www.elsevier.com / locate / bres
Research report
Expression of vascular endothelial growth factor by reactive astrocytes
and associated neoangiogenesis
a a,b a a c
Bodour Salhia , Lilyana Angelov
, Luba Roncari , Xiaoli Wu , Patrick Shannon ,
a,b ,
*
Abhijit Guha
a
Labatts Brain Tumor Center, Hospital for Sick Children, Toronto, Ontario, Canada b
Division of Neurosurgery, University Health Network, University of Toronto, Western Division, Toronto, Ontario, Canada c
Division of Neuropathology, University Health Network, University of Toronto, Toronto, Ontario, Canada Accepted 8 August 2000
Abstract
Injury to the central nervous system (CNS) invokes a reparative response known as astrogliosis, characterized largely by hypertrophy, proliferation and increased expression of glial fibrillary acidic protein (GFAP), resulting in reactive astrocytosis. Based on our prior observation that peritumoral reactive astrocytes express Vascular Endothelial Growth Factor (VEGF), a highly potent and specific angiogenic growth factor, we have hypothesized that reactive astrocytosis also contributes to the neovascularization associated with astrogliosis. To evaluate this hypothesis we evaluated human surgical / autopsy specimens from a variety of CNS disorders that induce astrogliosis and an experimental CNS needle injury model in wild type and GFAP:Green Fluorescent Protein (GFP) transgenic mice. Using computer image semi-quantitative analysis we evaluated the number of GFAP-positive reactive astrocytes, degree of VEGF expression by these astrocytes, associated Factor VIII-positive microvascular density (MVD) and Ki-67 proliferating endothelial cells. The degree of reactive astrocytosis correlated to levels of VEGF immunoreactivity and MVD in the neuropathological specimens. The mouse–needle–stick brain injury model demonstrated this correlation was temporally and spatially related and maximal after 1 week. These results, involving both human pathology specimens augmented by experimental animal data, supports our hypothesis that the neoangiogenesis associated with reactive astrogliosis is correlated to increased reactive astrocytosis and associated VEGF expression.
2000 Elsevier Science B.V. All rights reserved.
Theme: Cellular and molecular biology
Topic: Neuroglia
Keywords: Astrogliosis; GFAP; Neoangiogenesis; Microvascular density; Reactive astrocytes; VEGF
1. Introduction cell processes and bodies with increased expression of the astrocyte specific cytoskeletal intermediate filament Glial Reactive astrogliosis is a normal reparative mechanism Fibrillary Acidic Protein(GFAP) [7,11,14,35,41,50,64,65]. induced in many CNS disease processes such as ischemia, This reactive astrocytic response has been noted to occur infection, trauma, degenerative diseases, epilepsy and brain within 2 days after experimental brain injury in rodents tumors [29,31,49,50,63]. Amongst the various cellular and approximately 1 week in humans and may persist for responses of reactive gliosis, there is a prominent as- several weeks with progressive decline over months to trocytic response [31,58] comprised of normally quiescent years [43,47,50,63]. The function of this florid reactive astrocytes proliferating, undergoing hypertrophy of their astrocytosis is not fully deciphered, but is postulated to play a role in the healing phase after insult to the CNS by monitoring and controlling the molecular and ionic
con-*Corresponding author. Division of Neurosurgery, University Health tents of the CNS extracellular space. This is likely Network, University of Toronto, Western Division, 399 Bathurst Street,
achieved via multiple membrane receptors for
neurotrans-Toronto, Ontario, Canada M5T 2S8. Tel.:11-416-603-5740; fax: 1
1-mitter peptides, growth factors and specific ion channels
416-603-5298.
E-mail address: [email protected] (A. Guha). that are known to be expressed by normal and reactive
astrocytes [31,50,63]. However, it is hypothesized that trocytosis, neoangiogenesis and VEGF expression from a even moderate reactive astrocytosis may interfere with spectrum of conditions including Alzheimer’s disease, eventual reconnection of functional neuronal circuitry by brain abscess, peritumoral brain from metastatic car-inhibiting axonal regeneration, preventing remyelination or cinoma, head injury and cerebral infarcts. Further, to promoting abnormal neuronal connections with increased evaluate the temporal relationship of reactive astrocytosis
seizure foci [57]. and neoangiogenesis, a stereotactic brain injury model was
There is currently little understanding of the molecular utilized. In order to obtain the proliferative index ([ Ki67 mechanisms regulating reactive astrocytosis. Several ob- positive cells / 1000 endothelial cells3100) of endothelial servations suggest that reactive astrocytosis is a regulated cells, surgical specimens from brain abscess and metastatic process involving cytokines, inflammatory mediators, carcinoma were stained for Ki-67. In addition, a CNS growth factors and physiological stimuli such as hypoxia injury model was utilized in transgenic mice which express [45,50,69]. For example, axonal growth and regeneration Green Fluorescent Protein (GFP) under the control of the after trauma and cerebral infarction are retarded by inhib- astrocyte specific GFAP promoter [5], to co-localize VEGF itory proteins associated with the intense astrogliotic scar expression in reactive astrocytes.
