1600 G
MP. 1200 G
MP. 800 G
MP. 400 G
Comparative Study of the Static Magnetic Field Effects on Growth Rate with Relative Antibiotic Susceptibility in Escherichia coli
693
Table 2 Optical density of E. coli growth before and after exposing to the magnetic fieldat different times.
Time (hr) Optical density (OD) at 600 nm
Control P1 P2 P3 P4
0 0.16 0.16 0.16 0.16 0.16
2 0.3 0.2 0.19 0.3 0.2
4 0.6 0.4 0.3 0.6 0.4
6 0.8 0.67 0.4 0.8 0.67
8 1 0.8 0.8 1 0.8
10 1.2 0.99 0.92 1.2 0.99
12 1.21 1 0.96 1.21 1
14 0.98 0.99 0.96 0.98 0.99
18 0.6 0.61 0.5 0.6 0.61
Table 3 The antibiotic test of exposed and unexposed E. coli.
Antibiotics
Inhibition antibiotics zone diameter in cm
Unexposed Exposed to 1
thP. Exposed to 2thP. Exposed to 3th P. Exposed to4th P.
6 hr 19 hr 6 hr 19 hr 6 hr 19 hr 6 hr 19 hr
Azithromycin 1.6 1.8 1.2 1.7 0.6 1.7 0.9 1.8 1.3
Trimethoprim 1.2 1.4 1.2 1.4 1.0 1.5 0.1 1.5 0.2
Amikacin 1.9 2.0 1.6 2.1 1.5 2.0 1.4 2.0 1.7
Tobramycin 1.9 2.2 1.9 2.0 1.9 2.0 1,9 2.0 1.9
Clindamycin 1.3 1.4 1.3 1.4 1.3 1.4 0.9 1.4 0.9
Vancomycin 1.1 1.3 1.0 1.3 0.8 1.2 0.8 1.2 0.8
Cefixime 0.9 1.3 1 1.2 0.1 1.0 r 1 0.8
Rifampin 1.9 2.0 0.8 2.2 1.1 2.1 0.9 2.3 0.9
Bacitracin 1.4 1.6 1.0 1.5 R R R R
Ciprofloxacin R R R R R R R R R
Carbencillin 1 1.1 0.8 1.1 0.8 1.0 R 1.0 0.7
exposure process compared with unexposed samples (Table 3). Further, changes in the antibiotic sensitivity was observed after exposure period of 6hrs since E. coli cells became more sensitive to certain antibiotics e.g. Azithromycin, Trimethoprim, Amikacin, Tobramycin, Clindamycin, Vancomycin, Cefixime, Rifampin, Bacitracin, Ciprofloxacin and Carbencillin as revealed in increase in their zone diameters while, after a 16 hrs exposure period, it became more resistant to the same antibiotics comparing with control group.
4. Discussion
These results suggest that the biological effects of magnetic fields may critically depend on the physical characteristics of the magnetic signal, in particular the wave forces. So treating enzyme with different magnetic fields can inhibit or promote enzyme activity
according to API 20 E. We could also identify the
Escherichia coli type 1 by this test.
The results in Fig. 2 indicate considerable changes in the growth curve characteristics for the exposure periods 6 and 16 hrs. Moreover, from these results one sees how the period of the active growth (log phase) decreased for the exposure groups than the unexposed cell in all period and also the lag phase was short.
5. Conclusions
We conclude that the magnetic fields have biological effects and that different force of magnetic fields decreases the growth rate of E. coli particularly increased the logarithmic phase.
Comparative Study of the Static Magnetic Field Effects on Growth Rate with Relative Antibiotic Susceptibility in Escherichia coli
694
after exposure period of 6 h to certain antibiotics, but become resistant after 16 hrs.
Acknowledgment
Thanks for the Physical Department and Medical Research staffs for their cooperation.
References
[1] Tega Jessa, What is a Magnetic Field?, September 29, 2010, http: //www.universetoday.com/74577/what-is-a- magnetic-field/.
[2] A.R. Davis, Jr. W.C. Rawls, Magnetism and Its Effects on the Living System, Acres, U.S.A., Kansas City, Mo., 1974.
[3] L. Fojt, L. Strav, V. Vetterl, Comparison of the low- frequency magnetic field effects on bacteria Escherichia coli, Feclercia adecarboxylata and Staphylococcus aureus, Bioelectrochemistry 63 (2004) 337-339.
[4] S. Ye, W. Huang, M. He, Q. He, L. Yang, Preliminary study on technology of magnetic field non- thermal sterilization, Transactions of the Chinese Society of Agricultural Engineering 19 (5) (2003) 156-160.
[5] L. Mei, Q. Jiu-Hui, P. Yong-Zheri, Sterilization of
Escherichia coli cells by the application of pulsed magnetic field, Journal of Environmental Sciences 16 (2004) 349-352.
[6] P.C. Appelbaum, J. Stavitz, M.S. Bentz, L.C. Von Kusyer, Four methods for identification of gram-negative non fermenting rods: Organisms more commonly encountered in clinical Specimens, J. Clin. Microbiol. 12 (1980) 271-278.
[7] A.A. Mohamed, F.M. Ali, E.A. Gaafar, H.R. Magda, Effects of magnetic field on the biophysical, biochemical properties and biological activity of Salmonella typhi, Master Thesis, Biophysics Department, Faculty of Science, Cairo University, Egypt, 1997.
[8] A.L. Koch, P. Gerhardt, Growth Measurement, “Methods for General and Molecular Bacteriology”, American Society for Microbiology, Washington, DC., 1994, pp. 248-277.
[9] Kirby-Bauer, Disk Diffusion Susceptibility Test Protocol 08, 2012.
[10] M. Haghi, M.J. Maghsoodi, M.B. Janipor, S.
Seyyedgholizadeh, Effect of static magnetic field on E. coli growth, International Journal of Advanced Biotechnology and Research 3 (4) (2012) 777-781. [11] A. Fulton, W. Isaacs, Titin, a huge, elastic sarcomeric
July 2013, Vol. 7, No. 7, pp. 695-699
Journal of Life Sciences, ISSN 1934-7391, USA
Use of Wood Fibre Compost for the Cultivation of
Trichoderma
sp. (Isolate Td
22
)
Yan Ramona1 and Martin A. Line2
1. Integrated Laboratory for Biosciences and Biotechnology, Udayana University, Bali 80362, Indonesia
2. School of Agricultural Science, University of Tasmania, Tasmania 7001, Australia
Received: February 16, 2013 / Accepted: May 15, 2013 / Published: July 30, 2013.
Abstract: The main objective of this research was to investigate the ability of a Trichoderma sp. (Td22), inhibitory to Sclerotinia
minor Jagger, to grow and survive in mature wood fibre waste (WFW) compost of paper mill origin following nutrient amendment. The growth and survival of the fungus in the WFW compost was assessed by serial dilution plate count method followed by confirmation of the fungal identity using pectic enzyme analysis as described in Cruickshank and Pitt [1]. It was found in this study that the population densities of Td22 achieved under non-sterile conditions in the WFW compost following nutrient amendment was
approximately in the range of 7.0 log10 CFU/g dw – 8.5 log10 CFU/g dw after 28 days, depending on pre-treatment. The efficacy of
this WFW compost-grown Td22 for protection of lettuce from attack by S. minor was also demonstrated in glasshouse trials. This
study indicates that cellulosic paper mill waste compost could provide an abundant low-cost growth medium for the large-scale cultivation of fungal antagonists, improving prospects for cost-competitiveness with chemical treatments.
Key words: Compost, biological control, Trichoderma sp., Sclerotinia minor.
1. Introduction
High concentration of pesticide usage often leads to increased pathogen resistance, resulting in ever higher pesticide applications in order to kill the same pathogen [2-4]. Furthermore, pesticide residue can have adverse effects on reproduction and developmental processes in a variety of animals including humans [5-7]. Due to these detrimental effects, reduced pesticide usage in agricultural practice has been championed [8, 9] and one of the best broad spectrum pesticide/fumigants (methyl bromide) is no longer used in developed countries. Alternatives have been proposed, but none appears to be as effective [10]. In anticipation of such future reduction, Painuly and Dev [11] proposed some possible alternative control, such as biological control, use of pathogen-resistant plant varieties, and integrated pest management.
Corresponding author: Yan Ramona, Ph.D., research fields: environmental microbiology, composting and biocontrol. E-mail: [email protected].
Problems related to the large-scale production of biological control agents in low cost materials include difficulties in handling, transport, and storage. These problems have been largely overcome by maintenance of bacterial antagonists in carriers such as peat or vermiculite for periods of five months or more [12-14]. Cost of the cultivation medium, its transport, and field dispersal are critical factors in any assessment of biological control relative to chemical treatments, a problem sometimes exacerbated by the perceived need for proprietary media formulations.
Norske-Skog Paper Mill Limited produces approximately 33,000 tonnes of wood fibre waste (WFW) per annum, to be dumped as landfill [15].
This cellulosic waste was considered worthy of examination in view of its attributes of excellent water-holding and/or aeration capacity, its freedom from toxic elements, its free availability, and its potential utilization as a source of energy and carbon by cellulose-utilizing fungi, including the Trichoderma sp.
DAVID PUBLISHING
Use of Wood Fibre Compost for the Cultivation of Trichoderma sp. (Isolate Td22)
696
isolate Td22.
Based on the above background, the potential use of wood fibre waste compost as a possible substrate for the low-cost cultivation of a fungal biological control agent (Trichoderma sp.isolate Td22) was investigated, together with assessments of the efficacy of the compost-grown Td22 to protect lettuce seedlings from attack by S. minor in pot trials or glasshouse level experiments.
2. Materials and Methods
2.1 Isolate Td22
Trichoderma sp., isolate Td22 was kindly provided by Dr. Dean Metcalf (DPIWE Tasmania). For long term storage, the fungus was maintained at 4 °C in sterilized and moist millet seeds.
2.2 Wood Fiber Waste
The WFW used in this study was the same as that used by Ramona and Line [15]. It is a mix of eucalypt and Pinus radiata, comprising holocellulose as its primary constituent with very low levels of metal contaminants. It was also deficient (from a recycling perspective) in N and P. In the present trial, composting of this material was for three months (followed the method specified by Jackson and Line [16] and Jackson [17]) until its toxicity to radish seeds was eliminated.
2.3 Inoculum Preparation
The active starters used in this experiment were prepared according to the methods as specified in Ramona [18]. The potency of this inoculum, measured in CFU, was determined by serial dilution plating (in triplicates) before being used to inoculate the WFW.
2.4 Assessment of Growth of the Td22
The WFW compost (at a self-generated temperature of 55-60 °C) was air-dried in a glasshouse, mixed with 2% (w/v) Phostrogen® solution, and placed into 750 mL flasks (100 g quantities each). These flasks were
then either briefly autoclaved for 5 minutes prior to inoculation (Compost A), or inoculated directly with
inoculum (Td22 suspension described above)
(Compost B) to give an initial density of 5.5 log10 CFU/g compost. Incubation was at 25 °C for 4 weeks with periodic assay of CFU on Potato Dextrose Agar (PDA) for Td22 and on 0.1% Trypticase Soya Agar (TSA) for check for bacterial contaminants. The identity of the Td22 was confirmed by analysis of pectic enzymes using the method as described by Cruickshank and Pitt [1].
2.5 The Efficacy of WFW Compost-Grown Td22
Lettuce seeds were sown in pots containing field soil amended with suppressive compost (Compost A) at levels of 2.5, 5.0, 10 and 20% v/v.
The spore density of the antagonist (Td22) in the
compost was 9.21 log10 spores/g wet weight of
compost. Lettuce pathogen (S. minor) grown on millet seed was evenly inoculated approximately 20 mm below the soil surface at the rate of 2.0 g inoculum per pot. Soil without compost amendment, amended with pathogen only, or without pathogen, served as controls. Five replicate pots per treatment, each containing five seeds, were maintained for four weeks in a shade house. Pots were irrigated as required and the germinated seeds counted one week after sowing. Disease incidence on the lettuce seedlings was recorded from two weeks after sowing.
2.6 Data Analysis
The data obtained from this experiment was statistically analyzed by using analysis of variance (ANOVA) using Minitab software for Windows. The significance of differences between means was further tested using the least significant different (LSD) test at
P < 0.05 following ANOVA.
3. Results
3.1 The Growth and Survival of Td22 in WFW Compost
Use of Wood Fibre Compost for the Cultivation of Trichoderma sp. (Isolate Td22) 697
days following different pre-treatments is presented in Fig. 1. Significant increases in CFU of Td22 in steam-treated or untreated composts were observed in the first seven days, after which time the populations remained relatively constant at approximately 8.5 log10 CFU/g dw in compost A or approximately 7.0 log10 CFU/g dw in compost B.
The identity of Td22 cultivated in non-sterile compost was confirmed by both morphological characteristics and isozyme profile (Fig. 2).
The initial bacterial populations (contaminants) in the mixes depended on the method of preparation. The population of the mesophilic bacteria in compost A was recorded at approximately one order of magnitude lower than that in compost B after two weeks incubation. However after four weeks of incubation, the growth rate of the indigenous mesophilic bacteria in compost A exceeded that of compost B, to result in a population of 9.3 log10 CFU/g in compost A, in comparison with 8.5 log10 CFU/g in compost B.
3.2 The Efficacy of Td22-Grown Compost to Protect Lettuce from S. minor Attack
The Td22-grown compost was found to be non toxic as between 88%-100% of the lettuce seeds were in three months old WFW compost, either briefly treated for 5 min at 121 °C prior to inoculation (Compost A), or directly inoculated following re-wetting (Compost B). Both compost types were brought to approximately field capacity with 2% (w/v) Phostrogen solution (~350 mL/L). Each value in the graph is an average of three replicate determinations ± standard error.
