Negative Variation Preceding Antisaccades in
Schizophrenia
Christoph Klein, Theda Heinks, Burghard Andresen, Patrick Berg, and
Steffen Moritz
Background: The contingent negative variation (CNV) is
considered to reflect prefrontal functioning and can be
observed before manual and ocular motor responses.
Schizophrenic patients exhibit reduced CNV amplitudes in
tasks requiring manual motor responses. A number of
studies has also found normal prosaccades, but delayed
antisaccades and an augmented rate of erroneous
prosac-cades during the antisaccade task in schizophrenia. In this
study we examined the CNV during pro- and antisaccade
tasks in schizophrenic patients and healthy control
subjects.
Methods: Data of 17 medicated schizophrenics (ICD-10,
F20) and 18 control subjects, matched with patients for
age, gender, and education were analyzed. Horizontal
pro- and antisaccades were elicited in four blocks, each
consisting of 80 trials. Electroencephalogram was
re-corded from 32 channels with a DC amplifier.
Results: Patients exhibited delayed correct responses and
more erroneous prosaccades during the antisaccade task
than control subjects, but normal prosaccadic reaction
times. In control subjects, the vertex-predominant
sac-cadic CNV was generally larger than in patients, and
larger during the anti- than during the prosaccade task.
This task-related amplitude augmentation was absent in
patients. Analyses of additional components suggested
specificity of impaired event-related potential modulation
to the saccadic CNV.
Conclusions: In accordance with the presumed prefrontal
dysfunction, our results suggest deficient preparation and
execution of antisaccades in schizophrenia. Biol
Psychi-atry 2000;47:978-990 © 2000 Society of Biological
Psychiatry
Key Words: Schizophrenia, event-related brain
poten-tials, saccadic CNV, antisaccadic task
Introduction
A
ccording to Fuster (1984, 1985, 1989), one of the
functions of the prefrontal cortex is the mediation of
cross-temporal contingencies for the temporal
organiza-tion of goal-directed behavioral sequences. This funcorganiza-tion
has been investigated most intensively with delay tasks. In
delay tasks, the information provided by a stimulus, which
is presented at the beginning of the trial, has to be retained
during a delay period in order to be able to select the
correct, rewarded response upon presentation of a second
stimulus. Unit recordings in primates have revealed
delay-related activity in the prefrontal cortex that seems to serve
two complementary cognitive functions: working memory
and preparatory set (Funahashi et al 1993; Fuster 1984,
1989). Working memory enables the organism not only to
retain behaviorally relevant information over time, but
also to select an appropriate action on the basis of an
internal representation; preparatory set involves the
ad-justment of the sensory and motor systems before an
ex-pected event in order to optimize the reception of stimuli
and the anticipated (motor) response (Fuster 1989, p. 163).
A rostro-caudal sequencing of motor response preparation
seems to take place, because response-related units in
the monkey principal sulcus (corresponding to Brodman’s
area 46 in humans) increase their firing before units in the
premotor and motor cortex (Fuster 1989, p. 103).
The two-stimulus paradigm is a variant of the delay task
(Fuster 1989): A warning stimulus (WS), presented at the
beginning of the trial, reliably signals the subsequent
presentation of an imperative stimulus (IS), which is
delayed by some seconds and associated with a certain
task (Rockstroh et al 1989). Between WS and IS, a
surface-negative potential arises referred to as the
contin-gent negative variation (CNV; Walter 1964). For a
num-ber of reasons, the CNV is considered to reflect the
delay-related activity of the prefrontal cortex; it is also
seen to reflect the activity of additional cortical areas that
are recruited by the prefrontal cortex as part of the
behavioral structures of delay tasks (Fuster 1984, 1985,
From the Forschungsgruppe Psychophysiologie, Universitaet Freiburg, Freiburg(CK, TH), Klinik fu¨r Psychiatrie und Psychotherapie, Universitaet Hamburg, Hamburg (BA, SM), and Fachgruppe Psychologie, Universitaet Konstanz, Konstanz (PB), Germany.
Address reprint requests to Christoph Klein, University of Freiburg, Psychophysi-ology Research Group, Department of PsychPsychophysi-ology, Belfortstrasse 20, D-79098 Freiburg, Germany.
Received June 21, 1999; revised December 10, 1999; accepted December 20, 1999.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
1989): 1) Between the WS and IS, a surface-negative
cortical potential can be recorded at many prefrontal sites
of the monkey cortex, including the dorsal and ventral
banks of the principal sulcus (Sasaki and Gemba 1991); 2)
subdural potentials similar to the CNV can be measured
preceding the IS at different prefrontal sites in epileptic
patients during presurgical evaluation (Hamano et al 1997;
Ikeda et al 1996); 3) the CNV amplitude is positively
related to the working memory load imposed by a delayed
matching-to-sample task (Klein et al 1996).
Slow surface-negative potential shifts have been
re-corded from the premotor, supplementary motor, motor,
and somatosensory cortices of monkeys (Sasaki and
Gemba 1991) and humans (Hamano et al 1997; Lamarche
et al 1995) before prewarned motor responses; they may
reflect the aforementioned preparatory adjustment of the
sensory and motor systems. These cortical potentials—
with the exception of the prefrontal potentials (Rektor et al
1994)— have also been found preceding self-paced hand
(Hamano et al 1997; Ikeda et al 1994; Neshige et al 1988;
Rektor et al 1994) or eye (Sakamoto et al 1991)
move-ments. They are considered to be the source of the
“Bereitschaftspotential” (“readiness potential,” RP;
Korn-huber and Deecke 1965) that can be measured at the scalp.
The RP has been suggested to be at least part of the
terminal phase of the CNV (e.g., Brunia 1988; Roesler
1991). Despite this partial overlap between CNV and RP
in their cortical generator structures, two arguments
sug-gest that these components should be distinguished: 1) In
neurological patients, degeneration of the basal ganglia
reduces or abolishes the CNV, but preserves the RP (Ikeda
et al 1997), whereas the reverse pattern is seen after
decussation of the superior cerebellar peduncle (Ikeda et al
1994). The cerebellum projects to the premotor and motor
cortex; the basal ganglia, however, also project to the
prefrontal cortex (Kandel et al 1991); 2) the structure of
the RP tasks (initiation of a movement) lacks the
compo-nents of establishment of cross-temporal contingencies or
sensory-motor integration that characterize delay tasks.
Saccadic eye movements are typically elicited within
the two-stimulus paradigm: A central fixation point is
presented as the WS. This is followed a few seconds later
by the peripheral cue, which serves as the IS and the
saccade goal, in the case of visually guided or
“prosac-cades.” Averaging time-locked to the onset of the saccade
(Evdokimidis et al 1992; Everling et al 1997; Klostermann
et al 1994), or to the onset of the peripheral cue (e.g.,
Evdokimidis et al 1996; Go´mez et al 1996) reveals
surface-negative potential shifts with a topographical
max-imum at anterior central locations. Unit recordings in
primates have revealed that neurons in the prefrontal
frontal eye fields (FEF) and the premotor supplementary
eye fields (SEF; Schall 1991; Schlag and Schlag-Rey
1987) show increased firing before visually guided
sac-cades. In addition, during the execution of visually guided
saccades as compared to a fixation control condition, in
human subjects augmented blood flow in the FEF and the
supplementary motor area (SMA) have been found in
positron emission tomography (PET; Anderson et al 1994;
Melamed and Larsen 1979; Petit et al 1993) and functional
magnetic resonance imaging (fMRI; Darby et al 1996)
studies.
During the “antisaccade” task (Hallett 1978; Hallett and
Adams 1980), subjects are instructed not to look at the cue
that is presented in the visual periphery, but to look
“voluntarily” in the opposite direction, that is, generate an
antisaccade. The initiation of anti- as compared to
prosac-cades is typically delayed (e.g., Reuter-Lorenz et al 1995),
and even normal subjects may happen to glance
uncon-sciously (Mokler and Fischer 1999) at the peripheral cue
in a number of trials. For some reason, this task seems to
“stress” prefrontal or frontal cortical functions more than
the prosaccade task: 1) Patients with large excisions of
frontal lobe tissue (Guitton et al 1985), or with
circum-scribed lesions of the dorsolateral (Pierrot-Deseilligny et
al 1991) or ventrolateral (Walker et al 1998) prefrontal
cortex, but not of the FEF or the SMA (Pierrot-Deseilligny
et al 1991), are impaired at inhibiting erroneous
prosac-cades during the antisaccade task; 2) patients with FEF
lesions, however, exhibit delayed antisaccade initiation
(Rivaud et al 1994). This clinical observation is
comple-mented by physiologic results showing increased blood
flow in the human FEF and SMA (Doricchi et al 1997;
Nakashima et al 1994; O’Driscoll et al 1995; Sweeney et
al 1996), augmented neuronal firing in the monkey SEF
(Amador et al 1995; Schlag-Rey et al 1997), and greater
slow negative potential shifts at anterior central sites in
human subjects (Evdokimidis et al. 1996; Everling et al
1998) before anti- as compared to prosaccades; 3)
inhibi-tion of a peremptory response (looking at the peripheral
cue) in favor of a “voluntary” response (looking at a
position where no stimulus is present) on the basis of an
instruction held in the working memory is per se a typical
prefrontal function (Frith et al 1991; Goldman-Rakic
1987; Roberts et al 1994), subsumed under the
neuropsy-chological concept of “executive functions” (Denckla
1996; Pennington and Ozonoff 1996).
been reported to increase the frontal brain metabolism in
schizophrenia (e.g., Berman et al 1986; Buchsbaum et al
1987). The CNV amplitude reduction may be considered
part of the frontal hypometabolism that has been supported
by regional cerebral blood flow (rCBF) studies during the
execution of tasks sensitive to frontal dysfunctions
(An-dreasen et al 1992; Frith et al 1991; Lewis et al 1992;
Paulman et al 1990; Weinberger et al 1986).
Impaired performance during antisaccade tasks (e.g.,
Crawford et al 1998; Sereno and Holzman 1995) seems to
be another consequence of the frontal dysfunction in
schizophrenia. Despite normal latencies of prosaccades,
schizophrenic patients need significantly more time than
healthy subjects to generate correct antisaccades
(Danck-ert et al 1998; Fukushima et al 1990; Karoumi et al 1998;
McDowell and Clementz 1997). Schizophrenic patients
also produce more erroneous prosaccades during the
antisaccade task than control subjects but are apparently
able to correct all or at least most of them (Clementz et al
1994; Fukushima et al 1988, 1990; Karoumi et al 1998;
McDowell and Clementz 1997). There is some direct
evidence that links the antisaccade deficit of schizophrenic
patients with dysfunctions of the frontal lobes. First, the
deficit is more frequently observed in schizophrenic
pa-tients with abnormal frontal computed tomography (CT)
scans (Fukushima et al 1988, 1990). Second, during a task
similar to the antisaccade task, the generation of
“volition-al” saccades was associated with an increase in FEF and
left dorsolateral prefrontal cortex (DLPFC) metabolism in
healthy participants but not in schizophrenic patients
(Nakashima et al 1994). Finally, in schizophrenic patients
the antisaccade task performance covaries with
perfor-mance in other tasks sensitive to frontal dysfunctions, such
as the smooth pursuit eye movement task (Schlenker and
Cohen 1995; Sereno and Holzman 1995) or the Wisconsin
Card Sorting Test (WCST; Karoumi et al 1998; Nkam et
al 1998; Rosse et al 1993; nonsignificant correlations with
WCST were reported by Schlenker and Cohen [1995]).
The aim of our study is the investigation of the CNV
preceding pro- and antisaccades in schizophrenic
pa-tients as compared to healthy control subjects. This
study expects the following results: 1) A slow
surface-negative potential shift, the saccadic CNV, with greater
amplitudes preceding anti- as compared to prosaccades,
should arise in healthy control subjects and patients; 2)
schizophrenic patients should exhibit reduced CNV
amplitudes during all saccade tasks, and a significantly
smaller CNV amplitude modulation than control
sub-jects when preceding antisaccades as compared to
prosaccades; and 3) normal prosaccadic but augmented
antisaccadic latencies along with augmented rates of
erroneous prosaccades during the antisaccade task
should be found in schizophrenic patients.
Methods and Materials
Participants
Data from 35 of a total of 39 subjects who participated in the experiment were analyzed. Data of three schizophrenic patients and one control participant had to be excluded because of artifacts (eye movement and other movement artifacts). The remaining sample comprised 17 patients treated for schizo-phrenic disorders (12 men, 5 women; mean age 29.967.9 years; mean education510.362.4 years; mean age of onset524.86 6.4 years; mean number of episodes53.062.6). All patients received an ICD-10 diagnosis of a schizophrenic disorder (F20), according to the International Diagnostic Checklist (Hiller et al 1993a, 1993b). Nine of these patients were recruited from the Psychiatric University Hospital of Hamburg-Eppendorf (six stationary patients, three day patients); the remaining eight outpatients were recruited from lodging houses for psychiatric patients in the Hamburg metropolitan area. All inpatients were tested after florid symptoms had largely disappeared. Patients’ current symptomatology was assessed with the Positive and Negative and Disorganized Symptom Scale (PANADSS; Andre-sen and Moritz 2000; Moritz et al 2000). The PANADSS is a standardized interview that covers negative (e.g., blunted affect, slowing, anhedonia, avolition, poverty of content of speech, nonparanoid social withdrawal), positive (e.g., auditory halluci-nations, mental control, bizarre delusion, ideas of reference, paranoid social avoidance, parathymia), and disorganized (e.g., loosening of associations, inadequate affect, eccentric behavior, attention deficits) symptoms. Principal components analyses confirmed the three-dimensional structure of the PANADSS, with the three factors negative, positive, and disorganized schizo-phrenic symptoms accounting for 23.7%, 14.6%, and 13.9% of the total variance in schizophrenia patients, respectively. Patients with a history of substance abuse or neurological disorders, as well as patients with electroencephalogram (EEG) or CT abnor-malities were excluded. All but two patients were under neuro-leptic medication (four patients high-potency, one patient low-potency, 12 patients atypical neuroleptics; mean chlorpromazine equivalents 416, range 300 –750). A group of 18 healthy control subjects (13 men, 5 women; mean age531.369.3 years; mean education 5 10.4 6 1.7 years) was selected on the basis of comparability to the patient group for age, gender, and education (ps . .20). Except for one patient, all participants were right-handed. None of the control subjects reported a history of psychiatric illness, psychotherapeutic treatment, or psychotropic medication.
Procedure
around a mean of 8 sec. For the prosaccade task, participants were instructed to look as quickly as possible at the peripheral stimulus as soon as it appeared; for the antisaccade task, participants were instructed to look as quickly as possible at the horizontal mirror position of the cue, that is, straight in the opposite direction. No frames or other visual cues were used to define the approximate landing point of the antisaccade. Four blocks of 80 trials were provided: two for the prosaccade and two for the antisaccade task. After each block the instruction (pro, anti) changed. Half of the participants of each group began with the prosaccade task, half with the antisaccade task.
At the beginning of a session the laboratory was shown and the forthcoming investigation explained to each participant. None of the subjects renounced participation, and all gave their informed written consent before the investigation could begin. During the experiment, the participant sat in a reclining chair in the dimly lit EEG laboratory, separated from the experimenter by a wall. After preparation for the physiologic recordings, participants performed an eye movement calibration task in order to allow the experimental data to be corrected for eye artifacts. In the calibration task, participants made 20 movements away from and back to the central fixation point in each of four directions (up, down, left, right), and 20 eye blinks while looking at the fixation point. After the calibration task, participants were informed about the tasks. They were instructed to adopt a relaxed position and to focus on the fixation cross in the center of the monitor in order to avoid head or eye movements (Weerts and Lang 1973). The same honorarium of DM 35—about $20 —was given to patients and control subjects. The entire experimental session lasted about 2 hours, including breaks between the task blocks.
Apparatus and Physiologic Recordings
The experimental stimuli were generated with a TURBO PASCAL program and presented on two videographic adapter monitors (participant, experimenter) simultaneously. The EEG was recorded over both hemispheres with 32 electrodes using an AC amplifier (NEUROFILE II, Nihon Kohden). Electrodes were attached with an electrode cap (FMS, Munich, Germany) to the following 10-10 positions (American Electroencephalographic Society 1991): Fp1, Fp2, F9, F7, F3, Fz, F4, F8, F10, FC5, FC3, FC4, FC6, T7, C3, Cz, C4, T8, TP9, CP1, CP2, TP10, P7, P3, Pz, P4, P8, O1, O2, and Iz, with mean mastoids ([Tp91TP10]/2) as recording reference. In addition, two infraorbital channels local-ized 2 cm vertically below each eye were attached. Recording reference was the mean of the channels TP9, TP10 (mastoids). The position FCz was interpolated for the statistical analyses (see below). A forehead electrode served as ground. The EEG was recorded with a 10 sec time constant using nonpolarizable AgAgCl electrodes with ABRALYT light as conducting agent (FMS). The skin under the electrodes was prepared by rubbing in abrasive paste (ABRALYT light, FMS). Data was sampled continuously at 256 Hz and stored on a Pentium microprocessor PC, amplified at 10bins/mV.
Data Reduction and Analysis
A trial was defined as a 7-sec EEG epoch, including a 1.5-sec baseline, 3.5-sec WS-IS interval, and 2 sec after the IS. Eye
maps, spherical spline interpolation was accomplished following the corrected algorithm of Perrin et al (1989). The applied inter-polation method uses a smoothing constant lambda of 0.00001.
Analysis of the ERP Data
The mean amplitudes during the intervals20.5– 0 sec relative to IS onset using a 1.0 sec pretrial baseline served as saccadic CNV scores. Using analysis of variance (ANOVA) we analyzed the amplitudes of the saccadic CNV at frontal (F3, Fz, and F4), fronto-central (FC3, FCz, and FC4), central (C3, Cz, and C4), and parietal (P3, Pz, and P4) channels. These 12 channels were selected, on the basis of the topographical maps, as those channels that covered the main activity of the saccadic CNV. The ANOVA comprised the between-subjects factor GROUP (pa-tients vs. control subjects), and the within-subjects factors CONDITION (prosaccade vs. antisaccade task), ELECTRODE (“left-sided” [F3, FC3, C3, P3] vs. “central” [Fz, FCz Cz, Pz] vs. “right-sided” [F4, FC4, C4, P4] sagittal rows), and ANTERIOR-POSTERIOR (frontal [F3, Fz, F4] vs. fronto-central [C3, FCz, FC4] versus central [C3, Cz, C4] vs. parietal [P3, Pz, P4] coronal rows). We used the mean mastoid reference data for our
statistical analyses; however, because our mapping programs use
exclusively the average reference, the graphical result presenta-tion is based on the average reference. To control for amplitude effects in the case of significant condition by topography interactions (McCarthy and Wood 1985), the statistical proce-dures were repeated after z transformation of the data (12 leads), for the two groups and two tasks separately. For all main and interaction effects including within-subject factors with more than two levels, violations of the sphericity assumption were controlled for by df adjustment. Hence, Greenhouse–Geisser epsilons and corrected p values will be reported. Planned contrasts (F tests) compare the lateral sagittal rows against the central one (ELECTRODE factor), and the frontal, fronto-central, and parietal coronal rows against the central one (ANTERIOR-POSTERIOR factor). Means and standard devia-tions are reported.
Additional negative potentials followed the IS, which are, how-ever, not the main topic of this article: First, a negative peak with a maximum at Cz arose in control subjects 73 msec and 85 msec after the IS during the pro- and the antisaccade task; this peak was discernible in patients only during the antisaccade task, with a maximum at Cz 77 msec after IS onset; second, a broad and large negative deflection was found in patients and control subjects, having a maximum at FCz except for the prosaccade task in control subjects, where it was at a maximum at Cz; third, a negative potential with smaller amplitudes than the previous component was found in patients and control subjects. This negativity was at a maximum at FCz except for the prosaccade task in control subjects, where it was at a maximum at Fz. Concerning these components, all mentioned results were statistically significant (ps,.05).
Results
Saccadic Eye Movements
Whereas prosaccade latencies were similar in
schizo-phrenic patients (238.8
6
34.6 msec, range 188 –317
msec) and in healthy control subjects (237.2
6
26.1 msec,
range 202–281 msec), antisaccade latencies were
signifi-cantly longer in patients (288.8
6
66.5 msec, range
187– 444 msec) compared to control subjects [259.1
6
48.4
msec,
range
188 –379
msec;
CONDITION:
F(1,33)
5
28.8, p
,
.001; CONDITION
3
GROUP:
F(1,33)
5
4.4, p
,
.05; GROUP: F
,
1.5]. Furthermore,
schizophrenic patients made more direction errors during
the antisaccade task than control subjects [patients: 11.8%
6
6.8%; control subjects: 7.6%
6
4.5%; GROUP: t(33)
5
2.24, p
,
.05]. The proportions of anticipations were
greater during the pro- as compared to the antisaccade
task, but similar in schizophrenic and healthy participants
[patients: prosaccade task: 13.7%
6
10.8%; antisaccade
task: 7.7%
6
2.8%; control subjects: prosaccade task:
10.7%
6
8.7%; antisaccade task: 6.9%
6
4.8%;
CONDI-TION: F(1,33)
5
14.1, p
,
.001]. Finally, the proportions
of express saccades during the prosaccade task were
almost identical in the two groups (patients: 14.3%
6
8.5%; control subjects: 14.1%
6
10.5%).
Event-Related Brain Potentials: Overview
The grand average curves of patients and control subjects
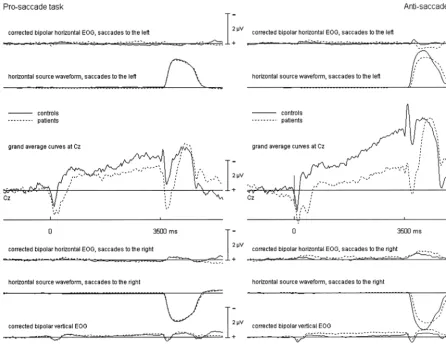
during the pro- and antisaccade tasks are shown in Figure
1 for Cz together with the corrected vertical and horizontal
EOG channels and the source waveforms for vertical and
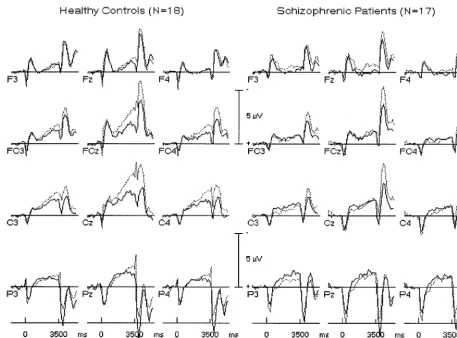
horizontal eye movements, and in Figure 2 for the 12
electrodes used for statistical analyses. As can be seen in
Figure 1, a slow potential shift developed between WS and
IS, which can be identified with the saccadic CNV.
by incomplete eye activity correction. Finally, at the end
of the trial a third negative potential arose. This potential
was at its maximum at fronto-central or frontal sites, with
a left-sided predominance in control subjects and a
bal-anced pattern in patients (see Figure 2). Its amplitudes
were significantly larger following antisaccades compared
to prosaccades. The horizontal source waveform depicted
in Figure 1 shows that this potential developed after the
eyes returned to the central position.
Event-Related Brain Potentials: Saccadic CNV
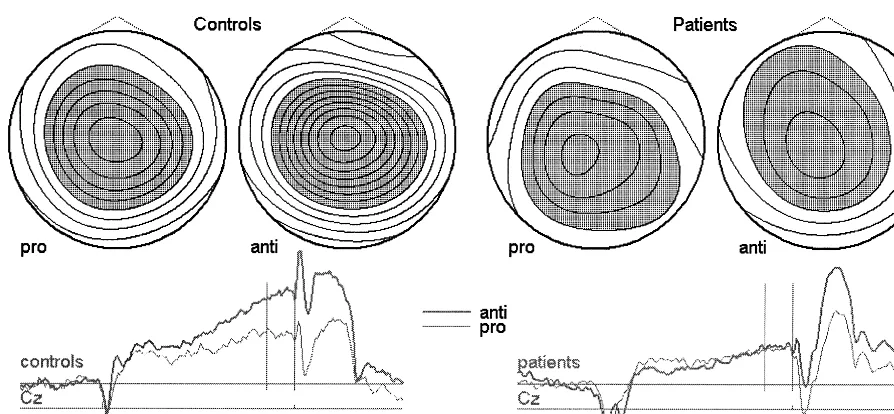
The topography of the saccadic CNV is shown in Figure 3
for control subjects and patients using scalp potential
maps; mean CNV amplitudes (
6
SD) are documented in
Table 1. The saccadic CNV was larger at the sagittal
midline than over the left or right hemisphere
[ELEC-TRODE: F(2,66)
5
11.1, p
,
.001,
e 5
0.89]. The
amplitude augmentation, during the anti- compared to the
prosaccade task, was also at a maximum at the sagittal
midline [CONDITION
3
ELECTRODE: F(2,66)
5
4.1,
p
5
.02,
e 5
0.98, after data normalization: F(2,66)
5
3.0,
p
,
.06].
Healthy control subjects exhibited generally larger
CNV amplitudes than schizophrenic patients [GROUP:
F(1,33)
5
7.7, p
,
.01]. Consistent with the assumption of
a topography change, healthy participants also showed
significantly larger CNV amplitudes at central and
pre-central leads during the anti- than during the prosaccade
task. This effect was missing in schizophrenic patients
[CONDITION
3
ANTERIOR-POSTERIOR
3
GROUP:
jects: F(3,51)
5
6.1, p
,
.002,
e 5
0.83, after data
normalization: F(3,51)
5
4.6, p
5
.01; patients: F(3,48)
5
1.9, p
.
