The Effects of Steroid Hormones in HIV-Related
Neurotoxicity: A Mini Review
Sheila M. Brooke and Robert M. Sapolsky
This review examines the interaction of steroid hormones,
glucocorticoids and estrogen, and gp120, a possible
causal agent of acquired immune deficiency syndrome–
related dementia complex. The first part of the review
examines the data and mechanisms by which gp120 may
cause neurotoxicity and by which these steroid hormones
effect cell death in general. The second part of the review
summarizes recent experiments that show how these
ste-roid hormones can modulate the toxic effects of gp120 and
glucocorticoids exacerbating toxicity, and estrogen
de-creasing it. We then examine the limited in vivo and
clinical data relating acquired immune deficiency
syndro-me–related dementia complex and steroid hormones and
speculate on the possible clinical significance of these
findings with respect to acquired immune deficiency
syn-drome–related dementia complex.
Biol Psychiatry 2000;
48:881– 893 ©
2000 Society of Biological Psychiatry
Key Words:
gp120, HIV, glucocorticoids, estrogen,
AIDS-related dementia (ADC), review
Introduction
A
mong patients with acquired immune deficiency
syn-drome (AIDS), AIDS-related dementia complex
(ADC) is a neurologic disorder that develops in the later
stages of AIDS in a small but significant number of
patients (Bacellar et al 1994; Budka 1991; Price et al
1988). It has been characterized by a variety of neurologic
and neuropsychologic impairments, including loss of
memory, confusion, motor deficits, delayed development,
and a wide range of behavioral abnormalities. There are
indications of neuropathologic changes and cell loss in
cortical and subcortical regions that may account for these
behavioral manifestations (Epstein and Gelbard 1999;
Gendelman et al 1994; Glass and Johnson 1996; Masliah
et al 1992; Michaels et al 1988; Price et al 1988). In some
cases, it has proven difficult to directly correlate the extent
of neuron loss with the severity of symptoms in ADC,
suggesting that dysfunction of neurons, rather than overt
neuron death, also may be an aspect of ADC
(Adle-Biassette et al 1999; Bell 1998; Everall et al 1999).
Because most readers are familiar with the broad aspects
of the disease, we will limit our discussion on the matter,
and refer the reader to recent reviews (Rafalowska 1998;
Saksena et al 1998; Vitkovic and Tardieu 1998).
HIV does not appear to infect and kill neurons directly
(Michaels et al 1988). Although glia (the other principal
cell type of the central nervous system [CNS]) can be
infected, the extent is insufficient to account for the cell
damage observed. The virus may enter the CNS in
association with macrophages or by disrupting the blood
brain barrier, however (Annunziata et al 1998; Haase
1986; Persidsky et al 1997b; Sharer 1992). Therefore, the
virus can be present in the brain within microglia and
macrophages (the disease-fighting proinflammatory cells
of the CNS), from whence it may cause neuron death and
dysfunction (Giulian et al 1990; Lipton 1994; Ohagen et al
1999; Pulliam et al 1993; Tan and Guiloff 1998).
In the first part of this review, we briefly summarize
current knowledge about the mechanisms underlying the
adverse effects of HIV on neurons, focusing on the actions
of gp120. This is a prelude to the core of this review,
which considers the modulatory effects of two steroid
hormones on the actions of gp120.
gp120
There are relatively few effective treatments for dementia
and prevention by impeding the initial neuron death or
dysfunction is perhaps the most promising treatment for
ADC. Because the intact virus does not infect and destroy
cells in the brain directly, the causal agents of
neurotox-icity were hypothesized to be factors that originated from
HIV, such as gp120, the major envelope glycopolypetptide
of HIV that acts as the binding protein for viral entry. It is
formed from the cleavage of gp160, leaving a gp41
fragment and can be readily shed to become a soluble
protein (Gelderblom et al 1987; Pahwa et al 1985;
Schnei-der et al 1986). The neurotoxicity of gp120 was first
demonstrated (Brenneman et al 1988) in mice retinal and
hippocampal tissue and then confirmed in human cortical
tissue (Pulliam et al 1991), and rodent spinal cord,
hippocampus, cortex, cerebellum, and retinal ganglion
with LD50’s in the picomolar range (Aggoun-Zouaoui et
al 1996; Bagetta et al 1996b; Barks et al 1997; Corasaniti
From the Department of Biological Sciences, Stanford University, Stanford, California.
Address reprint requests to Sheila M. Brooke, Stanford University, Department of Biological Sciences, Stanford CA 94305.
Received January 5, 2000; revised April 17, 2000; accepted May 2, 2000.
et al 1998a; Dawson et al 1993; Diop et al 1994; Dreyer et
al 1990; Lipton 1994; Savio and Levi 1993). Other HIV
proteins that are also toxic in vitro are gp160 (Lannuzel et
al 1995; Sasaki et al 1996), gp41 (Adamson et al 1996;
Kort 1998; Wiley et al 1994), and the nuclear protein Tat
(Bartz and Emerman 1999; Cheng et al 1998; Wang et al
1999). All of these initial demonstrations of gp120
neuro-toxicity were done in vitro.
The neurotoxic effects of gp120 in vivo after
intrahip-pocampal injection of nanomolar quantities have been less
consistent than in vitro, with demonstrations of such
toxicity in neonatal and adult rats (Glowa et al 1992; Hill
et al 1993), lack of toxicity in fetal rats (Bussiere et al
1999), and toxicity only in conjunction with other
neuro-toxins (Barks et al 1997). These differences may arise
because of technical difficulties in the handling of gp120,
which is labile and easily sticks to plastics; differences in
age of rats; or, more likely, gp120 from different sources.
Some of the most convincing in vivo data linking gp120 to
neuronal toxicity have been reported from transgenic mice
that express gp120. These animals show neuronal
degen-eration similar to that seen in human ADC, as well as
disruption of calcium homeostasis in cells (Krucker et al
1998; Toggas et al 1994, 1996; Wyss-Coray et al 1996).
One of the main arguments against gp120 being involved
in neurotoxicity associated with ADC is the fact that
gp120 has not been detectable in brain. A recent paper
described a breakthrough in determination of gp120 levels
in the CNS (Altmeyer et al 1999). Another recent study
has shown that there is a correlation between severity of
dementia and the amount of gp41 in the brain after autopsy
of HIV patients (Adamson et al 1999). Therefore, we
would hope soon to have definitive answers on the
relationship between HIV coat proteins and ADC.
With the demonstration of gp120 neurotoxicity in the
brains of animals has come an understanding of several
mechanism by which gp120 can cause neuron death. The
first mechanism involves the release of cytokines, such as
TNF-
a
, and members of the interleukin family by
macro-phages and microglia as part of an inflammatory response
that alerts the immune system to a foreign body.
Research-ers have reasonably documented that gp120 releases such
cytokines in the periphery with increasing evidence for
similar effects within the CNS (Adamson et al 1996; Ilyin
and Plata-Salaman 1997; Koka et al 1995; Kong et al
1996; Wiley et al 1994; Yeung et al 1998). Paradoxically,
within the CNS these compounds can kill the neurons they
were released to protect (Bjugstad et al 1998; