1 8 8 0169-5347/00/$ – see front matter © 2000 Elsevier Science Ltd. All rights reserved. PII: S0169-5347(00)01821-8 TREE vol. 15, no. 5 May 2000
31 Yamamoto, S. (1996) Gap regeneration of major tree species in different forest types of Japan. Vegetatio 127, 203–213
32 Runkle, J.R. (1998) Changes in southern Appalachian canopy tree gaps sampled thrice. Ecology 79, 1768–1780
33 van der Meer, P.J. et al. (1998) Tree seedling performance in canopy gaps in a tropical rain forest at Nouragues, French Guiana. J. Trop. Ecol. 14, 119–137
34 Brokaw, N.V.L. and Scheiner, S.M. (1989) Species composition in gaps and structure of a tropical forest. Ecology 70, 538–541
35 Zagt, R.J. and Werger, M.J.A. (1998) Community structure and the demography of primary species in tropical rain forest. In Dynamics of Tropical Communities (Newbery, D.M. et al., eds), pp. 193–219, Blackwell Science
36 Lieberman, M. et al. (1995) Canopy closure and the distribution of tropical forest tree species at La Selva, Costa Rica.J. Trop. Ecol. 11, 161–178 37 Welden, C.W. et al. (1991) Sapling survival, growth, and recruitment:
relationship to canopy height in a neotropical forest.Ecology 72, 35–50 38 Dalling, J.W. et al. (1998) Seed dispersal, seedling establishment and
gap partitioning among tropical pioneer trees. J. Ecol. 86, 674–689 39 Hubbell, S.P. et al. (1999) Light-gap disturbances, recruitment limitation,
and tree diversity in a neotropical forest. Science 283, 554–557 40 Denslow, J.S. (1995) Disturbance and diversity in tropical rain forests:
the density effect. Ecol. Appl. 5, 962–968
41 Busing, R.T. and White, P.S. (1997) Species diversity and small-scale disturbance in an old-growth temperate forest: a consideration of gap partitioning concepts. Oikos 78, 562–568
42 Reader, R.J. et al. (1995) Interspecific variation in tree seedling establish-ment in canopy gaps in relation to tree density. J. Veg. Sci. 6, 609–614 43 Wright, E.F. et al. (1998) Regeneration from seed of six tree species in the interior cedar-hemlock forests of British Columbia as affected by substrate and canopy gap position. Can. J. For. Res. 28, 1352–1364 44 Ribbens, E. et al. (1994) Seedling recruitment in forests: calibrating models
to predict patterns of tree seedling dispersion. Ecology 75, 1794–1806 45 Hurtt, G.C. and Pacala, S.W. (1995) The consequences of recruitment
limitation: reconciling chance, history, and competitive differences among plants. J. Theor. Biol. 176, 1–12
46 Duncan, R.P. et al. (1998) Small-scale species richness in forest canopy gaps: the role of niche limitation versus the size of the species pool. J. Veg. Sci. 9, 455–460
47 Terborgh, J. et al. (1996) Tropical tree communities: a test of the nonequilibrium hypothesis. Ecology 77, 561–567
48 Yu, D.W. et al. (1998) Can high tree species richness be explained by Hubbell’s null model? Ecol. Lett. 1, 193–199
49 Grubb, P.J. (1977) The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biol. Rev. 52, 107–145
I
t has long been known that the Y chromosome is crucial for development of the male pheno-type in mammals. Intensive search for the testis-determining factor culminated in the early 1990s with the identification of the Y-linkedSry gene1, present on the Y chromosome of most mammalian species studied so far. Srytriggers a cascade of proteins involved in male development that are en-coded by autosomal, as well as X-and Y-linked, genes. However, the role of Sryas the key to sex differ-entiation does not extend outside mammals2. For instance, birds appear to have a different system for sex differentiation, although the knowledge of how this system operates is lagging far behind what we know about mammals. Importantly, avian sex chromo-somes show a reversed organ-ization compared with mammals, females being heterogametic ZW
and males homogametic ZZ. A long-standing issue in avian genetics has been whether the W chromosome is crucial for female development or whether it is the number of Z chromosomes that regulates male development3. The sex chromosome aneuploids (Box 1) required to answer this question have yet to be identified, but recent molecular analyses and gene mapping data have given the first hints
to the process of avian sex deter-mination. Moreover, these new data give insights into the evo-lution of heteromorphic sex chro-mosomes in general, and in birds in particular. Here, I will dis-cuss these recent achievements and make comparisons with mammals, birds’ closest relatives, for which detailed knowledge on sex differentiation processes is available.
The avian sex chromosomes
The Z and W chromosomes of birds share many features with mammalian X and Y chromo-somes, respectively. Both avian sex chromosomes are metacen-tric. They pair during meiosis and a synaptonemal complex (Box 1) is formed at the end of the short arms of the two chromosomes; therefore, a small pseudoautos-omal region (Box 1) exists. Typically, the Z chromosome is comparable in size with the fourth or the fifth chromosome pair, constituting some 7–10% of the total genome size4 (which in birds is only one-third of that in mammals). In most species, the W chromosome is considerably smaller and, without appropriate staining techniques, is some-times difficult to distinguish from the many microchromo-somes. In some species, from taxa as diverse as Piciformes,
Evolution of the avian sex chromosomes
and their role in sex determination
Hans Ellegren
Is it the female-specific W chromosome of birds that causes the avian embryo to develop
a female phenotype, analogous to the dominance mode of genic sex differentiation
seen in mammals? Or is it the number of Z chromosomes that triggers male development,
similar to the balance mode of differentiation seen in Drosophila and Caenorhabditis elegans? Although definite answers to these
questions cannot be given yet, some recent data have provided support for the latter hypothesis. Moreover, despite the potentially
common features of sex determination in mammals and birds, comparative mapping shows that the avian sex chromosomes have
a different autosomal origin than the mammalian X and Y chromosomes.
Hans Ellegren is at the Dept of Evolutionary Biology, Evolutionary Biology Centre, Uppsala University,
Falconiformes and Gruiformes, enlarged sex chromosomes can be seen. In these cases, the Z chromosome is the largest in the karyotype, and the W chromosome is also unusually large. This suggests a coordinated mechanism for chromo-some expansion operating on the two sex chromochromo-somes. Another exception to the general pattern of avian sex chromo-some characteristics is found in ratites, where Z and W are similar in size (Box 2).
The W chromosome contains few genes. The staining technique C-banding indicates that W is rich in constitut-ive heterochromatin (Box 1), which is otherwise only found in high densities at the centromeres of microchromo-somes and at one of the ends of the Z chromosome, at least in chickens. The heterochromatic region of the W chromo-some consists mainly of late-replicating repetitive satellite DNA, which has been cloned and characterized in a few species5. Generally, one or a few repeat types prevail within each species and the repeats appear to be poorly conserved across species.
Avian sex determination: is it the Z or the W that matters?
It has been tempting to assign a similar dominance role for the avian W chromosome as found in the mammalian Y chromosome – that it is a chromosome unique to one sex, the presence of which triggers a sex differentiation process (to females in birds)6. This type of sex determin-ation traditionally has been demonstrated by studying sex chromosome aneuploids. Thus, in mammals, the fact that 2A:X0 individuals develop a female phenotype, but 2A:XXY individuals show male characteristics, illustrates that the presence of Y determines maleness, rather than the bal-ance between X chromosomes and autosomes determin-ing femaleness (the latter is the case in Drosophila and
C. elegans). However, in birds the corresponding aneu-ploids are extremely rare, if they exist at all. If a male pheno-type develops from 2A:ZZW birds or a female phenopheno-type from 2A:Z0 individuals, it would be the balance between the number of Z chromosomes relative to the number of autosomal complements that matters. But, if a female pheno-type develops from 2A:ZZW birds, it would be the mere presence of a dominant W chromosome that drives the ZW embryo towards female development.