.14,
e 5
0.94].
Although schizophrenic patients exhibited significantly
more direction errors during the antisaccade task than
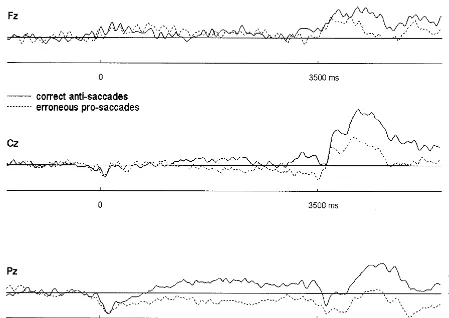
control subjects, only one of them committed enough
errors that the average ERP before correct anti- and
erroneous prosaccades could be compared with a
reason-able signal-to-noise ratio. This comparison is documented
in Figure 4 and reveals smaller CNV amplitudes preceding
erroneous prosaccades as compared to correct antisaccades.
Neuroleptic Medication
Neuroleptic dose correlated by
2
.18 and
2
.15 with the
CNV amplitude at Cz during the pro- and antisaccade
tasks, respectively. These coefficients were nonsignificant.
Discussion
Overview
The present study yielded the following main results: 1)
Antisaccades were significantly slower than prosaccades
in both groups; 2) in comparison to healthy control
subjects, schizophrenic patients exhibited delayed
re-sponding only during the anti-, but not during the
prosac-cade task. During the antisacprosac-cade task, patients committed
more erroneous prosaccades than control subjects; 3) a
centrally predominant saccadic CNV was found in both
groups and during both tasks; 4) control subjects’ saccadic
CNV had larger amplitudes during the anti- than during
the prosaccade task; and 5) schizophrenics’ saccadic CNV
was generally smaller than that of control subjects;
fur-thermore, patients failed to show the CNV augmentation
during the anti- compared to the prosaccade task.
Saccadic Eye Movements
In both groups, correct antisaccades were significantly
slower than prosaccades, confirming results of numerous
other studies (e.g., Klein and Foerster, in press;
Reuter-Lorenz et al 1995). A number of time-consuming
pro-cesses putatively contributing to this latency augmentation
have been suggested (Everling and Fischer 1998; Klein
and Foerster, in press), but shall not be discussed here.
mal latencies in the initiation of prosaccades but
aug-mented latencies in the initiation of correct antisaccades.
This pattern of response latencies had already been
re-ported by others (e.g., Fukushima et al 1990; Karoumi et
al 1998) and has two important implications. First, the
presence of normal prosaccadic response latencies
sug-gests that patients were not less motivated than control
subjects during task execution. Second, the coexistence of
normal prosaccadic but augmented antisaccadic response
latencies suggests a deficit specific to the generation of
antisaccades in schizophrenic patients. To the extent that
the execution of visually guided prosaccades reflects
“controlled” (instead of “automatic”) processes, this result
would argue against the assumption of a generalized
deficit (Chapman and Chapman 1978).
Along with the delayed initiation of correct
antisac-cades, schizophrenic patients committed more direction
errors, that is, reflexive prosaccades, during the
antisac-cade task, again confirming results of other studies (e.g.,
Clementz et al 1994; Danckert et al 1998; Fukushima et al
1990; Karoumi et al 1998; Sereno and Holzman 1995) and
suggesting an impaired inhibition of a “stimulus-driven”
response. Both the augmented antisaccade latencies and
the greater rate of erroneous prosaccades support the
assumption of impaired (pre-) frontal functioning in
schizophrenic disorders (Buchsbaum et al 1987).
Saccadic CNV
The presentation of the saccade-initiating cue was
pre-ceded by a vertex-predominant slow negative potential
shift during both tasks and in both groups, which we
identified with the saccadic CNV. This component had
been observed by others as well (e.g., Go´mez et al 1996).
In healthy control subjects, the saccadic CNV was
significantly greater during the anti- as compared to the
prosaccade task, consistent with the results of other studies
(Evdokimidis et al 1996; Everling et al 1997). This effect
was at a maximum at central and precentral sites. After
having normalized the data in the present study, the effect
Figure 3. Scalp potential maps of the saccadic contingent negative variation. Negative potentials are depicted in gray, positive potentials in white. Isocontour lines with 0.5-mV difference. The mapped interval corresponds to the vertical bars in the grand averages curves below.Table 1. Amplitudes of the Saccadic Contingent Negative Variation in Patients and Control Subjects at the Sagittal Midline
Prosaccade task Antisaccade task
Fz FCz Cz Pz Fz FCz Cz Pz
Patients
Mean 20.83 21.81 22.14 22.01 21.55 22.07 22.35 22.14
SD 1.9 2.4 2.3 2.1 2.4 2.8 3.0 2.1
Controls
Mean 22.62 23.53 23.80 22.89 23.14 25.05 25.66 23.67
SD 2.6 2.5 2.5 2.7 2.7 3.2 3.3 2.5
remained statistically significant as a task by topography
interaction. This suggests that the effect may be
inter-preted as a change in topography according to McCarthy
and Wood (1985). A topographical change, however,
means that different generators or the same generators
with different strengths contribute to the surface potential.
It is tempting to relate the augmentations of the anterior
portions of the CNV to the greater activity of prefrontal
and frontal cortical areas during the anti- rather than
during the prosaccade task. This increase in activity was
reported in the regional cerebral blood flow (rCBF) and
unit recording studies reviewed in the introduction. These
studies revealed greater metabolism or neuronal firing in
areas of the motor or premotor cortex and, some of these,
in prefrontal regions. A saccadic readiness potential had
also been recorded in the FEF and SMA regions in
epileptic patients (Sakamoto et al 1991). As outlined in the
introduction, RP and terminal CNV share cortical
gener-ators in the preparation of manual motor responses. Hence,
the task-related modulation of the CNV found in this study
and by Evdokimidis et al (1996) and Everling et al (1997)
may reflect task-related activity differences in the FEF and
SMA regions and possibly in other cortical areas.
“stressing” of frontal or prefrontal areas by the task
(Berman 1987). In line with this reasoning, schizophrenic
patients lacked the task-related augmentation of the CNV
amplitude during the anti- compared to the prosaccade
task that was found in control subjects. The deficient
task-related CNV modulation as well as the generally
reduced CNV amplitudes, however, must be considered
rather specific to the preparation of pro- and, in particular,
antisaccades, because normal or even augmented
ampli-tudes or task-related amplitude modulation was found for
the components that followed the execution of the primary
saccade.
If “preparatory set,” as one of the functions triggered by
the prefrontal cortex during delay tasks, means adjustment
of the sensory and motor systems in order to optimize
responding (Fuster 1989) and is reflected by the CNV, the
amplitude of this component should covary with task
performance. Indeed, there is evidence that slow negative
potential shifts reflect increased cortical excitability (e.g.,
Rockstroh et al 1994). The amplitude of the negativity
preceding the presentation of a Sternberg task was also
predictive of the subsequent short-term memory
perfor-mance (Morgan et al 1992). Concerning the antisaccade
task, in comparison to correct antisaccades erroneous
prosaccades were preceded by smaller slow negative
potential deflections at anterior central sites in humans
(Everling et al 1998) and by lower firing rates in the
supplementary eye field of monkeys (Schlag-Rey et al
1997). Furthermore, greater firing in build-up neurons of
the monkey superior colliculus was found before
errone-ous prosaccades compared with correct antisaccades
(Ev-erling et al 1998), this being possibly due to reduced
inhibition of these neurons by the cortical areas involved
in correct antisaccade generation. We could confirm these
results in the present study, although only with data of a
single patient due to the low absolute number of erroneous
prosaccades.
Compared to other studies, we found relatively low
error rates in patients and control subjects. Two reasons
may explain the discrepancy: First, although stimulus
conditions may influence task performance (Fischer and
Weber 1997), there is currently no standardized testing
protocol for antisaccade task studies, and error proportions
may vary greatly between different laboratories (reviewed
in Everling and Fischer 1998); second, all patients and
control subjects were “pretrained” by having participated
in an ocular motor experiment including a total of 240
antisaccade trials 3– 4 weeks prior to the study.
Perfor-mance during the antisaccade task may improve with
practice, and such effects could reduce differences
be-tween patients and control subjects if the initial error level
is low in control subjects. This is generally the case with
our experimental setup (Klein and Foerster, in press).
Neuroleptic Medication
Although most of our patients were receiving neuroleptic
medication when tested, previous reports revealed that
antisaccade task performance and neuroleptic medication
are not correlated (Clementz et al 1994; Fukushima et al
1990; Karoumi et al 1998; Sereno and Holzman 1995).
This was also the case in our study.
Conclusions
The present study revealed impaired ocular motor control
and ERP modulation in schizophrenic patients during the
antisaccade task which cannot be considered to be part of
a generalized deficit. The antisaccade task may, hence, be
a useful paradigm in the investigation of prefrontal
dys-functions in schizophrenic disorders.
Research was supported by the Deutsche Forschungsgemeinschaft (DFG; Kl 985/6-1).
The authors are grateful to two anonymous reviewers as well as Rolf Verleger and Rudolf Cohen for helpful comments on an earlier version of the manuscript.
References
Abraham P, McCallum WC (1976): The CNV and its relation to specific psychiatric syndromes. In: McCallum WC, Knott JR, editors. The Responsive Brain. Bristol, UK: Wright, 144 – 149.
Amador N, Schlag-Rey M, Schlag J, Sanchez H (1995): Supple-mentary eye field activity during monkey performance of antisaccadic tasks. Soc Neurosci Abstr 21:1195.
Anderson TJ, Jenkins IH, Brooks DJ, et al (1994): Cortical control of saccades and fixation in man: A PET study. Brain 117:1073–1084.
Andreasen NC, Rezai K, Alliger R, et al (1992): Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia. Arch Gen Psychiatry 49:943–958.
Andresen B, Moritz S (1999): Positive, and Negative, and
Disorganized Syndrome Scales for Schizophrenia (PAN-ADSS)/Manual. Westerau, Germany: PPV.
Berg P, Scherg M (1994): A multiple source approach to the correction of eye artifacts. Electroencephalogr Clin
Neuro-physiol 90:1–13.
Berman KF (1987): Cortical “stress tests” in schizophrenia: Regional cerebral blood flow studies. Biol Psychiatry 22: 1304 –1326.
Berman KF, Zec RF, Weinberger DF (1986): Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. II. Role of neuroleptic treatment, attention, and mental effort.
Arch Gen Psychiatry 43:126 –135.
Buchsbaum MS (1990): The frontal lobes, basal ganglia, and temporal lobes as sites for schizophrenia. Schizophr Bull 16:379 –389.
somato-sensory cortex neuroleptic drug effects: Differences between normal controls and schizophrenic patients. Biol Psychiatry 22:479 – 494.
Chapman LJ, Chapman JP (1978): The measurement of differ-ential deficit. J Psychiatr Res 14:303–311.
Clementz BA, McDowell JE, Zisook S (1994): Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol 103:277–287. Cohen R (1989): Event-related potentials and cognitive dysfunc-tion in schizophrenia. In: Haefner H, Gattaz WF, editors.
Search for the Causes of Schizophrenia. Berlin: Springer,
342–360.
Crawford TJ, Sharma T, Puri BK, Murray RM, Berridge DM, Lewis SW (1998): Saccadic eye movements in families multiply affected with schizophrenia: The Maudsley Family Study. Am J Psychiatry 155:1703–1710.
Danckert J, Maruff P, Pantelis C, Currie J (1998): Saccadic and attentional abnormalities in patients with schizophrenia.
Schizophr Res 29:115.
Darby DG, Nobre AC, Thangaraj V, et al (1996): Cortical activation in the human brain during lateral saccades using EPISTAR functional magnetic resonance imaging.
Neuroim-age 3:53– 62.
Denckla MB (1996): A theory and model of executive function: A neuropsychological perspective. In: Lyon GR, Krasnegor NA, editors. Attention, Memory, and Executive Function. Baltimore: Paul H. Brookes, 263–278.
Doricchi F, Perani D, Inoccia C (1997): Neural control of fast-regular saccades and antisaccades, an investigation using positron emission tomography. Exp Brain Res 116:50 – 62. Evdokimidis I, Liakopoulos D, Constantinidis TS, et al (1996):
Cortical potentials with antisaccades. Electroencephalogr
Clin Neurophysiol 98:377–384.
Evdokimidis I, Mergner T, Lucking CH (1992): Dependence of presaccadic cortical potentials on the type of saccadic eye movement. Electroencephalogr Clin Neurophysiol 83:179 – 191.
Everling S, Dorris MC, Munoz DP (1998): Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. J Neurophysiol 80:1584 –1589.
Everling S, Fischer B (1998): The antisaccade: A review of basic research and clinical studies. Neuropsychologia 36:885– 899. Everling S, Krappmann P, Flohr H (1997): Cortical potentials preceding pro- and antisaccades in man. Electroencephalogr
Clin Neurophysiol 102:356 –362.