Fig. 2 Contact print following polyacrylamide-gel electrophoresis to confirm survival of Td22 in three-month old WFW compost. Dark bands indicate polygalacturonase and light bands indicate pectinesterase. Wells 1, and 9 – 14 contained the control reference, Td22, while wells 2 – 8 contained re-isolated fungi from compost B on day 7 of sample collection.
Fig. 3 Protection of lettuce seedlings from attack by S. minor. Each value in the graph ± standard error bar is an average of 5 replicates. Bars with the same letter are not statistically significant at P < 0.05.
1. Nil pathogen and antagonist addition; 2. Pots amended with S. minor only;
3. Pots amended with 2.5% compost-grown Td22 and S. minor;
4. Pots amended with 5% compost-grown Td22 and S. minor;
5. Pots amended with 10% compost-grown Td22 and S. minor;
6. Pots amended with 20% compost-grown Td22 and S. minor.
observed to germinate in soil amended with
5-20% (v/v) of this compost. No inhibition of seed germination was evident even higher rate of
Td22-grown compost was applied 4 weeks after
sowing.
Use of Wood Fibre Compost for the Cultivation of Trichoderma sp. (Isolate Td22)
698
amendment to protect lettuce seedlings from attack by
S. minor is presented in Fig. 3. As indicated in Fig. 3, the rate of amendment of the compost/fungus was proportional to disease control, ranging from 32% provided by 2.5% (v/v) amendment to 100% protection provided by 20% (v/v) amendment at four weeks after sowing. This was statistically significant when compared to the control treatment (pots inoculated with S. minor only) where 100% mortality was observed at four weeks after sowing (Fig. 3).
4. Discussion and Conclusion
Air drying compost in a glasshouse for three weeks or brief steam-treatment (rather than sterilizing) with a view to minimizing the indigenous microbiota, prior to inoculation with Td22, gave encouraging results. The CFU of this fungus cultured under non-sterile conditions increased by two to three orders of magnitude, although they were always lower than those obtained under sterile conditions (Fig. 1). The CFU were found to be higher in the briefly autoclaved compost (compost A) than those in the directly inoculated compost (compost B) following re-wetting. Application of heat treatment to the WFW compost (by briefly autoclaving at 121 °C) may have changed its composition. However the most probable reason for the better growth of Td22 in this medium was the near-elimination of competition by mesophilic bacterial survivors in compost A relative to compost B. Similar results were also demonstrated by Nakasaki et al. [19] who found a reduced growth rate of a strain of
Bacillus subtilis following inoculation of this antagonist into non-sterile (as compared with sterile), but freshly-cut grass clipping compost. This reinforces the notion that competition between the indigenous microbiota and the inoculated antagonists is important in the directed establishment of such antagonists in a compost matrix.
The ability of Trichoderma sp. to produce cellulase and pectolytic enzymes illustrated in Fig. 2 has been reported previously by Metcalf [20], Oksanen et al. [21], Domingues et al. [22], and Lee et al. [23]. These
and other enzymes are important in the degradation of complex carbon sources contained in WFW and the ability to produce them is advantageous when in competition for available major energy sources.
Assay of isoenzyme profiles (e.g. pectolytic enzyme profiles as undertaken in the present study) of fungal isolates provided a convenient tool to differentiate Td22 from other isolates of Trichoderma sp. following re-isolation from non-sterile samples. This assay has been applied by many workers [24-26] to distinguish their fungal isolates from other related fungi.
The effectiveness of Trichoderma sp. (Td22) grown in wood fibre waste compost to protect against S. minor
in glasshouse trials has been demonstrated in the present study. Application of compost-grown Td22 at rates up to 20% (v/v) was non-toxic (relative to controls) to lettuce seed in all treatments (Fig. 3).
The utilization of composted WFW as the authors described could be achieved in large scale at moderate cost while avoiding the problems of liquid cultures as outlined by Hadar et al. [27]. Application of compost-grown Td22 after allowing growth for two weeks or more appeared to be advantageous, because it was mostly in the form of spores (unpublished data). This reduces concerns relating to viability in the field, because fungal spores will be relatively more resistant than mycelia to environmental stress [28].
Acknowledgment
The authors would like to acknowledge Dr. Dean Metcalf, Norske Skog Paper Mill Limited, and AusAid for the provision of Trichoderma sp. isolate Td22, wood fiber waste (WFW), and financial support, respectively.
References
[1] R.H. Cruickshank, J.I. Pitt, Identification of species in
Penicillium subgenus Penicillium by enzyme electrophoresis, Mycologia 79 (1987) 614-620.