Given that mass screenings for these aneuploids have been made without great success in poultry breeding, they might be lethal or at least severely deleterious. There is a sin-gle (and somewhat uncertain) report of a ZZW male chicken from 1954 (Refs 3,7), but nothing else. However, there is some circumstantial evidence supporting the Z-autosome balance view from gynandromorphic birds (which are lateral sex chimeras, where one side of the bird has a male pheno-type and the other a female phenopheno-type). In at least a few such cases, ZZ/Z0 constitutions have been identified, the male half of the bird being ZZ and the female half Z0 (Refs 3,8). Although this provides some information, firmly establishing the role of the avian sex chromosome in sex determination will require techniques other than aneuploids.
Clues from comparative mapping
Comparative mapping reveals the chromosomal location of orthologous DNA fragments, commonly genes, in differ-ent taxa. This approach is one of the most important in genome projects, allowing map information to be trans-ferred from map-rich species (typically human or mice) to map-poor species (less well characterized genomes). For instance, once a trait locus has been mapped by linkage analysis to a particular chromosomal region, candidate
genes (Box 1) can be searched for within the corresponding region in a species with more extensive map information. Comparative mapping can also be of great importance for addressing the evolution of chromosomes, genomes or, as in this case, patterns of sex determination.
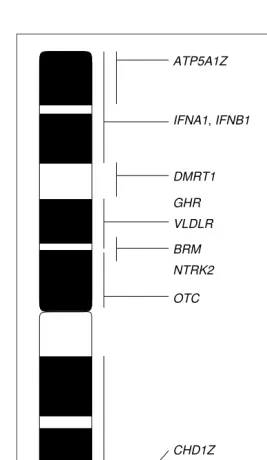
First, we can ask whether avian and mammalian sex chromosomes share a common ancestry. If they evolved from the same pair of autosomes in an ancestral verte-brate, this would be suggestive of the two taxa sharing mol-ecular or chromosomal principles for sex determination. One suitable way to address this is to find out whether genes on the avian Z chromosome are similarly sex-linked in mammals (or vice versa) – comparative mapping shows that they are not (Fig. 1). Genetic, as well as physical, map-ping places genes from the chicken Z chromosome on sev-eral different human autosomes9. So far, only one Z-linked gene (OTC) has been found on the human X chromosome,
Box 1. Glossary
Aneuploidy: an abnormal chromosomal condition where one or more chromosome from a haploid set is either absent or present more than once.
Candidate gene:a gene that potentially could be responsible for a particular trait, but where functional or mutant studies have not yet provided a causal relationship. Often, researchers search for candidate genes among those known to reside within a chromosomal region to which a trait locus has been mapped by linkage analysis or association studies.
Dosage compensation:many organisms with heteromorphic sex somes try to balance the expression of genes on one of the sex chromo-somes so that the expression does not differ between sexes. In organisms with XY/XX sex chromosomes, this can take place by more or less completely inactivating gene expression on one of a female’s two X chromosomes (as in mammals) or by upregulating gene expression on a male’s single X chromosome (as in Drosophila).
Haploinsufficiency: the loss of function when a protein is expressed from only one copy of the gene. Haploinsufficiency can arise from a deletion of one of the two gene copies.
Heterochromatin:chromosomal region with a compact structure (heavily folded) during most parts of the cell cycle. Generally, heterochromatic regions contain few genes (which require less compact structures for their regulation and expression).
Pseudoautosomal region: the part of the sex chromosomes that recom-bine. A marker from the pseudoautosomal region will show no sex linkage.
Retroposition:a mobile genetic element that results in a copy of a certain RNA sequence being inserted elsewhere in the genome. The inserted sequence might differ from the original DNA sequence because the mobile element is an RNA molecule, which generally lacks introns and contains post-transcriptional modifications (e.g. polyadenylation).
Synaptonemal complex: the structure of proteins joining homologous chromosomal regions at meiosis before recombination.
Transposition:a mobile genetic element that results in a copy of a certain DNA sequence being inserted elsewhere in the genome. The mobile element is in the form of DNA.
Box 2. The undifferentiated sex chromosomes of ratites
1 9 0 TREE vol. 15, no. 5 May 2000 but given the size of X this could be expected by chance.
The few genes mapped to the chicken W chromosome are not sex-linked in mammals either9. Thus, in spite of the sex chromosomes being a common means for sex determin-ation among vertebrates, it appears that sex chromosomes have evolved independently in different lineages.
The absence of significant homologies between Z/W and X/Y might suggest that there are no links between sex determination in birds and mammals. However, this
conclusion is probably premature. Notably, through com-parative mapping, the chicken Z chromosome has recently been found to be homologous to a large part of human chromosome 9, including 9p (Refs 9,10). At first glance, the significance of this observation is obscure, but it becomes clearer when it is noted that 9p is one of the chromosomal regions implicated in female sex reversal and gonadal dys-genesis among XY humans11. Specifically, a small region at 9p24.3 (close to the telomere) has been found to be deleted in XY sex reversals, suggesting that one or several genes present within this region are required in two copies for normal male development in XY individuals [otherwise haploinsufficiency (Box 1) is caused]. If such a gene is located on the Z chromosome of birds and has similar functions as those in mammals, one could speculate that it might play a key role in avian sex determination.
What is the role of DMRT1?
Most genes involved in vertebrate sex determination seem not to be conserved across taxa. For instance, the genes involved in sex differentiation processes in Drosophilaand
C. elegansare essentially specific to the respective organ-ism2. Recently, however, evidence for evolutionary con-servation of a sex-determining gene has been found. The
mab-3male sexual regulatory gene of C. elegans contains a DNA-binding domain (DM-domain) with significant amino acid homology to the Drosophilasexual regulatory gene
doublesex(dsx)12. A common feature of the two proteins is that they control sex-specific neuroblast differentiation and regulate transcription of yolk proteins. Transgenic studies reveal that the DSX protein can restore male differ-entiation in mab-3 C. elegansmutants12; thus, mab-3 and dsx are functionally interchangeable.
A gene with a DM-domain similar to mab-3and dsxhas also been identified in humans, and has been designated
DMRT1(originally DMT1)12. Interestingly, DMRT1maps to the small region on 9p24.3 implicated in sex reversal and is a strong candidate gene for this trait13. DMRT1is expressed exclusively in the genetical ridge before sex differentiation (the only other gene which shows this pattern is Sry) and soon after is expressed only in the testes14; thus, DMRT1 seems to be involved in mammalian sexual development. Moreover, the conserved function of DMRT1-related pro-teins in downstream sexual development in divergent phyla, the probable requirement of two expressed copies of DMRT1 for an XY human to develop male phenotype, and the recent mapping of chicken DMRT1to the Z chro-mosome10, suggest that DMRT1might also be involved in regulating sexual differentiation in birds, through Z chro-mosome dosage. Recently, support for this has been obtained through whole-mount in situhybridization and reverse transcriptase PCR (RT-PCR) studies of chicken embryos14. DMRT1is expressed in the genetical ridge from the time it starts to form, as well as in the Wolffian ducts, which become the male-specific internal reproductive structures. Moreover, DMRT1expression is higher in male embryos than in female embryos. The expression of
DMRT1 might occur before the expression of the anti-Müllerian hormone (AMH), which is an early marker of testis differentiation3,15,16, thus inhibiting the development of female reproductive structures.