Everling S, Spantekow A, Krappmann P, et al (1998): Event-related potentials associated with correct and incorrect re-sponses in a cued antisaccade task. Exp Brain Res 118:27–34. Fischer B, Gezeck S, Hartnegg K (1997): The analysis of saccadic eye movements from gap and overlap paradigms.
Brain Res Protocols 2:47–52.
Fischer B, Weber H (1997): Effects of stimulus conditions on the performance of antisaccades in man. Exp Brain Res 116:191– 200.
Frith CD, Friston K, Liddle PF, et al (1991): Willed action and the prefrontal cortex in man: A study with PET. Proc R Soc
Lond B 244:241–246.
Fukushima J, Fukushima K, Chiba T, et al (1988): Disturbances
of voluntary control of saccadic eye movements in schizo-phrenic patients. Biol Psychiatry 23:670 – 677.
Fukushima J, Fukushima K, Morita N, et al (1990): Further analysis of the control of voluntary saccadic eye movements in schizophrenic patients. Biol Psychiatry 28:943–958. Funahashi, S, Chafee MV, Goldman-Rakic PS (1993): Prefrontal
neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature 365(6448):753–756.
Fuster J (1984): Behavioural electrophysiology of the prefrontal cortex. Trends Neurosci 23:408 – 414.
Fuster JM (1985): The prefrontal cortex, mediator of cross-temporal contingencies. Hum Neurobiol 4:169 –179. Fuster JM (1989): The Prefrontal Cortex: Anatomy, Physiology
and Neuropsychology of the Frontal Lobe, 2nd ed. New
York: Raven.
Goldman-Rakic PS (1987): Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, Mountcastle V, editors. Handbook of Physiology:
The Nervous System. Baltimore: Wiliams and Wilkins, 373–
417.
Go´mez C, Atienza M, Go´mez GJ, et al (1996): Response latencies and event-related potentials during the gap paradigm using saccadic responses in human subjects. Int J
Psycho-physiol 23:91–99.
Guitton D, Buchtel HA, Douglas RM (1985): Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain
Res 58:455– 472.
Hallett PE (1978): Primary and secondary saccades to goals defined by instructions. Vision Res 18:1279 –1296.
Hallett PE, Adams BD (1980): The predictability of saccadic latency in a novel voluntary oculomotor task. Vision Res 20:329 –339.
Hamano T, Luders HO, Ikeda A, et al (1997): The cortical generators of the contingent negative variation in humans: A study with subdural electrodes. Electroencephalogr Clin
Neu-rophysiol 104:257–268.
Hiller W, Dichtl G, Hecht H, et al (1993a): An empirical comparison of diagnoses and reliabilities in ICD-10 and DSM-III-R. Eur Arch Psychiatr Clin Neurosci 242:209 –217. Hiller W, Zaudig M, Mombour W, et al (1993b): Routine psychiatric examinations guided by ICD-10 diagnostic check-lists (international diagnostic checkcheck-lists). Eur Arch Psychiatr
Clin Neurosci 242:218 –223.
Ikeda A, Lueders HO, Collura TF (1996): Subdural potentials at orbitofrontal and mesial prefrontal areas accompanying an-ticipation and decision making in humans: A comparison with bereitschaftspotential. Electroencephalogr Clin Neurophysiol 98:206 –212.
Ikeda A, Shibasaki H, Kaji R, et al (1997): Dissociation between contingent negative variation (CNV) and Bereitschaftspoten-tial in patients with Parkinsonism. Electroencephalogr Clin
Neurophysiol 102:142–151.
Ikeda A, Shibasaki H, Nagamine T (1994): Dissociation between contingent negative variation and Bereitschaftspotential in a patient with cerebellar efferent lesion. Electroencephalogr
Clin Neurophysiol 90:359 –364.
Kandel ER, Schwartz JH, Jessell TM (1991): Principles of
Karoumi B, Ventre-Dominey J, Vighetto A, et al (1998): Saccadic eye movements in schizophrenic patients. Psychiatr
Res 77:9 –21.
Klein C (1997): The Post-Imperative Negative Variation in
Schizophrenic Patients and Healthy Subjects. Frankfurt: Peter
Lang.
Klein C, Cohen R, Berg P, et al (1996): Determinants of the post-imperative negative variation in schizophrenic patients and controls. Schizophr Res 21:97–110.
Klein C, Foerster F (in press): Development of pro- and antisac-cade performance in participants aged 6 to 26 years.
Psycho-physiology.
Klostermann W, Koempf D, Heide W, et al (1994): The presaccadic cortical negativity prior to self-paced saccades with and without visual guidance. Electroencephalogr Clin
Neurophysiol 91:219 –228.
Knott JR, Peters JF, Robinson MD, et al (1976): The contingent negative variation (CNV) in schizophrenic and depressed patients. Electroencephalogr Clin Neurophysiol 40:329 –330. Kornhuber HH, Deecke L (1965): Hirnpotentialaenderungen bei Willkuerbewegungen und passiven Bewegungen des Men-schen: Bereitschaftspotential und reafferente Potentiale.
Pflugers Arch 284:1–17.
Lamarche M, Louvel J, Buser P, et al (1995): Intracerebral recordings of slow potentials in a contingent negative varia-tion paradigm: An exploravaria-tion in epileptic patients.
Electro-encephalogr Clin Neurophysiol 95:268 –276.
Lewis SW, Ford RA, Syed GM, et al (1992): A controlled study of 99m-Tc-HMPAO single-photon emission imaging in chronic schizophrenia. Psychol Med 22:27–35.
McCallum WC, Abraham P (1973): The contingent negative variation in psychosis. Electroencephalogr Clin Neurophysiol
Suppl 33:329 –335.
McCarthy G, Wood CC (1985): Scalp distributions of event-related potentials: An ambiguity associated with analysis of variance models. Electroencephalogr Clin Neurophysiol 62: 203–208.
McDowell JE, Clementz BA (1997): The effect of fixation condition manipulations on antisaccade performance in schizophrenia: Studies of diagnostic specificity. Exp Brain
Res 115:333–344.
Melamed E, Larsen B (1979): Cortical activation pattern during saccadic eye movement in humans: Localization by focal cerebral blood flow increases. Ann Neurol 5:79 – 88. Mokler A, Fischer B (1999): The recognition and correction of
involuntary prosaccades in an antisaccade task. Exp Brain Res 125:511–516.
