Supporting Information
The Reaction of Cp*(Cl)M(Diene) (M = Ti, Hf) with Isonitriles
Travis N. Valadez, Jack R. Norton,* and Michelle C. Neary
Department of Chemistry, Columbia University, New York, New York 10027, United States
Table of Contents:
General Methods S2
Materials S3
Synthetic Procedures S3
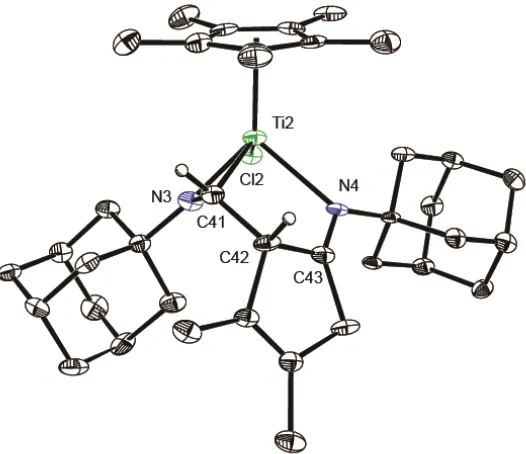
Figure S1. ORTEP view of other enantiomer of the titanaaziridine 3 S7
References S8
General Methods. Unless otherwise noted, all manipulations were carried out in an inert atmosphere box (O2 < 1 ppm) or under Ar by standard Schlenk techniques.
Glassware was flame or oven-dried immediately prior to use. NMR spectra were recorded on a Bruker 300 MHz, 400 MHz, or 500 MHz instrument. High resolution mass spectra were acquired on a Agilent 6220 Acccurate-Mass Time-of-Flight LC/MS. X-ray diffraction data were collected on a Bruker Apex II diffractometer. Crystal data, data collection and refinement parameters are summarized in Table 1. The structure was solved using direct methods and standard difference map techniques, and was refined by full-matrix least-squares procedures on F2 with SHELXTL (Version
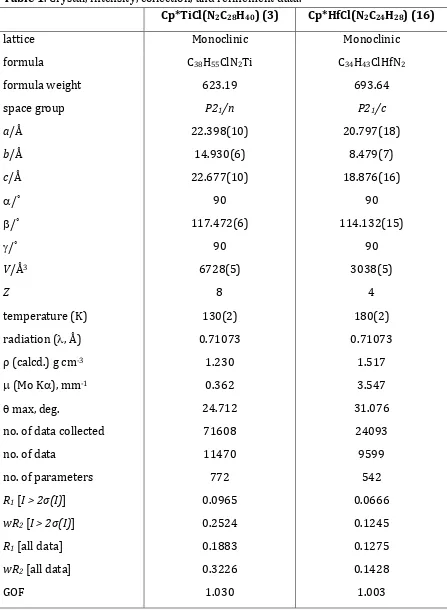
Table 1. Crystal, intensity, collection, and refinement data.
no. of data collected 71608 24093
Rint 0.2420 0.0861
Materials. Tetrahydrofuran and benzene were distilled from
sodium/benzophenone ketyl under N2. Pentane was stirred over sulfuric acid for
several days to remove any olefins, and then was distilled from sodium under Ar. Deuterated solvents (Cambridge Isotopes) were purified by vacuum transfer from the appropriate drying agent (Na/Ph2CO for C6D6 and THF-d8, CaH2 for Tol-d8).
Synthetic Procedures. Cp*TiCl3 was generously donated by Boulder
Scientific. Cp*HfCl3 was purchased from Strem Chemicals and used as received.
2,3-Dimethylbutadiene was purchased from Acros and vacuum transferred from BHT immediately prior to use. Cp*(Cl)Ti(2,3-dimethylbutadiene) (1) and Cp*(Cl)Hf(2,3-dimethylbutadiene)(THF) (14-THF) were prepared by modification of the literature procedures.2 14-THF was prepared according to the literature method and purified
by recrystallization from pentane. 2,6-(CH3)2C6H3N13C was prepared according to the
literature procedure using 13C labeled formic acid.3
(C5(CH3)5)(Cl)Ti(2,3-dimethylbutadiene) (1).2b To a red solution of Cp*TiCl3 (2.80 g, 9.67 mmol) and 2,3-dimethylbutadiene (1.7 mL, 12.0 mmol) in THF
(1.40 g, 48% yield). 1H NMR (500 MHz, C6D6) δ 2.72 (d, J = 8.2 Hz, 2H), 1.89 (s, 6H),
1.87 (s, 15H), 1.38 (d, J = 8.2 Hz, 2H).
(C5(CH3)5)(Cl)Ti(N-(tert-butyl)-2-((tert-butylimino)methyl)-3,4-dimethyl cylopent-3-en-1-imine) (2). To a solution of Cp*(Cl)Ti(2,3-dimethylbutadiene) (1) (1.20 g, 4.0 mmol) in C6H6 (40 mL) was added tert-butyl isonitrile (905 µL, 8 mmol).
The solution color immediately changed from deep blue to dark green-brown. After 10 min at RT, the solution was concentrated in vacuo to give a dark green powder. The green solid was dissolved in pentane (50 mL) and the resulting solution was cooled to –78 °C for 5h. The resulting olive green powder was isolated via cannula filtration and rinsed with cold pentane (1.22 g, 2.64 mmol, 66% yield). 1H NMR (400
MHz, C6D6, 298 K) δ 2.97 (bs, 1H), 2.75 (d, J = 21.8 Hz, 1H), 2.50 (d, J = 4.2 Hz, 1H), dimethylcylopent-3-enimine) (3). To a solution of
Cp*(Cl)Ti(2,3-dimethylbutadiene) (1) (200 mg, 0.668 mmol) in C6H6 (15 mL) was added
1-adamantyl isonitrile (216 mg, 1.34 mmol) in C6H6 (15 mL). After 10 min, removal of
solvent in vacuo gave a dark residue. Washing the residue with pentane (25 mL) afforded 3 as a green powder (291 mg, 70% yield). 1H NMR (500 MHz, C6D6) δ 3.07
(d, J = 12.2 Hz, 3H), 1.41 (s, 3H). 13C NMR (126 MHz, C6D6) δ 191.08, 134.67, 127.79,
122.53, 74.95, 73.37, 63.00, 61.51, 45.13, 42.02, 40.02, 37.69, 36.78, 31.22, 30.75, 14.29, 13.04, 12.74. Single crystals suitable for X-ray diffraction were grown from a concentrated pentane solution at –35 °C over 6h.
(C5Me5)(Cl)Ti(NtBu)(NC5H5) (10) + 3,4-dimethyl--methylene
cyclopentenimine (11). Pyridine (0.0275 mmol, 27.5 µL of 1M solution in C6D6) was
added to solution of 2 in C6D6 (600 µL). The resulting dark green solution was heated
at 55°C for 2h to give a red solution of 10 and 11 (yield of both 10 and 11 >90% from
153.20 (C=CH2), 137.13 (C(Me)C(Me)), 134.41 (C(Me)C(Me)), 102.38 (C=CH2), 56.36
(NCMe3), 41.18 (CH2), 30.68 (NCMe3), 14.58 (CH3) 10.44 (CH3). 11 can be separated
from (C5Me5)(Cl)Ti(NtBu)(NC5H5) (10) via vacuum transfer. However, material
obtained in this way is not analytically pure and cannot be purified by
chromatography. HRMS (11) m/z calculated for C12H20N+ (M+H)+ 178.15903, found
178.15917. IR (NaCl) (11) 1618 cm-1 (C=N stretch).
Cp*HfCl(N2C24H28) (16). The diazahafnacyclopentane 16 was prepared according to the procedure of Teuben.5 A solution of 2,6-dimethylphenyl isonitrile
(10.5 mg, 0.08 mmol) in C6D6 (200 µL) was added dropwise to a solution of
µL). The resulting red solution was left overnight. The 1H NMR spectrum of the
product was identical to that of compound 15 reported by Teuben.5 1H NMR (500
MHz, C6D6) δ 7.17-6.84 (m, 6H), 3.03 (d, 17.0 Hz, 1H), 2.77 (d, J = 2.3 Hz, 1H), 2.67 (d,
17.0 Hz, 1H), 2.53 (s, 3H), 2.46 (s, 3H), 2.43 (s, 3H), 2.36 (s, 3H), 1.78 (s, 15H), 1.70 (bs, 1H), 1.33 (s, 3H), 1.12 (s, 3H). Single crystals suitable for X-ray diffraction were obtained by slow concentration of a pentane solution.
[31,32-13C]Cp*HfCl(N2C24H28) (1613C) was prepared from 14-THF and 2,3-Me2PhN13C in a manner analogous to 16.
Figure S1. ORTEP view of one enantiomer of the titanaaziridine 3. Thermal
REFERENCES
1. (a) Sheldrick, G. M. SHELXTL, An Integrated System for Solving, Refining, and Displaying Crystal Structures from Diffraction Data, University of Gottingen: Gottingen, 1981; (b) 1333031523 Sheldrick, G., Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 112.
2. (a) 1423599412 Blenkers, J.; Hessen, B.; Vanbolhuis, F.; Wagner, A. J.; Teuben, J. H., Organometallics 1987, 6, 459. ; (b) 1434986557 Chen, J.; Kai, Y.; Kasai, N.;
Yamamoto, H.; Yasuda, H.; Nakamura, A., Chem. Lett. 1987, 1545.
3. 1434990272 Nanjo, T.; Tsukano, C.; Takemoto, Y., Org. Lett. 2012, 14, 4270. 4. 1433445749 Dunn, S. C.; Mountford, P.; Robson, D. A., J. Chem. Soc., Dalton Trans. 1997, 293.