[6,45,50]. Second, reactive astrocytosis although most intense immediately surrounding the area of injury, is
found at much removed distances suggestive of soluble 2. Methods
trophic factors acting in a paracrine fashion [50]. While a
variety of these trophic factors have been studied in 2.1. Evaluation of human neuropathological diseases astrocyte cell culture experiments, their role and
interac-tions in the astrogliotic response in vivo is poorly under- In order to evaluate neoangiogenesis and VEGF expres-stood. Specifically, polypeptide growth factors such as sion in a variety of reactive astrogliotic specimens, 23 basic Fibroblast Growth Factor (bFGF), Epidermal Growth cases of neurosurgical and autopsy specimens obtained Factor (EGF), Transforming Growth Factor (TGF-a), from our neuropathology department were examined. Platelet Derived Growth Factor (PDGF), Cilliary Neuro- These cases represented a spectrum of benign and malig-trophic Factor (CNTF) and cytokines such as Interleukin-1 nant diseases that are known to induce reactive astrocytosis (IL1) and Tumor Necrosis Factor (TNF-a), have all been in the surrounding brain and included: four Alzheimer’s implicated in the astrogliotic response [3,13,32,37, Disease (AD), three bacterial abscesses, five carcinomas,
49,50,59,68,69]. three head injuries and seven infarcts (two acute:,7 days,
(S.E.M.) and also represented as a percentage of the endogenous GFAP was performed by incubating with a maximum value obtained. The extent of astrocytosis was rabbit anti-GFAP antibody (1:10, Dako) or mouse mono-assessed by averaging the number of GFAP-positive clonal anti-VEGF antibody (1:10, UBI) for 1 h at room reactive astrocytes / 4–6 high-powered fields (HPF). MVD temperature. After three washes in PBS containing 1% counts were derived by averaging the number of Factor BSA and 0.9% NaCl, the sections were incubated with VIII-positive vessels / 4–6 HPF and VEGF staining was either Cy5-conjugated goat anti-mouse IgG (1:50, Jackson quantified by averaging the percentage VEGF immuno- ImmunoResearch Laboratories Inc.) or Cy3-conjugated
reactivity / 4–6 HPF. goat anti-rabbit IgG (1:20, Jackson ImmunoResearch
In three cases of brain abscess and two cases of Laboratories Inc.) for 1 h at room temperature. Sections metastatic carcinoma, sections were stained for Ki-67 were washed in PBS and mounted for immunofluorescent (MIB-1, 1:50, Immunotech) after microwave antigen re- microscopy. Sections were evaluated with the green trieval and the proliferative index of the endothelium was immunofluorescence filter to detect expression of the GFP obtained. The proliferative index was determined by transgene under the GFAP promoter and for either endog-counting 1000 endothelial cell nuclei and determining the enous GFAP (Cy3) or VEGF (Cy5).
percentage staining positive for Ki-67.
2.2. Wild type and transgenic GFAP:GFP mouse 3. Results
stereotactic needle brain injury
3.1. Reactive astrocytosis, VEGF immunoreactivity and To determine the temporal association between reactive neoangiogenesis in human CNS diseases
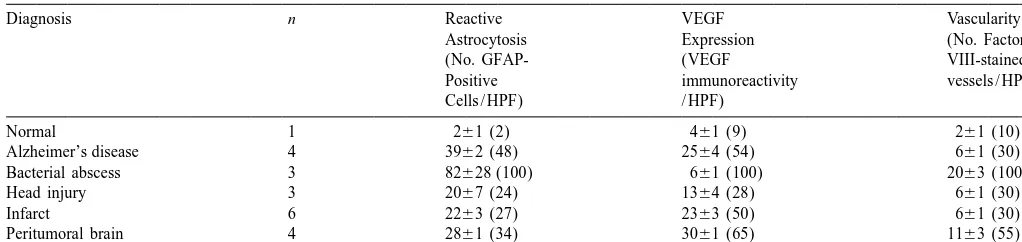
astrocytosis, neoangiogenesis and VEGF expression, a
reproducible stereotactic needle stick injury model to the The mean age of patients with the different diseases frontal cerebral cortex of mice was used [42,47,50]. A total were 79 years — AD; 38 years — Abscess; 65 years — of 27 CD1 mice were anesthetized with 0.5 ml of 2.5% Peri-metastatic carcinoma brain; 52 years — Head Injury; Avertin (2,2,2-tribromoethanol and 2-methyl-2-butanol) 71 years — Cerebral infarcts. Reactive astrocytosis with (Sigma-Aldrich Chemical Company Inc., WI) by intra- increased numbers of large and darkly stained GFAP-peritoneal (i.p.) injection. The head was stabilized in a positive reactive astrocytes were a prominent feature of all stereotactic frame and a burr hole drilled 2 mm anterior to these disease processes compared to normal brain, al-the coronal suture and 2 mm lateral to al-the saggital suture. though the degree of reactive astrocytosis varied with the The needle of a 5-ml Hamilton syringe was then inserted type of pathology, as demonstrated in Table 1, Fig. 1. The through the burr hole to a depth of 2.5 mm for a duration most exuberant reactive astrocytosis was induced in the of 1 min, then withdrawn. The contra-lateral frontal lobe brain surrounding bacterial abscesses (Table 1, Fig. 1-Row was used as the non-injured control side in all evaluations. A), with an average raw value of 82628 GFAP-positive The track was made in cortical gray matter, not penetrating astrocytes / HPF. In comparison, diffuse head injury (Fig. sub-cortical areas that normally contain higher numbers of 1-Row C), induced the lowest amount of reactive as-GFAP-positive cells [8]. All mice tolerated the procedure trocytosis, with a raw value 2067 GFAP-positive as-well. Three mice were sacrificed on each of days 1–9 trocytes / HPF, though this result should be tempered due to post-injury by cervical dislocation and the brains fixed in our lack of knowledge of severity or the time of the formalin for 24 h and embedded in paraffin for histological autopsy after the head injury. The amount of VEGF processing. Axial, 6-mm thick paraffin sections at right immunoreactivity paralleled the degree of induced reactive angles to the needle tract were cut, stained with anti-GFAP, astrocytosis, except for cerebral infarcts and peri-tumoral anti-Factor VIII and anti-VEGF antibodies and analyzed brain, where VEGF immunoreactivity was disproportion-with the computer assisted image analysis system as ately higher (Table 1). The number of Factor VIII-positive
above. blood vessels (MVD), as a measure of neoangiogenesis,
Three FVB / N background GFAP:GFP transgenic mice was related to the degree of reactive astrocytosis and (a gift from Dr. A. Messing, Madison, WI, USA) under- VEGF immunoreactivity. This is best exemplified by went a similar needle tract injury and were evaluated 7 MVD, raw counts of 2063 vessels / HPF, associated with days after injury with immunofluorescence microscopy. the florid reactive astrocytosis in bacterial abscess, com-Mice were sacrificed by cervical dislocation. Brains were pared to only 661 vessels / HPF in brains with prior head removed within 5 min of sacrifice, embedded in OCT injury, AD or infarcts. In these latter conditions, the degree (Tissue Tek) embedding medium on dry ice and stored at of reactive astrocytosis and associated neoangiogenesis
Table 1
a Semi-quantitative analysis of immunohistochemical staining in a variety of human neuropathologies
Diagnosis n Reactive VEGF Vascularity
Reactive astrocytosis, VEGF expression and vascularity in a spectrum of human neuropathological specimens. Raw mean values6S.E.M. are shown and values are reported as a percentage of the maximum in brackets. All slides were quantified at 4003magnification. VEGF, vascular endothelial growth factor; S.E.M., standard error of the mean; GFAP, glial fibrillary acidic protein; HPF, High Power Field.
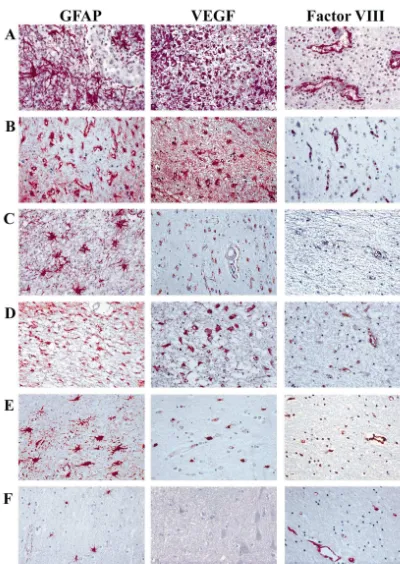
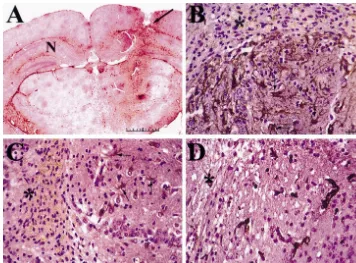
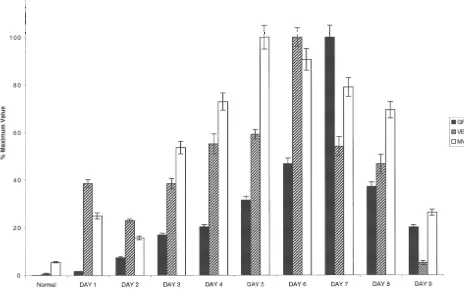
appeared larger and more robust than other CNS diseases parameters subsequently decreased towards normal levels investigated and the blood vessels associated with head by day 9. While the peak days for each parameter analyzed injury seem much smaller compared to other conditions. In do not occur on the same day in this experimental the astrogliotic response associated with AD and cerebral paradigm, the overall temporal correlation between them is infarcts, the vascularity, although increased from normal clearly demonstrated. The GFAP:GFP transgenic mice brain, was relatively less compared to the degree of VEGF were used for co-localization experiments in a needle stick immunoreactivity by reactive astrocytes. The proliferative injury model to confirm that the reactive astrocytes in-index of endothelial cells ranged from 8 to 12% in brain duced by the needle tract injury (* in Fig. 4) were abscesses (n53) and from 2 to 6% (n52) near metastatic expressing VEGF. Green immunofluorescence denoted the tumor. These results clearly demonstrate active neoan- expression of the GFP transgene under regulation of the giogenesis in these two conditions. Material taken from GFAP promoter (Fig. 4A). These same GFP expressing autopsy specimens was not used to determine proliferative cells were reactive astrocytes, as they also expressed index because, in our experience, detection of this antigen endogenous GFAP as detected by double immunofluores-is not reliable in timmunofluores-issue undergoing prolonged fixation. cence, where the cells are yellow due to the combination of green and the Cy3 secondary antibody used to detect 3.2. Temporal and spatial expression of reactive endogenous GFAP (Fig. 4B). On adjacent sections, the astrocytosis, VEGF immunoreactivity and GFP reactive astrocytes are the same cells that are express-neoangiogenesis ing VEGF, where they appear red due to combination of green and Cy5 immunofluorescence used to detect VEGF The mouse stereotactic needle stick injury resulted in a (Fig. 4C). There were no significant reactive GFP-positive distinct tract that could be consistently visualized on the astrocytes in the non-injured contra-lateral hemisphere injured side with a surrounding reactive astrogliotic re- (Fig. 4D).
sponse (Fig. 2A). The injury induced GFAP-positive astrocytes (Fig. 2B) to express VEGF (Fig. 2C) with
accompanying neoangiogenesis, as marked by Factor VIII- 4. Discussion
positive endothelial cells (Fig. 2D). The number of
Fig. 2. Visualization and characterization of mouse–needle–stick injury. Immunohistochemical sections of mouse brain stained for GFAP, VEGF and Factor VIII. (A) Coronal section through the injury site (scale bar depicts 2 mm in length) displaying GFAP immunoreactivity (brown staining) in reactive astrocytes straddling the tract site (arrow). The contralateral non-injured site (N) is GFAP negative in the same cortical region. Mouse brain sections at higher power magnification at the site of the needle stick injury (day 7 post-injury) (scale bar depicts 50mm in length) stained with (B) anti-glial fibrillary acidic protein (GFAP) antibody; (C) anti-vascular endothelial growth factor (VEGF) antibody; (D) anti-Factor VIII antibody. Day 7 post-injury is the maximum GFAP response, also shown in Fig. 3. The injury tract (*) can be seen in the upper left corner of each section. Arrows in B and C are pointing to positively stained reactive astrocytes.
form scars by extending their long, thick cytoplasmic neoangiogenesis (MVD) and VEGF immunoreactivity in a processes toward the site of the lesion [29]. It is likely that variety of human CNS pathologies (see Table 1, Fig. 1). the glial scar can serve to separate normal brain from the Reactive astrocytosis, neoangiogenesis and increased surrounding damaged tissue, but its formation may also be VEGF was maximal in response to bacterial brain abscess harmful by essentially creating a mechanical barrier [50] and probably reflects the acuteness of the illness and the which may impede neuronal dendritic growth, remodeling type of response that it induces in the CNS. In an abscess, and axonal regeneration [25,29,50,63,71]. In addition, the in response to the released toxins, there is an inflammatory glial scar may contribute to electrical instability in the cellular response, components of which themselves release region and in turn promote seizure activity [50]. However, VEGF and other inflammatory cytokines modulators. This more recent evidence suggests that astrogliosis may actual- leads not only to a vigorous reactive astrogliotic response ly facilitate CNS recovery through neurotrophic factor but it is also one of the few CNS diseases that induces a production around the area of the lesion [2,34,67]. In collagen fibrovascular response.
summary, like the common collagen fibrovascular wound The amount of VEGF immunoreactivity and MVD healing process, reactive astrogliosis serves a beneficial exceeded the degree of reactive astrocytosis in the brain CNS reparative function, however, under certain circum- around carcinomas (Table 1), perhaps attributable to the stances it may be detrimental much like exuberant scar and additive expression of VEGF from the GFAP positive
keloid formation in other tissues. reactive astrocytes and the carcinoma cells themselves
Fig. 3. Temporal association of astrocytosis, VEGF immunoreactivity and neoangiogenesis in the needle stick injury model. Histogram (depicting percentage of the maximum mean6S.E.M.) demonstrating the temporal association in the expression patterns of GFAP, VEGF and MVD following mouse–needle–stick injury. The maximal number of GFAP-positive astrocytes, VEGF expression and neoangiogenesis was temporally correlated and occurred between days 5–7 post-injury supporting a relationship in the time course of the astrogliotic and neoangiogenic responses.
artery occlusion in rats, VEGF mRNA expression was positive reactive astrocytes. Our results confirm previous consistently greater across different time points than the findings that reactive astrocytes after a stab wound injury number of CD31 stained vessels [54]. In neurodegenera- are VEGF positive [46,52] and also concur with the tive CNS diseases like AD, characterized by b-amyloid observation that VEGF mRNA was overexpressed in deposits, neuronal death is accompanied by proliferation of retinal astrocytes of diabetic rats [46].
astrocytes, which react in an attempt to detoxify neuronal Although our study focuses on increased expression of debris, toxins and produce various trophic substances VEGF in reactive astrocytes, it is evident that there are [33,63]. Furthermore, decreased cerebral perfusion and several cell types capable of producing VEGF, that are also glucose metabolism found in AD may also induce the prevalent in reactive astrocytosis. These include, platelets, reactive astrocytes to up-regulate VEGF in an attempt to neurons, macrophages / microglia, smooth muscles around
increase neoangiogenesis (Fig. 1) [33,63]. vessel walls, megakaryocytes and polymorphonuclear
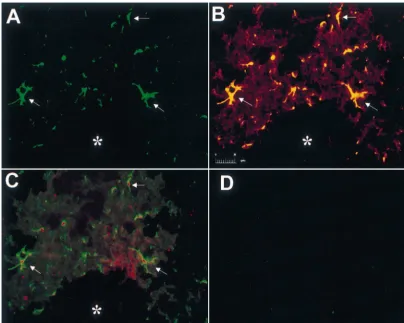
con-Fig. 4. Co-localization of VEGF in reactive astrocytes of GFAP:GFP transgenic mice. Photomicrograph (scale bar depicts 20mm in length) demonstrating the co-localization of reactive astrocytes and VEGF expression in GFAP:GFP transgenic mouse brains post-stereotactic needle stick injury. (*) denotes the area of the tract injury. (A) Endogenous reactive astrocytes (arrows) emit intense green fluorescence. Typical astrocyte morphology with cell bodies and numerous processes can be seen. (B) Double labeling on the same section with anti-GFAP antibody and endogenous GFP immunofluorescence. The reactive astrocytes are stained yellow (arrows) due to the combination of the GFP green fluorescence and the anti-GFAP antibody detected via a Cy3-conjugated secondary antibody. (C) Double labeling on an adjacent section showing the co-localization of GFP-positive reactive astrocytes and VEGF protein expression as demonstrated by the red immunofluorescence staining (arrows). (D) The non-injured contra-lateral hemisphere of the brain in the same area demonstrated no significant GFP-positive green astrocytes.
con-[3] F. Besnard, F. Perraud, M. Sensenbrenner, G. Labourdette,
Platelet-ditions is uncertain. For example, experiments which
derived growth factor is a mitogen for glial but not for neuronal rat
modulate VEGF in the zone of cerebral infarction as to
brain cells in vitro, Neurosci. Lett. 73 (1987) 287–292.
rescue the penumbra by neoangiogenesis [33], similar to [4] N. Bouk, Tumour Angiogenesis: the role of oncogenes and tumour experiments with VEGF gene therapy of ischemic car- suppressor genes, Cancer Cells 6 (6) (1990) 179–185.
diomyocytes [39], are under current study in our labora- [5] M. Brenner, A. Messing, GFAP transgenic mice, Methods 10 (1996) 351–364.
tory.
[6] J.A. Brodkey, E.D. Laywell, T.F. O’Brien, A. Faissner, K.
Stefan-This study has demonstrated using several experimental
sson, U. Derries, M. Schachner, D.A. Steindler, Focal brain injury
and neuropathological models that reactive astrocytosis is and upregulation of a developmentally regulated extracellular matrix accompanied by neoangiogenesis, part of which we can protein, J. Neurosurg. 82 (1995) 106–112.
postulate is based on VEGF expression by the reactive [7] J.L. Calvo, A.L. Carbonell, J. Boya, Co-expression of glial fibrillary acidic protein and vimentin in reactive astrocytes following brain
astrocytes. The process of reactive gliosis involves many
injury in rats, Brain Res. 566 (1991) 333–336.
different cell types (with reactive astrocytes being a major
[8] P. Carmeliet, V. Ferrara, G. Breirer, S. Pollefeyt, L. Kieckens, M.
component), several of which are capable of secreting
Gertsenstein, M. Fahrig, A. Vandenhoeck, K. Harpal, C. Eberhardt,
VEGF. Therefore, the functional importance of reactive Abnormal blood vessel development and lethality in embryos astrocytes and VEGF towards the neoangiogenesis accom- lacking a single VEGF allele, Nature 380 (1996) 435–439.
[9] R.A. Clark, Wound Repair, Curr. Opin. Cell Biol. 1 (1989) 1000–
panying reactive gliosis requires further experimentation.
1008.
Presently hypomorph (under-expressing) and hypermorph
[10] E. Connolly Jr., C. Winfree, D. Stern, R. Solomon, D. Pinsky,
(over-expressing) VEGF transgenic animals are being used
Procedural and strain-related variables significantly affect outcome
to address this issue, since both the homozygous and in a murine model of focal cerebral ischemia, Neurosurgery 38 heterozygous VEGF knockouts are embryonically lethal (1996) 523–532.
[9,17]. Another ongoing strategy utilizes small molecule [11] A. da Cunha, J.J. Jefferson, W.R. Tyor, J.D. Glass, F.S. Jannotta, L. Vitkovic, Gliosis in human brain: relationship to size but not other
inhibitors of the VEGF receptors expressed by the
endo-properties of astrocytes, Brain Res. 600 (1993) 161–165.
thelial cells to inhibit the VEGF mediated neoangiogenic
[12] S. David, C. Bouchard, O. Tsatas, N. Giftochristos, Macrophages
response in reactive astrocytosis. These and other potential can modify the nonpermissive nature of the adult mammalian central strategies will help to further our molecular understanding nervous system, Neuron 5 (1990) 463–469.
of reactive astrocytosis and associated neoangiogenesis and [13] F. Eclancher, F. Perraud, J. Faltin, G. Labourdette, M. Sensenbren-ner, Reactive astrogliosis after basic fibroblast growth factor (bFGF)
may provide therapeutic reagents to modulate this process
injection in injured neonatal rat brain, Glia 3 (1990) 502–509.
favorably where indicated in the future.
[14] L.F. Eng, A. Yu, Y.L. Lee, Astrocytic response to injury, Progress Brain Res. 94 (1992) 353–365.
[15] M.M. Feldkamp, N. Lau, J. Rak, R.S. Kerbel, A. Guha, Normoxic and hypoxic regulation of vascular endothelial growth factor
Acknowledgements
(VEGF) by astrocytoma cells is mediated by ras, Int. J. Cancer 81 (1999) 118–124.
The authors would like to thank Dr. A. Messing [16] N. Ferrara, Vascular endothelial growth factor, Eur. J. Cancer 32A (Madison, Wisconsin) for the GFAP:GFP mice. We wish (1996) 2413–2422.
[17] N. Ferrara, K. Carver-Moore, H. Chen, M. Dowd, L. Lu, K.S.
also to thank Ms. T. Nicklee (Toronto, ON) for her
O’Shea, L. Powell-Braxton, K.J. Hillan, M.W. Moore, Heterozygous
assistance with the image analysis system. LA was
sup-embryonic lethality induced by targeted inactivation of the VEGF
ported by a National Cancer Institute of Canada and an
gene, Nature 380 (1996) 439–442.
American Association of Neurological Surgeons Research [19] G. Finkenzeller, A. Technau, D. Marme, Hypoxia induced transcrip-Fellowship. BS was supported by the Lunenfeld Research tion of the vascular endothelial growth factor gene is independent of functional AP-1 transcription factor, Biochem. Biophys. Res.
Com-Institute summer studentship. AG is a Medical Research
mun. 208 (1995) 432–439.
Council of Canada Clinician Scientist and this work was
[20] J. Folkman, Toward an understanding of angiogenesis: search and
supported by operating grants to AG from Physician
discovery, Perspect. Bio. Med. 29 (1) (1985) 10–37.
Scientist Inc. and Heart & Stroke Fund of Ontario. [21] J. Folkman, Angiogenesis in cancer, vascular, rheumatoid and other
diseases, Nature Med. 1 (1995) 27–31.
[22] J. Folkman, M. Klagsbrun, Angiogenic factors, Science 235 (1987) 442–447.
References [23] J. Folkman, Y. Shing, Angiogenesis, J. Biol. Chem. 267 (1992)
10931–10934.
[1] L.P. Aiello, R.L. Avery, P.G. Arrigg, B.A. Keyt, H.D. Jampel, S.T. [24] A. Fontana, W. Fierz, The endothelium–astrocyte immune control Shah, L.R. Pasquale, H.T. Mami, A. Iwamoto, E.J. Park, Vascular system of the brain, Immunopath. 5 (1985) 57–70.
endothelial growth factor in ocular fluid of patients with diabetic [25] R. Ghirnikar, A. Yu, L.F. Eng, Astrogliosis in culture: III. Effect of retinopathy and other retinal disorders, New England J. Med. 331 recombinant retrovirus expressing antisense glial fibrillary acidic (1994) 1480–1520. protein RNA, J. Neurosci. Res. 38 (1994) 376–385.
endotheli-al growth factor and erythropoietin, J. Biol. Chem. 269 (1994) [50] M.D. Norenberg, Astrocyte response to CNS injury, J. Neuropathol.
4355–4359. Exp. Neurol. 53 (1994) 213–220.
[28] D. Gospodarwicz, J.A. Abraham, J. Schilling, Isolation and charac- [51] K. Norrby, Angiogenesis: new aspects relating to its initiation and terization of a vascular endothelial cell mitogen produced by control, APMIS 105 (1997) 417–437.
pituitary-derived follicle stellate cells, Proc. Natl. Acad. Sci. USA [52] E. Papavassiliou, N. Gogate, M. Proescholdt, J.D. Heiss, S. Wal-86 (1989) 7311–7315. bridge, N.A. Edwards, E.H. Oldfield, M. Merrill, Vascular endotheli-[29] V. Guenard, G. Frisch, P. Wood, Effects of axonal injury on astrocyte al growth factor (vascular permeability factor) expression in injured
proliferation and morphology in vitro: implications for astrogliosis, rat brain, J. Neurosci. Res. 49 (1997) 451–460.
Exp. Neurol. 137 (1996) 175–190. [53] V.H. Perry, M.C. Brown, S. Gordon, The macrophage response to [31] M.E. Hatten, R.K.H. Liem, M.L. Shelanski, C.A. Mason, Astroglia central and peripheral nerve injury, J. Exp. Med. 165 (1987)
in CNS injury, Glia 4 (1991) 233–243. 1218–1223.
[32] M.A. Kahn, J.A. Ellison, G.J. Speight, J. de Vellis, CNTF regulation [54] K.H. Plate, H. Beck, S. Danner, P.R. Allegrini, C. Wiessner, Cell of astrogliosis and the activation of microglia in the developing rat type specific upregulation of vascular endothelial growth factor in an central nervous system, Brain Res. 685 (1995) 55–67. MCA-occlusion model of cerebral infarct, J. Neuropathol. Exp. [33] R. Kalaria, D. Cohen, D. Premkumar, S. Nag, J. LaManna, W. Lust, Neurol. 58 (6) (1999) 654–666.
Vascular endothelial growth factor in Alzheimer’s disease and
[55] K.H. Plate, G. Breirer, H.A. Weich, W. Risau, Vascular Endothelial experimental cerebral ischemia, Mol. Brain Res. 62 (1998) 101–
Growth Factor is a potential tumor angiogenesis factor in human 105.
gliomas in vivo, Nature 359 (1992) 845–848. [34] S. Komoly, L.D. Hudson, H. de Webster, C.A. Bondy, Insulin-like
[56] J. Provius, K. Claffey, L. delAguila, N. Lau, M. Feldkamp, A. Guha, growth factor I gene expression is induced in astrocytes during
Meningiomas: the role of vascular endothelial growth factor / Vascu-experimental demyelination, Proc. Natl. Acad. Sci. USA 89 (1992)
lar permeability factor (VEGF / VPF) in angiogenesis and peri-1894–1898.
tumoral edema, Neurosurgery 40 (5) (1997) 1016–1026. [35] N. Latov, G. Nilaver, E.A. Zimmerman, W.G. Johnson, A.J.
Silver-[57] P.J. Reier, S. Fedooroff, A. Vernadakis, in: Gliosis Following CNS manm, R. Defendini, L. Cote, Fibrillary astrocytes proliferate in
Injury: The Anatomy of Astrocytic Scars and their Influences on response to brain injury. A study combining immunoperoxidase
Axonal Elongation, Vol. 3, Academic Press, Orlando, Florida, 1986, technique for glial fibrillary acidic protein and radioautography of
pp. 263–324. tritiated thymidine, Devel. Biol. 72 (1979) 381–384.
[58] The glial scar: Its bearing on axonal elongation and transplantation [36] F. Lennmyr, K. Ahmad, K. Funa, Y. Olsson, A. Terent, Expression
approaches to CNS repair, in: P.J. Reier, J.D. Houle, S.G. Waxman of vascular endothelial growth factor (VEGF) and its receptors
(Eds.), Functional Recovery in Neurological Disease, Advances in (Flt-1 and Flk-1) following permanent and transient occlusion of the
Neurology, Vol. 47, Raven Press, New York, 1988, pp. 87–137. middle cerebral artery in the rat, J. Neuropathol. Exp. Neurol. 57 (9)
[59] M. Rostworowski, V. Balasingam, S. Chabot, T. Owens, V.W. Yong, (1997) 874–882.
[37] S. Levison, M. Ducceschi, G. Young, T. Wood, Acute exposure to Astrogliosis in the neonatal and adult murine brain post-trauma: CNTF in vivo induces multiple components of reactive gliosis, Exp. elevation of inflammatory cytokines and the lack of requirement for Neurol. 141 (1996) 256–268. endogenous interferon-gamma, J. Neurosci. 17 (10) (1997) 3664– [38] A.P. Levy, N.S. Levy, M.A. Goldberg, Post-transcriptional regula- 3674.
tion of vascular endothelial growth factor by hypoxia, J. Biol. Chem. [60] K. Samoto, K. Ikezaki, M. Ono, T. Shono, K. Kimitoshi, M. 271 (1996) 2746–2753. Kuwano, M. Fukui, Expression of vascular endothelial growth factor [39] D.W. Losordo, P.R. Vale, J.F. Symes, Gene therapy for myocardial and its possible relation with neovascularization in human brain
angiogenesis: Initial clinical results with direct myocardial injection tumors, Cancer Res. 55 (1995) 1189–1193.
of phVEGF165as a sole therapy for myocardial ischemia, Circulation [61] D.R. Senger, S.J. Galli, A.M. Dvorak, C.A. Perruzzi, V.S. Harvey, 98 (25) (1998) 2500–2504. H.F. Dvorak, Tumor cells secrete a Vascular Permeability Factor that [41] A.J. Mathewson, M. Berry, Observations on the astrocyte response promotes accumulation of ascites fluid, Science 219 (1983) 983–
to a cerebral wound in adult rats, Brain Res. 327 (1985) 61–69. 985.
[42] T. Miyake, T. Hattori, M. Fukuda, T. Kitamura, S. Fujita, Quantita- [62] D. Shweiki, A. Itin, D. Soffer, E. Keshet, Vascular Endothelial tive studies on proliferative changes of reactive astrocytes in mouse Growth Factor induced by hypoxia may mediate hypoxia-initiated cerebral cortex, Brain Res. 451 (1988) 133–138. angiogenesis, Nature 359 (1992) 843–845.
[43] T. Miyake, M. Okada, T. Kitamura, Reactive proliferation of [63] M. Tacconi, Neuronal Death: Is there a role for astrocytes?, astrocytes studies by immunohistochemistry for proliferating cell Neurochem. Res. 23 (5) (1998) 759–765.
nuclear antigen, Brain Res. 590 (1992) 3300–3302. [64] Y. Takamiya, S. Kohsaka, S. Toya, M. Otani, Y. Tsukada, Immuno-[44] R. Mohle, D. Green, M.A. Moore, R.L. Nachman, S. Rafii, histochemical studies on the proliferation of reactive astrocytes and Constitutive production and thrombin-induced release of vascular the expression of cytoskeletal proteins following brain injury in rats, endothelial growth factor by human megakaryocytes and platelets, Devel. Brain Res. 38 (1988) 201–210.
Proc. Natl. Acad. Sci. USA 94 (1997) 663–668. [65] V.K. Vijayan, Y.L. Lee, L.F. Eng, Increase in glial fibrillary acidic [45] M.K. McMillian, L. Thai, J.S. Hong, J.P. O’Callaghan, K.R. protein following neural trauma, Neuropathology 13 (1990) 111–
Pennypacker, Brain injury in a dish: a model for reactive gliosis, 122.
Trends Neurosci. 17 (4) (1994) 138–142. [66] U. Wartiovaara, P. Salven, H. Mikkola, R. Lassila, J. Kaukonen, V. [46] S. Nag, J.L. Takahashi, D. Kilty, Role of vascular endothelial Joukov, A. Orpana, M. Heikinheimo, H. Joensuu, K. Alitalo, A. growth factor in blood brain barrier breakdown and angiogenesis in Palotie, Peripheral blood platelets express VEGF-C and VEGF brain trauma, J. Neuropathol. Exp. Neurol. 56 (8) (1997) 912–921. which are released during platelet activation, Thromb. Haemost. 80 [47] M. Nieto-Sampedro, R.P. Saneto, J. de Vellis, C.W. Cotman, The (1998) 171–175.
[69] V.W. Yong, R. Moumdjian, F.P. Yong, T.C. Ruijs, M.S. Freedman, N. [71] A. Yu, Y.L. Lee, L.F. Eng, Astrogliosis in culture: I. The model and Cashman, J.P. Antel, Gamma interferon promotes proliferation of the effect of antisense oligodendrocytes on glial fibrillary acidic adult human astrocytes in vitro and reactive gliosis in the adult protein synthesis, J. Neurosci. Res. 34 (1993) 295–303.
mouse brain in vivo, Proc. Natl. Acad. Sci. USA 88 (1991) 7016– [72] M. Xiong, G. Elson, D. Legarda, S.J. Leibovich, Production of
7020. vascular endothelial growth factor by murine macrophages, Am. J.
[70] H. Yoshiji, D.E. Gomez, M. Shibuya, U.P. Thorgeirsson, Expression Pathol. 153 (2) (1998) 587–598. of vascular endothelial growth factor. Its receptor and other