Morgan JM, Wenzl M, Lang W, Lindinger G, Deecke L (1992): Frontocentral DC-potential shifts predicting behavior with and without a motor task. Electroencephalogr Clin
Neuro-physiol 83:378 –388.
Moritz S, Andresen B, Jacobsen D, et al (2000): Neurokognitive Korrelate des Drei-Faktoren-Modells der Schizophrenie und Schizotypie/Evaluation eines Instrumentes zur Erfassung schizophrener Symptomatik (PANADSS). In: Andresen B, Mass R, editors. Schizotypie. Psychometrische und
biopsy-chologische Forschungsansaetze. Goettingen, Germany: Hogrefe, 571–584.
Nakashima Y, Momose T, Sano I, et al (1994): Cortical control
of saccade in normal and schizophrenic subjects: A PET study using task-evoked rCBF paradigm. Schizophr Res 12:259 –264.
Neshige R, Luders H, Shibasaki H (1988): Recording of move-ment-related potentials from scalp and cortex in man. Brain 111:719 –736.
Nkam I, Thibaut F, Denise P, Levillain D, Segard L, Langlois-Thery S (1998): Saccadic eye movements and Wisconsin Card Sorting Test in deficit and nondeficit schizophrenia.
Schizophr Res 29:117.
O’Driscoll GA, Alpert NM, Matthysse SW, et al (1995): Func-tional neuroanatomy of antisaccade eye movements investi-gated with positron emission tomography. Proc Natl Acad Sci
U S A 92:925–929.
Paulman RG, Devous MD, Gregory RR, Herman JH, Jennings L, Bonte FJ, et al (1990): Hypofrontality and cognitive impair-ment in schizophrenia: Dynamic single-photon tomography and neuropsychological assessment of schizophrenic brain function. Biol Psychiatry 27:377–399.
Pennington BF, Ozonoff S (1996): Executive functions and the developmental child psychopathology. J Child Psychol
Psy-chiatry 37:51– 87.
Perrin F, Pernier J, Bertrand O, et al (1989): Spherical spline for scalp potential and current density mapping.
Electroencepha-logr Clin Neurophysiol 72:184 –187.
Petit L, Orssaud C, Tzourio N, et al (1993): PET study of voluntary saccadic eye movements in humans: Basal ganglia-thalamocortical system and cingulate cortex involvement.
J Neurophysiol 69:1009 –1017.
Pierrot-Deseilligny C, Rivaud S, Gaymard B, et al (1991): Cortical control of reflexive visually-guided saccades. Brain 114:1473–1485.
Pierrot-Deseilligny C, Rivaud S, Gaymard B, et al (1995): Cortical control of saccades. Ann Neurol 3:557–567. Rektor I, Feve A, Buser P, et al (1994): Intracerebral recording
of movement related readiness potentials: An exploration in epileptic patients. Electroencephalogr Clin Neurophysiol 90: 273–283.
Reuter-Lorenz PA, Oonk HM, Barnes LL, et al (1995): Effects of warning signals and fixation point offsets on the latencies of pro- versus antisaccades: Implications for an interpretation of the gap effect. Exp Brain Res 103:287–293.
Rivaud S, Muri RM, Gaymard B, et al (1994): Eye movement disorders after frontal eye field lesions in humans. Exp Brain
Res 102:110 –120.
Roberts RJ, Hager LD, Heron C (1994): Prefrontal cognitive processes: Working memory and inhibition in the antisaccade task. J Exp Psychol Gen 123:374 –393.
Rockstroh B, Elbert T, Canavan A, et al (1989): Slow Cortical
Potentials and Behaviour, 2nd ed. Baltimore: Urban and
Schwarzenberg.
Rockstroh B, Mueller M, Wagner M, et al (1994): Event-related and motor responses to probes in a forewarned reaction time task in schizophrenic patients. Schizophr Res 13:23–34. Roesler F (1991): Perception or action: Some components on
Rosse RB, Schwartz BL, Kim SY, et al (1993): Correlation between antisaccade and Wisconsin Card Sorting Test per-formance in schizophrenia. Am J Psychiatry 150:333–335. Sakamoto A, Lueders H, Burgess R (1991): Intracranial
record-ing of movement-related potentials to voluntary saccades.
J Clin Neurophysiol 8:223–233.
Sasaki K, Gemba H (1991): Cortical potentials associated with voluntary movements in monkeys. In: Brunia CMH, Mulder G, Verbaten MN, editors. Event-Related Brain Research. Amsterdam: Elsevier Science, 80 –96.
Schall JD (1991): Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: Com-parison with supplementary eye rields. J Neurophysiol 66: 559 –579.
Schlag J, Schlag-Rey M (1987): Evidence for supplementary eye field. J Neurophysiol 57:179 –200.
Schlag-Rey M, Amador N, Sanchez H, et al (1997): Antisaccade performance predicted by neuronal activity in the supplemen-tary eye field. Nature 390:398 – 401.
Schlenker R, Cohen R (1995): Smooth pursuit eye-movement dysfunction and motor control: A follow-up study. Eur Arch
Psychiatr Clin Neurosci 245:125–126.
Sereno AB, Holzman PS (1995): Antisaccades and smooth pursuit eye movements in schizophrenia. Biol Psychiatry 37:394 – 401.
Sweeney JA, Mintun MA, Kwee S, et al (1996): Positron emission tomography study of voluntary saccadic eye
move-ments and spatial working memory. J Neurophysiol 75:454 – 468.
Tecce JJ, Cole JO (1976): The distraction-arousal hypothesis, CNV, and schizophrenia. In: Mostofsky DJ, editor.
Behav-ioral Control and Modification of Physiological Activity.
London: Prentice Hall, 162–220.
Timsit-Berthier M, Gerono M, Rousseau J, et al (1984): An international pilot study of CNV in mental illness—second report. In: Karrer R, Cohen J, Tueting P, editors. Brain and
Information. New York: New York Academy of Science,
629 – 637.
van den Bosch RJ (1983): Contingent negative variation and psychopathology: Frontal-central distribution, and association with performance measures. Biol Psychiatry 18:615– 634. Walker R, Husain M, Hodgson TL, et al (1998): Saccadic eye
movements and working memory deficits following damage to human prefrontal cortex. Neuropsychologia 36:1141–1159. Walter WG (1964): The contingent negative variation: An electrical sign of significance of association in the human brain. Science 146:434.
Weerts TC, Lang PJ (1973): The effects of eye fixation and stimulus and response location on the contingent negative variation (CNV). Biol Psychol 1:1–19.
Weinberger DR, Berman KF, Zec RF (1986): Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen