Modification Effect of Saccharomyces cerevisiae To Fungi and Yeast Growth Dynamics and β-Glucan Content of Tempeh During Fermentation
Samsul Rizal, Murhadi, Maria Erna Kustyawati, Udin Hasanudin, and Lia Dahliani Pratiwi
Department of Agricultural Product Technology, Agricultural Faculty, University of Lampung, Indonesia
Email: marrizal@yahoo.com
Abstract
This study aimed to determine the dynamics of fungi and yeast growth during tempeh fermentation process with addition of Saccharomyces cerevisiae and know the effect of addition of Saccharomyces cerevisiae to the β-glucan content of tempeh. The research was done by Randomized Complete Blo ck Design (RAKL) with two factors and three replications. The first factor was the type of tempeh inoculum, consist of 3 levels i.e. S.cerevisiae, R.oligosporus, and mixture of R.oligosporus and S.cerevisiae. The second factor was fermentation duration, consist of 6 levels i.e. 0, 8, 16, 24, 32, and 40 hours. The results showed that fungi grew during the soybean fermentation with R.oligosporus inoculum and mixture of R.oligosporus and S.cerevisiae inoculum, but fungi did not grow during soybean fermentation with S.cerevisiae only. The growth of fungi increased until the end of fermentation periode with maximum specific growth rate of fungi in all of inoculum type, consecutive i.e. 0.113 cells/hours and 0.016 cells/hours, although the growth delayed at the beginning of fermentation at 0 to 16 hours. Yeast grew during soybean fermentation with S.cerevisiae inoculum and mixture of R.oligosporus and S.cerevisiae inoculum, but did not grow in soybean fermentation with R.oligosporus only. The growth of yeast increased until the end of fermentation periode with maximum specific growth rate in all of inoculum type, consecutive i.e. 0.012 cells/hours and 0.013 cells/hours, although in the soybean fermentation with mixture of R.oligosporus and S.cerevisiae, yeast growth decreased at fermentation time of 32 hours. The β-glucan content of all tempeh is higher than soybean without inoculum. The highest β-glucan content was shown in tempeh with addition of mixture inoculums of R.oligosporus and S.cerevisiae at 40 hours of fermentation time, i.e. 0.578% (w/w). This study showed that the addition of S. cerevisiae in making of tempeh can increase yeast growth and β-glucan content of tempeh.
Keywords: Tempeh modification, fungi growth dynamics, yeast growth dynamics, Saccharomyces cerevisiae, β-glucan.
Introduction
The quality of tempeh is influenced by raw materials, processing, and the type of inoculum or yeast used. The use of inoculum plays an important role in making tempeh because it affects the quality of tempeh produced. Inoculum of tempeh with a lot of amounts cause fermentation time becomes too critical, because the fermentation process takes place quickly. On the other hand, the use of yeast or tempeh inoculum with less amount of microbial cause contaminants can grow, because fungi can not conduct the fermentation process thoroughly on materials.
The preparation of tempeh usually uses inoculum containing R. oligosporus as a converting agent of soybean raw material into tempeh (Kasmidjo, 1990). Microorganisms that play an important role in the process of fermentation in the manufacture of tempeh are R. oligosporus, R. oryzae and R. stolonifer. All three have the potential to ferment soybeans into tempeh. Rhizopus oligosporus plays a major role in the manufacture of tempeh because it can retain most of the nutrients contained in soybeans, increase protein digestibility, and increase levels of some vitamin B (Muchtadi, 2010 in Mursyid, 2014). In the process of tempeh fermentation, R. oligosporus has more role to synthesize protease enzyme, whereas R. oryzae has more role to synthesize α-amylase enzyme (Triwibowo, 2011).
The growth of microflora in tempeh is not only dominated by fungis, but other microorganisms are also present during the tempeh fermentation process (Barus et al., 2008; Seumahu et al., 2013). Efriwati et al. (2013), reported that other microorganisms found during tempeh fermentation were lactic acid bacteria (BAL) and yeasts. Mulyowidarso et al. (1989) in Kustyawati (2009), found that bacteria are indigenous microflora that significantly grows during the manufacture of tempeh and has an important role. In addition to bacteria, yeasts are also found to be present in the fermentation of tempeh. One type of yeast found in tempeh fermentation is S. cerevisiae (Kustyawati et al., 2016) known as a beta-glucan source (Pengkumsri et al., 2017). The growth interaction of Saccharomyces cerevisiae with other microflora in the manufacture of tempeh is not known, so microbiological analysis is needed to reveal in detail the involvement of each type of microorganism in the manufacture of tempeh.
Materials and Methodes
Materials
The main ingredients used in this study include pure cultures Rhizopus oligosporus and Saccharomyces cerevisiae, soybean brand "Soybean USA" no. 1, Nutrient Broth (NB), Nutrient Agar (NA), Malt Extract Agar (MEA), De Man Rogosa and Sharpe Broth (MRSB), Potato Dextrose Agar (PDA), and other materials for chemical analysis.
Methodes
The study was arranged in a Factorial Randomized Block Design with three replications. The first factor was the type of inoculum with 3 levels of inoculum: S. cerevisiae (negative control), R.oligosporus, and R.oligosporus + S. cerevisiae. The second factor was the duration of fermentation with 6 levels i.e. 0, 8, 16, 24, 32, and 40 hours.
During fermentation, samples were analyzed for microbial (fungi and yeast), pH, and β -glucan content at 0, 8, 16, 24, 32, and 40 hours of fermentation time. The homogeneity of the data obtained was tested by Bartlett's test and the addition of the data was tested by the Tuckey test. The observed data were analyzed to determine whether there was any difference between treatments. Then the data were tested further by Orthogonal Polynomial and Orthogonal Comparison. The observations were done for number of microorganisms, pH, and β-glucan content of tempeh.
Preparation of S. cerevisiae culture
Isolate of S. cerevisiae was cultured into sterile Malt Extract Agar (MEA) medium using a sterilized ose needle with a scratch plate method then incubated for 24-48 hours at 28oC to form colonies. The S. cerevisiae colonies were then harvested by adding 5-10 ml of sterile aquadest into the plate disk. Saccharomyces cerevisiae cells were harvested using drygalski sticks and poured into a 50 mL centrifuge tube. The centrifuge tube was then weighed and centrifuged at 3000 rpm for 10 minutes to obtain a separate solid from the supernatant. The supernatant was discarded and the remaining solids were added again by 25-30 ml of sterile aquadest. The S. cerevisiae cells were transferred into a test tube containing a 9 ml diluent solution and then homogenized using a vortex.
Furthermore, the number of S. cerevisiae cells present in the diluent solution was
calculated using a hemacytometer until the amount of S. cerevisiae reached 107 cells/ml.
Preparation of R. oligosporus culture
Production of Soybean Tempeh
The process of making tempeh was done according to the procedure of Kustyawati (2009). Three hundred grams of soybeans were soaked in the clean water at room temperature overnight. The soybean husk was removed and then boiled using clean water with a ratio of 1 : 3 (soybean : water) for 30 minutes, drained and then aerated until the soybean temperature reaches room temperature and ready to be inoculated with inoculum according to the treatment. Preparation of tempeh was made by duplo. The inoculation stage was carried out by mixing 100 g of boiled soybeans with inoculum: (1) 1 ml suspension of 105 spores/ml of R. oligosporus, (2) 1 ml suspension of 107 cells/ml of S. cerevisiae, and (3) 1 ml suspension of 105 spores/ml R. oligosporus + 1 ml suspension of 107 cells/ml S.cerevisiae. Furthermore, inoculated soybeans were packaged in plastics that have been drilled regularly for aeration purposes and then incubated at 32oC for 40 hours and observed every 8 hours.
Degree of acidity (pH) (AOAC, 2005)
The pH value was measured with pH-meter according to AOAC procedure (2005). The pH value was measured at the same temperature. Prior to measurement, pH-meters were standardized using standard buffers pH 4 and pH 7. The pH value was evaluated by duplo.
Analysis of β-glucan
Analysis of β-glucan was performed following the procedure of Kusmiati et al. (2007). The ß-glucan analysis was done every 8 hours during tempeh fermentation process up to 40 hours. One gram of samples was added 30 ml of NaOH 0.7 N. Subsequently the sample was hydrolyzed for 6 hours at temperature of 75°C and then centrifuged at 10,000 rpm at 25°C for 30 minutes. The supernatant was removed and the obtained residue was washed with 30 ml of 0.5 M acetic acid solution and centrifuged again at 10,000 rpm at 25°C for 30 minutes. The supernatant was removed again, the washing with acetic acid was performed three times. The obtained residue was then washed with 20 ml of aquadest and centrifuged at 5,000 rpm for 10 minutes twice.
The obtained residue was added with 20 ml of ethanol and then centrifuged at 5000 rpm for 10 minutes, resulting in wet β-glucan (crud). The biomass was stored in a 45°C oven for 1 day and weighed as the dry weight of β-glucan (crud)/weights β-gross roughage. The dry residue was added with 4 ml of 1M NaOH and left for 1 hour. The sample was then diluted with aquadest and shaken with Orbital Shaker. The solution was then added with 2 ml of Pb-Acetate and allowed to stand for ±30 minutes. Furthermore, the solution was given with 1 g of sodium oxalate to obtain a clear solution, the solution then was taken 2 ml and added by phenol and sulfuric acid. The samples were then tested using a sugar free containt spectrophotometer with a wavelength of 490 A.
Enumeration of Microorganisms
inoculum was analyzed in fermentation time 0, 8, 16, 24, 32, and 40 hours. Each of the tempeh was sampled and diluted from 10-1 to 10-10 in duplicate. The growth of microorganisms during soybean fermentation includes the colony forming units (CFUs) of yeast and fungi carried out during fermentation of soybean. Preparation of test samples follow the method of Kustyawati et al. (2009). A total of 10 g of sample was mixed with 90 ml of peptone water, homogenized with stomacher for 5 minutes, then diluted to concentration series. Then 1 mL of a certain dilution was taken and carried out the planting of microorganisms by surface plate count method on a suitable agar medium. Incubation was carried out at 32oC to grow fungi and 30oC to grow yeast for 24-48 hours.
RESULTS AND DISCUSSION
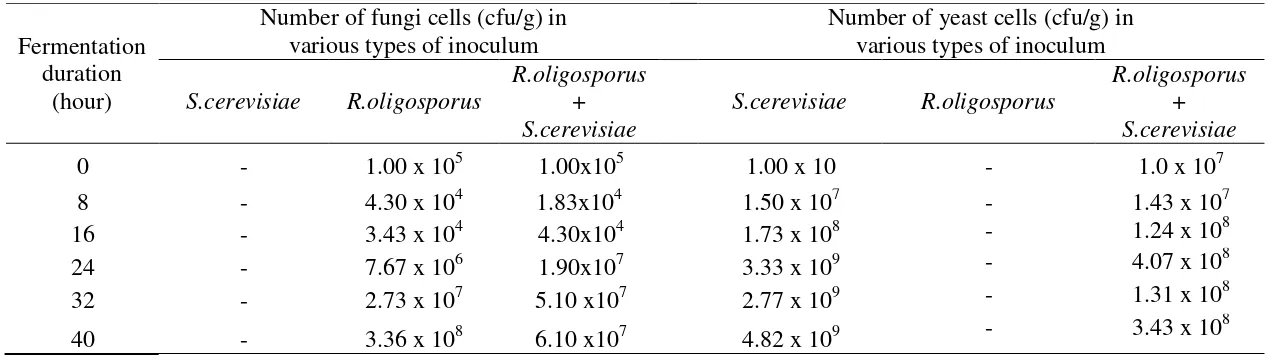
Tabel 1. Growth of fungi and yeast during soybean tempeh fermentation inoculated with S.cerevisiae, R.oligosporus, and a mixture of R.oligosporus dan S.cerevisiae inoculum.
Fermentation duration
(hour)
Number of fungi cells (cfu/g) in various types of inoculum
Number of yeast cells (cfu/g) in various types of inoculum
S.cerevisiae R.oligosporus
R.oligosporus + S.cerevisiae
S.cerevisiae R.oligosporus
R.oligosporus + S.cerevisiae
0 - 1.00 x 105 1.00x105 1.00 x 10 - 1.0 x 107
8 - 4.30 x 104 1.83x104 1.50 x 107 - 1.43 x 107
16 - 3.43 x 104 4.30x104 1.73 x 108 - 1.24 x 108
24 - 7.67 x 106 1.90x107 3.33 x 109 - 4.07 x 108
32 - 2.73 x 107 5.10 x107 2.77 x 109 - 1.31 x 108
40 - 3.36 x 108 6.10 x107 4.82 x 109 - 3.43 x 10
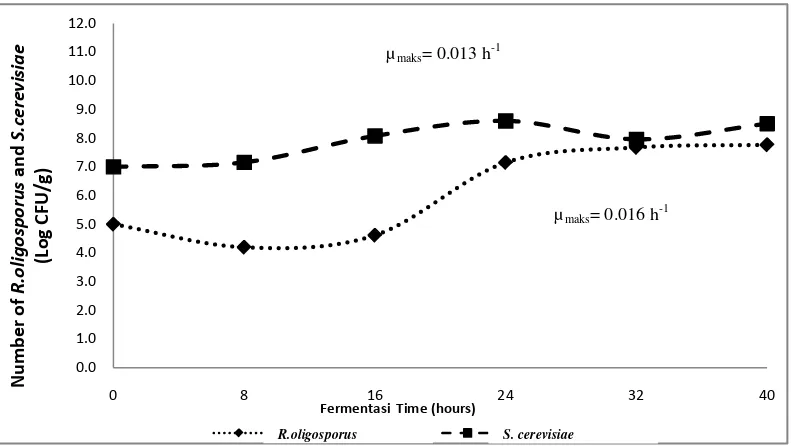
Growth dynamics of fungi and yeast during tempeh fermentation inoculated with S. Cerevisiae.
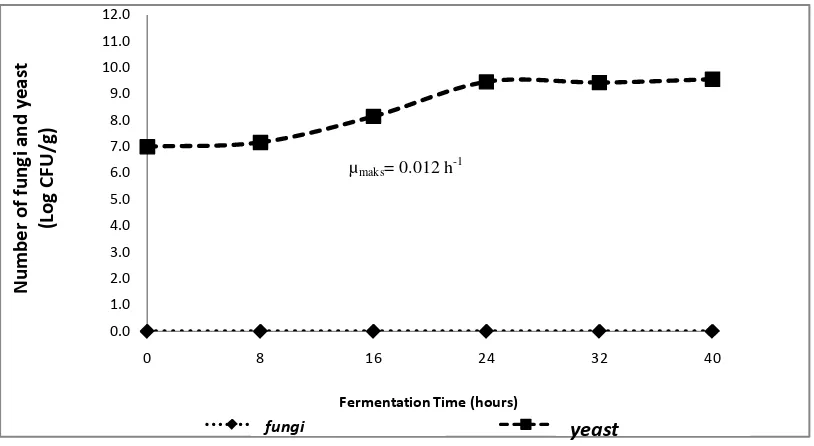
Saccharomyces cerevisiae grew during soybean fermentation process which was inoculated with S. cerevisiae. This was because S. cerevisiae uses carbon derived from soybeans as a nutrient for its growth. Figure 1 shows that the adaptation phase of S. cerevisiae occured in 0 to 8 hours of fermentation with a population of 107 cfu/g. Sugoro et al. (2006), stated that the adaptation phase of S. cerevisiae in modified 1% tapioca solution medium containing 10.21% glucose was at 6th hour of fermentation. Meanwhile, the adaptation phase of S. cerevisiae in the media using carbon sources of glucose was at 4 hours of fermentation (Kusmiati et al., 2011), and on YNB medium containing 30% of glucose was at fermentation 6 hour (Ishmayana et al ., 2012). This phase of S. cerevisiae adaptation is longer than the S. cerevisiae adaptation phase in the research conducted by Sugoro et al. (2006), Kusmiati et al. (2011), and Ishmayana et al. (2012). This is because there was no addition of carbon sources on the substrate needed for the growth of S. cerevisiae during fermentation in this study so that the phase of adaptation of S. cerevisiae is longer.
Figure 1. Growth curve of fungi and yeast during tempeh fermentation inoculated with S. cerevisiae
After 8 hours of fermentation, S. cerevisiae experienced an increase in the number of cells from 1.73 x 108 cfu/g at 16 hours of fermentation to 3.33 x 109 cfu/g at 24 hours of fermentation. This increase indicated that S. cerevisiae had entered an exponential phase. Kavanagh (2005) states that in the exponential phase, Saccharomyces
cerevisiae reproduces to form buds. Based on the exponential phase, the maximum specific growth rate (μmaks) of S. cerevisiae was 0.012 cells/hours. This means that the maximum increase rate of yeast cells is 0.012 cells/hour. Furthermore, S.
cerevisiae experienced a stationary phase until fermentation at the 40th hour with a population of 4.82 x 109 cfu/g. The death phase of S. Cerevisiae is estimated to occur after fermentation lasting more than 40 hours. The appearance of soybeans during
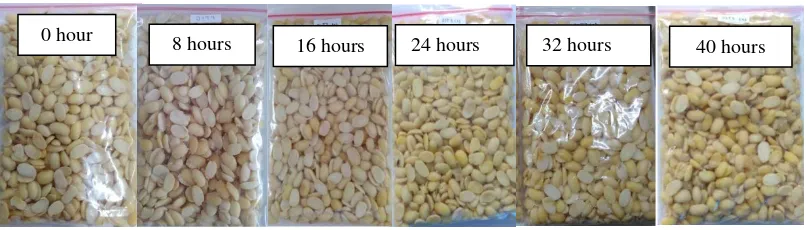
fermentation inoculated with S. cerevisiae remains intact and slightly slimy at the end of 40 hours of fermentation (Figure 2).
Figure 2. The appearance of soybean inoculated with Saccharomyces cerevisiae during fermentation.
The addition of S. cerevisiae as a single inoculum without the addition of yeast tempeh in tempeh fermentation during 40 hours of fermentation did not cause the formation of tempeh. This was because the added S. cerevisiae is not an agent that converts raw soybeans into tempeh, so that the soybeans remain intact until the end of fermentation. According to Sarwono (2004), in the manufacture of tempeh, the tempeh inoculum containing fungi and yeast spores is needed. Without the
inoculums soybeans will experience decay. Meanwhile, the growth of S. cerevisiae during fermentation continues to increase, but no growth of R.oligosporus is found. This research is in line with the research of Wahono et al. (2011), who reported that during fermentation of sorghum seeds with S. cerevisiae in bioethanol production there was an increase in the growth rate of S. cerevisiae.
The growth dynamics of fungi and yeast during tempeh fermentation inoculated with R.oligosporus
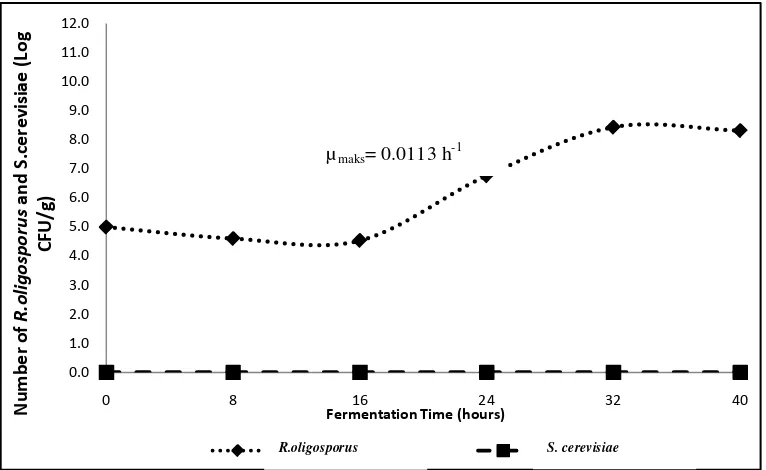
Based on growth of R.oligosporus and S. cerevisiae during tempeh fermentation (Table 1), the growth patterns of R.oligosporus and S. cerevisiae during fermentation inoculated with S. cerevisiae are presented in Figure 3.
0 hour 8 hours
Figure 3. Growth curves of R. oligosporus and S. cerevisiae during tempeh fermentation inoculated with Rhizopus oligosporus
Based on the Figure 3, R.oligosporus adaptation phase in the tempeh fermentation inoculated with R.oligosporus as a single inoculum was at a fermentation time of 0 to 16 hours, with a marked decrease in the number of R.oligosporus cells from 1 x 105cfu/g at the 0 h of fermentation time to 3.43x104cfu/g at hours of fermentation. Bintari, et al. (2008), Pagarra (2009) and Kustyawati (2009), stated that
R.oligosporus growth decreases at the beginning of fermentation, which indicates that R.oligosporus is in the adaptation phase. The growth of R. oligosporus had not been seen clearly, which was characterized by the appearance of intact soybeans at 0 to 16 hours of fermentation (Figure 4). After 16 hours, the total amount of
R.oligosporus is 7.67 x 106 cfu /g at 24 hours fermentation time to 2.73 x 107cfu/g at 32 hours of fermentation. The increase of number of R.oligosporus indicates that after 16 hours fermentation time R.oligosporus entered into an exponential growth phase. Based on the exponential phase, the maximum specific growth rate of R.oligosporus (μmaks) is 0.0113 hours-1. This means that the maximum growth rate of fungi is 0.0113 colonies/hour. The amount of fungi continues to increase until the end of fermentation (40 hours).
The Increasing of number of fungi spores after 16 hours was able to produce tempeh mycelium which covered part of the surface of soybeans for 24 hours to 32 hours of fermentation and covered all of the surface of soybeans at 40 hour of fermentation period (Figure 4). The mycelium formed is grayish white and there is a black color on the surface. The black color is R.oligosporus spores that grow due to over oxygen and have not formed hyphae threads. This is presumably due to too large aeration holes which cause R.oligosporus metabolism is too fast. According to Bintari et al. (2008) and Winanti et al. (2014), tempeh fungi is microaerophilic so that sufficient oxygen is needed for R.oligosporus growth. The black spores in the tempeh were reduced at fermentation time of 40 hours. This is presumably because in the 40th hour fermentation the spores have formed mycelium which increasingly solidifies and covers the surface of the soybean, resulting in the appearance of white tempeh.
0.0
KapangR.oligosporus KhamirS. cerevisiae
Figure 4. The appearance of soybean inoculated with R. oligosporus during fermentation.
In addition treatment of R. oligosporus as a single inoculum in making tempeh was known to increase the number of R.oligosporus cells, but the presence of S.
cerevisiae was not found. This was because only R.oligosporus which was inoculated as inoculum and there was no addition of S. cerevisiae. This study is in line with the research of Kustyawati, et al. (2009), stating that there was no yeast growth during tempeh fermentation which was inoculated with R.oligosporus. This indicates that the presence of S. cerevisiae during tempeh fermentation can be found if the addition of S. cerevisiae in the making of tempeh is done.
The growth dynamics of fungi and yeast in soybean inoculated with a mixture of R.oligosporus and S.cerevisiae during tempeh fermentation
Based on the growth of R.oligosporus and S. cerevisiae during tempeh fermentation (Table 1), the growth dynamics of R.oligosporus and S.cerevisiae during tempeh fermentation inoculated with a mixture of R.oligosporus and S. cerevisae are presented in Figure 5.
Figure 5. The growth curves of R. oligosporus and S. cerevisiae during tempeh fermentation inoculated with a mixture of R. oligosporus and S. cerevisiae.
0.0
KapangR.oligosporus KhamirS. cerevisiae
Figure 5 shows that the R. oligosporus adaptation phase in the mixture addition treatment of R.oligosporus and S. cerevisiae as inoculum in tempeh manufacture has the same adaptation phase with the addition of R.oligosporus treatment. The
adaptation phase of R.oligosporus occured at growth time of 0 to 16 hours. It was indicated by a decrease in the number of R.oligosporus cells from 1 x 105 cfu/g at 0 hour of fermentation time to 3.43 x 104 cfu/g at the 16th hour of fermentation period. Pagarra (2009) stated that in the fermentation period of 0 to 16 hours there has not been the growth of fungi spores of Rhizopus sp. in making of mungbean tempeh. The appearance of tempeh inoculated with a mixture of R.oligosporus and S. cerevisiae during fermentation showed that in the fermentation period of 0 to 16 hours there was not significantly R.oligosporus growth indicated by the appearance of intact soybeans (Figure 6). After 16 hours of fermentation periode, R.oligosporus experienced an exponential growth phase marked by an increase in the number of R.oligosporus spores to 7.67x106 cfu/g at 24 hours fermentation time, and 2.73 x 107cfu/g during 32 hours of fermentation time. Based on the exponential phase, the maximum specific growth rate of R.oligosporus (μmaks) was 0.0113 cell/hours. This means that the maximum growth rate of fungi was 0.0113 colonies/hour. At the end of 40 hours of fermentation, the amount of R.oligosporus increased to 3.36
x108cfu/g.
The time for mycelium formation during fermentation of soybean inoculated with R.oligosporus and S. cerevisiae was similar to the time of mycelium formation in soybean inoculated with R.oligosporus only. As presented in Figure 6, formation of mycelium occured at 24 hours to 32 hours of fermentation and the mycallium
partially covered the surface of the soybean. Mycellium covered the entire surface of the soybean at 40 hours of fermentation. The mycelium formed was grayish white color and partially was a black color. The black color was R.oligosporus spores that grow due to excessive oxygen and have not formed hyphae threads. Bintari et al. (2008) and Winanti et al. (2014) states that tempeh fungi is microaerophilic. If the tempe fermentation process is less oxygen, Rhizopus sp. will be hampered.
Conversely, if there is too much oxygen, the metabolism will run too fast. The spores will form mycelium which is increasingly compacted and produces white tempeh. The appearance of soybean inoculated with a mixture of R.oligosporus and
S.cerevisiae in making tempeh is presented in Figure 6.
v
Figure 6. The appearance of soybeans inoculated with a mixture of R.oligosporus and S.cerevisiae during fermentation.
Based on the treatment of mixture inoculums of R.oligosporus and S. cerevisiae, it was known that both microorganisms can grow together during fermentation.
According to Kustyawati (2009), there is a mutually beneficial symbiosis in terms of
the availability of nutrients between the two microorganisms, so that both have synergistic growth. During fermentation, it was suspected that S. cerevisiae absorbed the elements C, H, O, N from soybeans after the breakdown of carbohydrate, fat, and protein compounds by R. oligosporus. Decomposition of carbohydrates from
soybeans is thought to have been initially carried out by R. oligosporus. After carbohydrates are broken down into simple compound forms, they are then broken down by S. cerevisiae with the enzymatic activity produced by S. cerevisiae and utilized for their growth. Tempe produced by adding a mixture of R.oligosporus and S. cerevisiae as inoculums has a sweet aroma. This is because the added S. cerevisiae has very high proteolytic and lipolytic activity which is capable of hydrolyzing proteins and fats (Villijoen et al. 1995, in Rizal et al. 2017). Kustyawati et al. (2016), states that tempe with the addition of S. cerevisiae produces more volatile
components such as alcohol, ketones, fatty acids, esters, sesquiterpenes, and benzenoids. This component is a component of tempe flavor and aroma formation. This research is in line with the results of research by Kustyawati et al. (2009), that in soybeans which were inoculated with R.oligosporus and S.boulardii produced tempeh with a fragrant aroma that covered the aroma of soybeans.
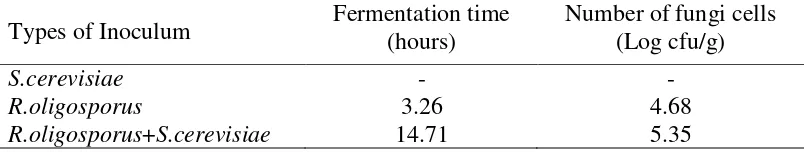
The addition of R.oligosporus and the mixture of R.oligosporus and S. cerevisiae as inculums in tempeh production produced fungi growth which tended to increase during fermentation, although at the beginning of the fermentation ist growth decreased due to fungi still in the adaptation phase at 0 to 16 hours of fermentation. Based on the linear regression equation, the optimum growth of fungi on the tempeh which was given the addition treatment of R. oligosporus and S. cerevisiae together occurred at the time of fermentation of 14.71 hours with a number of mold cells of 5.35 log cfu/g. The optimum growth of fungi in the addition treatment of R.
oligosporus as a tempeh inoculum occurred at 3.26 hours with number of fungi cells of 4.68 log cfu/g. The optimum growth of fungi in each type of inoculum during fermentation is presented in Table 2.
Table 2. The optimum growth of fungi during tempeh fermentation incolated with S. cerevisiae, R.oligosporus, and a mixture of R.oligosporus and S. cerevisiae.
Types of Inoculum Fermentation time (hours) Number of fungi cells (Log cfu/g)
S.cerevisiae - -
R.oligosporus 3.26 4.68
R.oligosporus+S.cerevisiae 14.71 5.35
Description: - There is no optimum growth of yeast
Table 2 shows that the fastest time of fungi to reach optimum growth occured in the inoculum addition of R.oligosporus namely at the fermentation time reach 3.26 hours. Meanwhile, the highest number of fungi cells was found in the addition of mixture inoculum of R. oligosporus and S. cerevisiae i.e. 5.35 log cfu/g. This is because S. cerevisiae mixed with R.oligosporus inoculum will stimulate the growth of tempeh fungi, and both can grow together (Kustyawati, 2009).
(2009) reported that yeast added to the tempeh inoculum can grow together with R. oligosporus and its growth can encourage the growth of fungi in tempeh.
Table 3 shows the optimum growth of yeast during tempeh fermentation inoculated with S. cerevisiae, R.oligosporus, and a mixture of R.oligosporus and S. cerevisiae culture. The optimum growth of yeast in soybean inoculated by mixture inoculums of R. oligosporus and S. cerevisiae occurred at the fermentation time reach 35.67 hours with a number of yeast cells of 8.41 log cfu/g, while the optimum growth of yeast in the addition treatment of S. cerevisiae as an inoculum occurred at fermentation time reach 51.75 hours, i.e. 9.92 log cfu/g. This is because during the fermentation of R. oligosporus, complex organic compounds are transformed into simpler compounds that can be used by S. cerevisiae for their growth, so that their growth becomes faster. Kustyawati et al. (2009) reported that during tempeh fermentation, inoculums of S.boulardii and R.oligosporus which were inoculated together showed mutually beneficial symbiosis in terms of nutrient availability between the two.
Table 3. The optimum growth of yeast during tempeh fermentation inoculated with S. cerevisiae, R.oligosporus, and a mixture culture of R.oligosporus and S.
Cerevisiae.
Types of Inoculum Fermentation time (hours)
Number of yeast cell (Log cfu/g)
S.cerevisiae 51.75 9.92
R.oligosporus - -
R.oligosporus+ S.cerevisiae 35.67 8.41
Description: -: There is no optimum growth of fungi
Degree of acidity (pH) of tempeh during fermentation
Table 4. The pH values of tempeh during fermentation inoculated with inoculum of S. cerevisiae, R.oligosporus, and a mixture of R.oligosporus + S.cerevisiae.
Fermentation time (hours)
Degree of acidity (pH) ± sd of tempeh with various types of inoculum
S.cerevisiae R.oligosporus R.oligosporus+ S.cerevisiae
The results of variance analysis showed that the treatment of inoculum types did not significantly affect the pH of tempeh, while the duration of fermentation had a significant effect on the pH of tempeh and there was interaction between the two. Further test results showed that the longer the fermentation time, the pH value of tempeh in all treatments increased. Kiers et al. (2003) explain that there is a
correlation between fungal growth and an increase in pH. Kustyawati (2009), stated that during tempeh fermentation there was no change in the compound that produced (H +) so that the pH value of tempeh was not acidic. This research is in line with the research of Nurrahman et al. (2012), which states that the longer the fermentation time the tempeh pH will increase. Suprihatin (2010) reported that with the presence of proteolytic activity of R.oligosporus, protein degradation occurs to amino acids which cause increase dissolved nitrogen. Increased dissolved nitrogen will cause the tempeh pH to increase. This is because fungi will actively hydrolyze protein during tempeh fermentation (Popoola et al., 2007). Further fermentation will form ammonia gas where tempeh will smell foul.
β-glukan content of tempeh
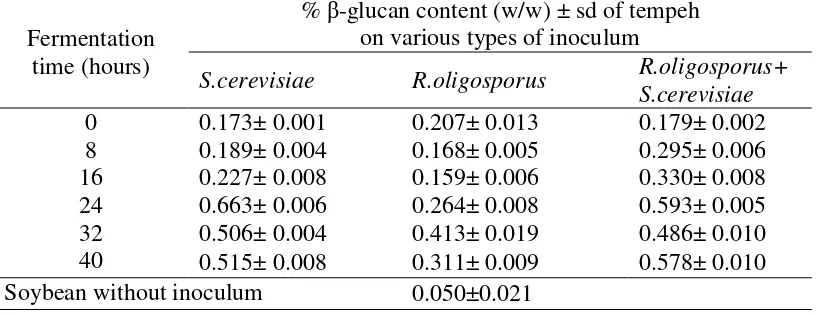
Analysis of β-glucan was carried out on control samples (soybean without inoculum) and tempeh inoculated with inoculums of S. cerevisiae, R.oligosporus, and mixture of R.oligosporus + S.cerevisiae, at fermentation time 0, 8, 16, 24, 32, and 40 hours. The β-glucan content of tempeh inoculated with various types of inoculum during fermentation is presented in Table 5.
Table 5. The β-glucan content of soybean inoculated with various types of inoculum during fermentation.
Fermentation time (hours)
% β-glucan content (w/w) ± sd of tempeh on various types of inoculum
S.cerevisiae R.oligosporus R.oligosporus+ S.cerevisiae
Based on the Table 5, the β-glucan content of tempeh obtained with addition of various types of inoculum during tempeh fermentation varied. The addition of all types of inoculums increased β-glucan content of tempeh with increasing
fermentation time and the β-glucan content was higher than the β-glucan content of soybean without inoculum. The soybeans inoculated with S. cerevisiae and a mixture inoculums of R.oligosporus and S. cerevisiae produced the highest β-glucan content. However, according to the appearance of tempeh produced with addition of
inoculums of S.cerevisiae only, the tempeh was not formed, while on the addition of mixture inoculums of R.oligosporus and S. cerevisiae, at 24 hours of fermentation, the tempeh was still half-finished (Figure 4 and 6). Therefore, based on the best tempeh appearance, the highest β-glucan content was found in soybean inoculated with a mixture of R.oligosporus and S.cerevisiae at the 40 hours of fermentation time i.e. 0.578% (w/w). While the lowest β-glucan content was found in soybeans without inoculum i.e. 0.05% (w/w).
β-glucans can be extracted from the cell wall of S. cerevisiae through alkaline extraction, but further purification is needed to obtain pure β-glucans. In this study, extraction was carried out through alkaline extraction using NaOH which was based on the properties of β-glucans which dissolve easily in alkaline solutions (Lee et al., 2001 in Thontowi et al., 2007). Javmen et al. (2012), stated that the extraction of β -glucan can be done quickly using NaOH for 4 hours. The β-glucan content in tempeh with the addition of commercial tempeh yeast as an inoculum tends to increase as fermentation takes place. This is because the yeast tempeh does not only contain R. oligosporus but also there are other microbes and fillers, namely rice flour (Sukardi, 2008). The β-glucan content in the addition of S. cerevisiae inoculum and the mixture of R. oligosporus and S. cerevisiae tended to increase during fermentation despite decreasing in the 32 hours fermentation period. The production of β-glucans increases with increasing the number of S. cerevisiae cells (Kusmiati, 2007). This is because the cell wall of S. cerevisiae contains β- (1,3) and β-1,6) glucans (M. Naruemon, et al., 2013).
The tempeh inoculated with S. cerevisiae only had a higher β-glucan content compared to the treatment without the addition of S. Cerevisiae (Table 5). The increase occurred due to the growth of S. cerevisiae cells during fermentation period. The β-glucan production from S. cerevisiae increased with the increasing number of S. cerevisiae cells in tempeh. The results of this study are in line with the research of Thontowi et al. (2007) which states that the β-glucan content of S. cerevisiae in cultures with N peptone sources tends to increase with fermentation duration and is relatively fixed at the end of fermentation time. Kusmiati et al. (2006) also reported that an increase in β-glucan production can occur with the use of different C sources, for example by utilizing sugar mill waste (molasses) as a fermentation medium. Increased β-glucan production occurs along with the increasing number of S. cerevisiae cells. Formation of β-glucans will continue and increase during
fermentation until S. cerevisiae reaches a stationary growth phase. Kim et al. (2014) reported that the β-glucan content of polysaccharides in black rice bran fermented by L. edodes increased with incrising the fermentation time.
studied by Rizal et al. (2018) was 0.076% (w/w). The difference in the β-glucan content is presumably because the inoculum used in tempeh making is different. This study used culture of S. cerevisiae, while Rizal, et al. (2018) used commercial instant yeast inoculum (Fermipan). It is suspected that in each inoculum used there is a difference in the number of S.cerevisiae cells at the same concentration of 3%. At the same concentration, in the instant yeast there is a filler or flour substrate so that there is a difference in the composition of the inoculum which causes different β-glucan content to be obtained.
Shokri et al. (2008) stated that the β-glucan content obtained from the isolation of S. cerevisiae cell wall by extraction method using alkaline solution (NaOH) was 27.5%, and the β-glucan content obtained from S.cerevisiae cell wall was extracted by the same was done by Varelas et al. (2016) by 40%. Meanwhile, the percentage of β -glucan content obtained in this study ranged from 0.05% - 0.663%, smaller than the β-glucan content obtained by Shokri et al. (2008) and Varelas et al. (2016). This is because there are differences when extracting β-glucan. In this study the β-glucan content investigated from fermented soybean flour was inoculated with the addition of tempeh yeast inoculums, S. cerevisiae, R.oligosporus, and a mixture of
R.oligosporus and S. cerevisiae , while the β-glucan content studied by Shokri et al. (2008) and Varelas et al. (2016) was directly isolated from the cell wall of S.
cerevisiae. In addition, the differences in strains used maight cause different levels of β-glucan produced. This study used strains of Saccharomyces cerevisiae FNCC 3012, while Shokri et al. (2008) used the strain Saccharomyces cerevisiae PTCC 5052, and Varelas et al. (2016) using a strain of Saccharomyces cerevisiae VIN 13. Alkaline concentration, extraction temperature, and extraction time affected the β -glucan content produced, in this study using 0.7 N NaOH with a temperature of 75 oC for 6 hours, while what was reported by Shokri et al. (2008) used 2% NaOH at 90 oC for 5 hours, and Varelas et al. (2016), using 1 M NaOH with a temperature of 90oC for 2 hours.
Conclusions
Authors’ Contribution
Samsul Rizal is the main author of this article and he is a candidate of doctor in Doctoral Program of Agricultural Faculty, University of Lampung. Murhadi, Maria Erna Kustyawati, and Udin Hasanudin are promotors of doctoral program for Samsul Rizal and they provide a lot of advice, input and improvements in writing this article. While Lia Dahliani Pratiwi, she is a student of Department of Agricultural Product Technology, Agricultural Faculty, University of Lampung who has been a partner for Samsul Rizal in conducting the research and writing this article.
References
Andriani, Yosie. (2007). Uji Aktivitas Antioksidan Ekstrak Betaglukan Dari Saccharomyces Cerevisiae. Jurnal Gradien. 3(1) : 226-230
AOAC. (2005).Official Methods of Analysis. Association of Official. Analytical Chemists. Benjamin Franklin Station. Washington
Barus T, Suwanto A, Wahyudi AT, Wijaya H. (2008). Role of bacteria in tempeh bitter taste formation and molecularbiological analysis base on 16S rRNA gene. J Microbiol Indon 2:17-21
Bintari, S.H., D.P. Anisa., E.J. Veronika., C.R. Rivana. (2008). Effect Inoculation of Micrococcus luteus to Growth of Mold and Content Isoflavone at Tempe Processing. Jurnal Biosantifika 1 (3): 1-8
Delatte, S. J., J. Evans, A. Hebra, W. Adamson, H. B. Othersen, E. P. Tagge. (2001). Effectiveness of beta-glucan collagen for treatment of partial-thickness burns in children. J. Pediatr. Surg. 36 :113.
Efriwati, A. Suwanto, G. Rahayu, dan L. Nuraida. (2013). Populations Dynamic of Yeast and Lactic Acid Bacteria (LAB) during Tempeh Production. Hayati Journal of Biosciences 20 (2) : 57-64. DOI: 10.4308/hjb.20.2.57
Hetland, G., E. Johnson, D.M. Eide, B. Grinde, A.B.C. Samuelsen, dan H. G. Wiker. (2013). Antimicrobial effects of β-glucans and pectin and of the Agaricus blazei Based Mushroom Extract, AndoSanTM. Examples of Mouse Models for Pneumococcal, Fecal Bacterial, and Mycobacterial Infections. Microbial Pathogens and Strategies for Combating Them : Science, Technology and Education (A. Méndez-Vilas, Ed.). Formatex. 889-898.
Ishmayana, S., Djajasoepana, A.S., Rachman, S.D. danSafari, A. (2012). Kinerja Fermentasi Ragi Saccharomyces cerevisiae Pada Media VHG dengan Variasi Konsentrasi Ekstrak Ragi sebagai Sumber Nitrogen Untuk Produksi Bioetanol. (Conference Paper). Universitas Padjadjaran. Bandung
Javmen, A., Grigiškis, S. and Gliebute, R. (2012). β-glucan extraction from Saccharomycescerevisiae yeast using Actinomyces rutgersensis 88 Yeast Lyzing Enzymatic Complex. BIOLOGIJA. 2 (58) 51–59
Kasmidjo, R.B. (1990). Tempe: Mikrobiologi dan Kimia Pengolahan serta Pemanfaatannya. PAU Pangan dan Gizi UGM. Yogyakarta.
Kavanagh, K. (2005). Fungi Biology and Applications. John Willey and Sons Ltd, England.
Kiers, J.L., J.C. Meijer, M.J.R. Nout, F.M. Rombouts, M.JA. Nabuurs, dan J.V. Meulen. (2003). Effect of Fermented Soya Beans on Diarrhoea and Feed Efficiency in Weaned Piglets. Journal of Applied Microbiology 95 : 545–552.
upregulation of the th1 immune reaction. Journal Agriculture and Food Chemistry. 62 :2384–2391
Kusmiati, F. Rachmawati., S. Siregar., S. Nuswantara, dan A. Malik. (2006). Production of beta-1,3 glucan from Agrobacterium and its wound healing activity on white rat. MAKARA, SAINS. 10(1): 24-29
Kusmiati, A., Swasono , Tamat, R S., Nuswantara, dan N. Isnaini. (2007). Produksi dan penetapan kadar β-glukan dari tiga galur Saccharomyces cerevisiae dalam media mengandung molase. Jurnal Ilmu Kefarmasian Indonesia, 5 (1): 7-16 Kusmiati, A. Thontowi, dan S. Nuswantara. (2011). Efek Sumber Karbon Berbeda
terhadap Produksi β-Glukan oleh Saccharomyces cerevisiae pada Fermentor Air Lift. Jurnal Natur Indonesia. 13(2) : 138-145.
Kustyawati, M.E. (2009). Study on the Role of Yeast in Tempe Production. Jurnal Agritech 29 (2) : 64-70.
Kustyawati, M.E., F. Pratama, D. Saputra, dan A. Wijaya. (2014). The Modification of Color, Texture, and Aroma of Tempe Processed with Supercritical Carbon Dioxide. Jurnal Teknologi dan Industri Pangan. 25 (2) : 168-175. ISSN 1979-7788.
Kustyawati, M.E., O. Nawansih, dan S. Nurdjannah. (2016). Profile of Aroma Compounds and Acceptability of Modified Tempeh. International Food Research Journal. 24 (2) : 734-740.
Lay, W., Bibiana dan Hastowo. (1994). Analisa Mikrobia di Laboratorium. PT. Raja Grafindo Persada. Jakarta. 61-71
Mursyid. (2014). Kandungan Zat Gizi dan Nilai Gizi Protein Tepung Tempeh Kedelai Lokal dan Impor serta Aktivitas Antioksidannya. (Tesis). Institut Pertanian Bogor. Bogor.
Nuraemon, M., Romanee, S., Cheunjit, P. Xiao, H., Mc, Landsborough., L. A, Pawadee. (2013). Influence of additives on Saccharomyces cerevisiae β -glucan production. International Food Research Journal. 20(4): 1953-1959 Nurrahman, M., Astuti, Suparmo, Marsetyawan, Soesatyo, HNE. (2012). The Mold
Growth, Organoleptic Properties and Antioxidant Activities of Black Soybean Tempe Fermented by Different Inoculums. AGRITECH. 32(1)
Pagarra, H. (2009). The Rate of Growth of Fungus Rhizopus sp. at Green Bean Tempe (Phaseolus radiatus L.). Bionature .ISSN: 1411-4720. 10 (2): 69 – 74 Pengkumsri, N., Sivamaruthi, B.S., Sirilun, S., Peerajan, S., Kesika, P., Chaiyasut, K.
and Chaiyasut, C.T. (2017). Extraction of Β-Glucan From Saccharomyces cerevisiae : Comparison of Different Extraction Methods and In Vivo Assessment of Immunomodulatory Effect in Mice. Journal of Food Sci. Technol, Campinas, 37 (1) : 124-130. DOI:http://dx.doi.org/10.1590/1678-457X.10716
Popoola, T. O., Kolapo, A., and Afolabi, O. 2007. Hanges in functional properties as a Measure of Biochemical Deterioration of Stored Soybean Daddawa
Condiment. Journal Acta Science PolytechnicTechnologia Almentaria. 6(3): 51-59.
Rizal, S., Kustyawati, M.E., and Ramadhani, I. (2017). Effect of Saccharomyces cerevisiae on The Organoleptic Properties of Soybean Tempeh. Prosiding Seminar Nasional Perhimpunan Ahli Pangan Indonesia; Bandar Lampung, 10-12 November 2017. Pp 1096-1105.
Nasional Dies Natalis Universitas Sebelas Maret ke-42 Tahun 2018. Pp 96-103.
Sarwono, B. (2004). Membuat Tempeh dan Oncom. Penebar Swadaya. Jakarta. Seumahu, C., Suwanto A., Rusmana., Solihin, D.D. (2013). Bacteria And Fungal
Communities In Tempeh As Reveal By Amplified Ribosomal Intergenic Sequence Analysis. J Hayati of Biosci. 20(2):65-71
Shokri, H., F. Asadi, and A. R. Khosravi. (2008). Isolation of β-glukan from The Cell Wall of Saccharomyces cerevisiae. Natural Product Research. 22 (5) : 414-421. DOI : 10.1080/14786410701591622
Sukardi, W., dan Purwaningsih, I. (2008). Uji coba penggunaan inokulum tempeh dari kapang Rhizopus oryzae dengan substrat tepung beras dan ubi kayu pada unit produksi tempeh Sanan Kodya Malang. Jurnal Teknologi Pertanian. 9(3), 207-215.
Suprihatin. 2010. Teknologi Fermentasi. UNESA Pres. Surabaya. Thontowi, A., Kusmiati., dan Nuswantara, S. (2007). Produksi β-Glukan
Saccharomyces cerevisiae dalam Media dengan Sumber Nitrogen Berbeda pada Air-Lift Fermentor. Biodiversitas. 8 (4):. 253-256
Triwibowo, R. (2011). Kajian Kimiawi Stakhiosa dan Asam Lemak Esensial pada Tempeh Kedelai (Glycine max) selama Proses Fermentasi. (Skripsi).
Universitas Sebelas Maret. Surakarta.
Varelas, V., Tataridis, P., Liouni, M., and Nerantzis, E. T. (2016). Application of different methods for the extraction of yeast β-glucan. e-Journal of Science & Technology (e-JST).
Wahono ,S.K., E, Damayanti., Vita,T.R., Evi, I., Sadyastuti. (2011). Laju
Pertumbuhan Saccharomyces cerevisiae pada Proses Fermentasi Pembentukan Bioetanol dari Biji Sorgum (Sorghum bicolor l.). Conference:Proceedings of National Seminar on Chemical Engineering and Processes - Diponegoro University, At Semarang, Indonesia, Volume: ISSN : 1411-4216
Widyastuti, N., T. Baruji., R. Giarni., H. Isnawan., P.Wahyudi., dan Donawati. (2011). Analisa Kandungan Beta-Glukan Larut Air dan Larut Alkali dari Tubuh Buah Jamur Tiram(Pleurotus ostreatus) dan Shitake (Lentimus edodes). J. Sains dan Teknologi. 13 (3): 182-191.