Although Sryis not conserved between mammals and birds, another testis promoter is. In mammals, the gene product of Sox9acts downstream of Sry, probably by defin-ing and maintaindefin-ing Sertoli cell identity in males17. Appar-ently triggered by a different mechanism, Sox9 also is involved in regulating gonadal development in birds. At
Fig. 1. Genes mapped to the chicken Z chromosome (GGAZ) and their chromosomal location in humans (HSA1chromosome number). Vertical bars depict physical assignments to GGAZ. Genes not associated with a horizontal bar have been mapped only by linkage analysis to GGAZ. Note that the order of genes along GGAZ might, in some cases, not be precisely as indicated in the figure. Locus designations are as follows: ATP5A1Z, ATP synthase a-subunit; IFNA1/IFNB1, interferon a1/b1; DMRT1, doublesex and mab-3 related in testis 1; GHR, growth hormone receptor; VLDLR, very low density lipoprotein receptor; BRM, SWI/SNF-related, matrix associ-ated, actin-dependent regulator of chromatin, subfamily A, member 2; NTRK2, neurotrophic tyrosine kinase receptor 2; OTC, ornithine transcar-bamylase; CHD1Z, chromodomain helicase DNA binding protein 1; CHRNB3, cholinergic receptor, neuronal nicotinic, bpolypeptide 3; ACO1, aconitase 1; ALDOB, aldolase B; GGTB2, b-1,4-galactosyltransferase, polypeptide 1; IREB1, iron-responsive element-binding protein 1; TMOD, tropomodulin; PTCH, homologue of Drosophila patched.
HSA18
HSA9
HSAX
HSA5
HSA8
HSA9
GGAZ Human chromosomes IFNA1, IFNB1
DMRT1
VLDLR
BRM
NTRK2
GGTB2 ACO1
TMOD ALDOB
PTCH ATP5A1Z
GHR
OTC
CHD1Z
CHRNB3
IREB1
the time when the gonads start to differ-entiate in the avian embryo, Sox9is ex-pressed exclusively in the testes18. This indicates further that birds and mammals might share molecular and physiological mechanisms for sex determination.
Evolution of the avian sex chromosomes from an ancestral pair of autosomes
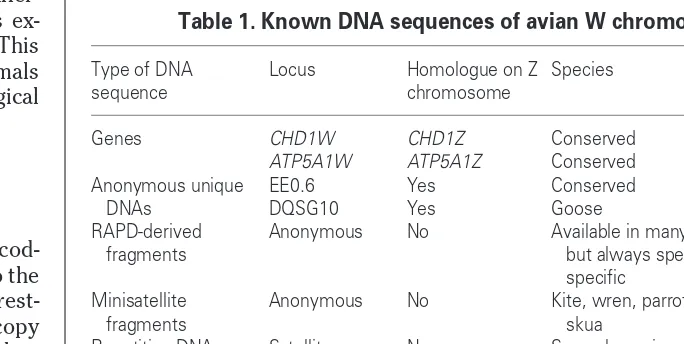
So far, only a few genes (or potential cod-ing sequences) have been assigned to the avian W chromosome (Table 1). Interest-ingly, these genes are present as one copy on W and one copy on Z, although they are not pseudoautosomal – they are pres-ent on the nonrecombining parts of the two types of sex chromosomes. The best characterized pair are the CHD1Z-CHD1W
genes19,20. They are Z- and W-linked, respectively, in all nonratite (Neognate) birds studied and sequence data sug-gest that the two genes are evolving independently21. The
CHD1Z-CHD1Wsystem offers a universal means for molecu-lar sexing of nonratite birds, using straightforward PCR analysis22. Because some introns differ in size between
CHD1Zand CHD1W, conserved primer pairs that amplify both copies will reveal one PCR fragment in males (CHD1Z) and two fragments in females (CHD1Z and CHD1W)23,24. In addition to their use in molecular sexing, the CHD1Z
and CHD1W genes have allowed investigation of sex-specific mutation rates in birds, thus providing evidence for a male-biased mutation rate (Box 3).
An intriguing issue in the evolution of the avian sex chromosomes is what types of genes have remained on the W chromosome. In mammals, we know that many genes present on the Y chromosome have male-specific func-tions, so obviously there has been selection for their re-tention. Moreover, male-specific genes also have been acquired by Y through transposition25and even retropos-ition26(Box 1) from autosomal origins. If sex determination in birds is principally a matter of the number of Z chromo-somes, it becomes less straightforward to invoke sex-spe-cific roles for genes retained on W. Perhaps the answer relates to the fact that dosage compensation (Box 1) of Z-linked genes has not yet been found in birds27. If correct, in cases where the lower amount of gene product produced by females would have an impact on fitness, there should be selection for retaining a functional homologue on the W chromosome. This hypothesis is supported by the fact that the few pairs of genes shared between Z and W, which have been characterized to date, show a high degree of sequence similarity12,21,28. However, overall little is known about how birds deal with the expression of Z-linked genes in males and females, respectively.
Prospects
Sexual reproduction is one of the most widespread features of life, yet an increasing body of evidence points to independent solutions to the physiology, genetics and molecular mechanisms of sexual development in different lineages. In fact, genes involved in sexual development seem to be among the most quickly evolving. However, in spite of these general differences, parts of the enzymatic cascades underlying sexual development can be con-served, as described above for DMRT1. Such common means will be important for dissecting the molecular basis of sexual development organisms that have not been well
characterized. This, together with increased efforts to map genes onto the sex chromosomes and the use of transgenic techniques to study functional properties of candidate genes, is likely to provide a detailed picture of avian sex deter-mination in the near future. In particular, the construction of a ZW bird transgenic for an additional copy of DMRT1would provide interesting information on Z-chromosome dosage and sex differentiation.
Recent observations on the evolution of the avian sex chromosomes have provided answers but also new ques-tions. Future research should focus on the molecular evo-lution of genes on the avian sex chromosomes to reveal what are the functional constraints associated with such genes. For instance, are nonsynonymous substitution rates equal in Z- and W-linked homologues of genes shared between the nonrecombining parts of the Z and W sex chromosomes? In the wider perspective, one of the most important questions is why some organisms have evolved male heterogamety, while in others females have become the heterogametic sex? In order to solve this, comparative approaches addressing how sex chromosomes have evolved
Box 3. Male-biased avian mutation rate
Because the two types of sex chromosomes spend different times in the sexes, any difference in the sex-specific mutation rates should be mani-fested by differing rates of sequence evolution on the two sex chromo-somes (given no selection)45. Specifically, in birds, the rate of neutral sequence evolution on the W chromosome unique to females should directly reflect the female mutation rate. The rate of evolution of neutral sequences on Z should be governed by a combination of the mutation rate in males and females; from this, the male mutation rate can be inferred indir-ectly given that a Z chromosome spends exactly two-thirds of its time in the male germline. Because the mutation rate might differ between genes, the best approach for analysing sex-specific mutation rates, in the absence of sequence data from a large number of genes, is to study genes shared between the sex chromosomes (yet independently evolving). Comparing the sequences of different bird species, introns, as well as silent sites of CHD1Z, are found to evolve faster than paralogous sequences of CHD1W (Ref. 21). From this, a male-to-female mutation rate ratio (a) of between three and six has been estimated. An excess of male mutations also has been revealed from studies of the ATP5A1Z/ATP5A1W gene pair28.
The avian sex chromosome system offers an advantage over the mam-malian X and Y chromosomes for addressing sex-specific mutation rates. It has been suggested that the mutation rate of the X chromosome might be reduced specifically, owing to the avoidance of exposure of recessive del-eterious mutations in hemizygote males46. If so, observations of a faster rate of neutral evolution on Y than on X might not necessarily be a result of vary-ing sex-specific rates. However, in birds, estimates of aare conservative because the effect of a potential reduction in the Z-chromosome mutation rate would be the opposite of that given by higher male mutation rates.
Table 1. Known DNA sequences of avian W chromosomes
Type of DNA Locus Homologue on Z Species Refs sequence chromosome
Genes CHD1W CHD1Z Conserved 19,20 ATP5A1W ATP5A1Z Conserved 9,28,32 Anonymous unique EE0.6 Yes Conserved 33,34
DNAs DQSG10 Yes Goose 35 RAPD-derived Anonymous No Available in many species, 36–38
fragments but always species-specific
Minisatellite Anonymous No Kite, wren, parrots and 39–42 fragments skua
Repetitive DNAs Satellite No Several species, but 5,43,44 repeats always species- or
1 9 2 TREE vol. 15, no. 5 May 2000 in a variety of lineages are needed, along with knowledge
of the mechanisms of sex determination in these lineages. Evolutionary biologists, geneticists and developmental biologists should join forces to accomplish this task.
Acknowledgements
I thank Ben Sheldon for comments on this article and members of my group for discussions. Financial support has been obtained from the Swedish Natural Sciences Research Council.
References
1 Koopman, P.J. et al. (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351, 117–121
2 Cline, T.W. and Meyer, B.J. (1996) Vive la différence: males vs females in flies vs worms. Annu. Rev. Genet. 30, 637–702 3 Clinton, M. (1998) Sex determination and gonadal development:
a bird’s eye view. J. Exp. Zool. 281, 457–465
4 Bloom, S.E. et al. (1993) Constant and variable features of avian chromosomes. In Manipulation of the Avian Genome (Etches, R.J. and Gibbins, A.M.V., eds), pp. 39–60, CRC Press
5 Tone, M. et al. (1984) Genus specificity and extensive methylation of the W chromosome-specific repetitive DNA sequences from the domestic fowl, Gallus gallus domesticus. Chromosoma 89, 228–237 6 Mittwoch, U. (1971) Sex determination in birds and mammals. Nature
231, 432–434
7 Crew, F.A.E. (1954) Sex Determination, John Wiley & Sons 8 Halverson, J.J. and Dvorak, J. (1993) Genetic control of sex
determination in birds and the potential for its manipulation. Poultry Sci. 72, 890–896
9 Fridolfsson, A-K. et al. (1998) Evolution of the avian sex
chromosomes from an ancestral pair of autosomes, Proc. Natl. Acad. Sci. U. S. A. 95, 8147–8152
10 Nanda, I. et al. (1999) 300 million years of conserved synteny between chicken Z and human chromosome 9. Nat. Genet. 21, 258–259 11 Bennet, C.P. et al. (1993) Deletion 9p and sex reversal. J. Med. Genet.
30, 518–520
12 Raymond, C.S. et al. (1998) Evidence for evolutionary conservation of sex-determining genes. Nature 391, 691–695
13 Raymond, C.S. et al. (1999) A region of human chromosome 9p required for testis development contains two genes related to known sexual regulators. Hum. Mol. Genet. 8, 989–996
14 Raymond, C.S. et al. (1999) Expression of Dmrt1 in the genetical ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev. Biol. 215, 208–220
15 Smith, C.A. et al. (1999) Conservation of a sex-determining gene. Nature 402, 601–602
16 Shimada, K. (1998) Gene expression of steroidogenic enzymes in chicken embryonic gonads. J. Exp. Zool. 281, 450–456
17 Swain, A. and Lovell-Badge, R. (1999) Mammalian sex determination: a molecular drama. Genes Dev. 13, 755–767
18 Morais da Silva, S. et al. (1996) Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat. Genet. 14, 62–68
19 Ellegren, H. (1996) First gene on the avian W chromosome provides a tag for universal sexing of non-ratite birds. Proc. R. Soc. London Ser. B 263, 1635–1641
20 Griffiths, R. et al. (1996) Sex identification in birds using two CHD genes. Proc. R. Soc. London Ser. B 263, 1251–1256
21 Ellegren, H. and Fridolfsson, A-K. (1997) Male-driven evolution of DNA sequences in birds. Nat. Genet. 17, 182–184
22 Ellegren, H. and Sheldon, B.C. (1997) New tools for sex identification and the study of sex allocation in birds. Trends Ecol. Evol. 12, 255–259
23 Griffiths, R. et al. (1998) A DNA test to sex most birds. Mol. Ecol. 7, 1071–1075
24 Fridolfsson, A-K. and Ellegren, H. (1999) A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 30, 116–121
25 Lahn, B.T. and Page, D.C. (1997) Functional coherence of the human Y chromosome. Science 278, 675–680
26 Lahn, B.T. and Page, D.C. (1999) Retroposition of autosomal mRNA yielded testis-specific gene family on human Y chromosome. Nat. Genet. 21, 429–433
27 Baverstock, P.R. et al. (1982) A sex-linked enzyme in birds – Z chromosome conservation but no dosage compensation. Nature 296, 763–766
28 Carmichael, A. et al. Male-biased mutation rates revealed from Z- and W-chromosome linked ATP synthase a-subunit (ATP5A1) sequences in birds. J. Mol. Evol. (in press)
29 Ansari, H.A. et al. (1988) Morphological differentiation of sex chromosomes in three species of ratite birds. Cytogenet. Cell Genet. 47, 185–188
30 Ogawa, A. et al. (1998) The location of Z- and W-linked marker genes and sequence on the homomorphic sex chromosomes of the ostrich and the emu. Proc. Natl. Acad. Sci. U. S. A. 95, 4415–4418
31 Härlid, A. and Arnason, U. (1999) Analyses of mitochondrial DNA nest ratite birds within the Neognathae: supporting a neotenous origin of ratite morphological characters. Proc. R. Soc. London Ser. B 266, 305–309
32 Dvorak, J. et al. (1992) cDNA cloning of a Z- and W-linked gene in Gallinaceous birds. J. Hered. 83, 22–25
33 Ogawa, A. et al. (1997) Molecular characterization and cytological mapping of a non-repetitive DNA sequence region from the W chromosome of chicken and its use as a universal probe for sexing Carinatae birds. Chromosome Res. 5, 93–101
34 Itoh, Y. et al. (1997) Identification of the sex of Oriental white stork, Ciconia boyciana, by the polymerase chain reaction based on its sex chromosome-specific DNA sequences. Genes Genet. Syst. 72, 51–56 35 Quinn, T.W. et al. (1990) Molecular sexing of geese using a cloned
Z chromosomal sequence with homology to the W chromosome. Auk 107, 199–202
36 Griffiths, R. and Tiwari, B. (1993) The isolation of molecular genetic markers for the identification of sex. Proc. Natl. Acad. Sci. U. S. A. 90, 8324–8326
37 Sabo, T.J. et al. (1994) PCR-based method for sexing Roseate terns (Sterna dougallii). Auk 111, 1023–1027
38 Lessells, C.M. and Mateman, A.C. (1998) Sexing birds using random amplified polymorphic DNA (RAPD) markers. Mol. Ecol. 7, 187–195 39 Rabenold, P.P. et al. (1991) Polymorphic minisatellite amplified on
avian W chromosome. Genome 34, 489–493
40 Miyaki, C.Y. et al. (1992) Sex typing of Aratinga parrots using the human minisatellite probe 33.15. Nucleic Acids Res. 19, 5235–5236 41 May, C.A. et al. (1993) Polymorphic sex-specific sequences in birds of
prey. Proc. R. Soc. London Ser. B 233, 271–276
42 Millar, C.D. et al. (1992) Sex-specific restriction fragments and sex-ratios revealed by DNA fingerprinting in the Brown skua. J. Hered. 83, 350–355
43 Griffiths, R. and Holland, P.W.H. (1990) A novel avian W chromosome DNA repeat sequence in the lesser black-backed gull (Larus fuscus). Chromosoma 99, 243–250
44 Saitoh, Y. and Mizuno, S. (1992) Distribution of XhoI and EcoRI family repetitive DNA sequences into separate domains in the chicken W chromosome. Chromosoma 101, 474–477
45 Hurst, L.D. and Ellegren, H. (1998) Sex biases in the mutation rate. Trends Genet. 14, 446–452
46 McVean, G.T. and Hurst, L.D. (1997) Evidence for a selectively favourable reduction in the mutation rate of the X chromosome. Nature 386, 388–392
Students
Did you know that you are entitled to a 50% discount on a subscription to
Trends in Ecology & Evolution? See the subscription order card in this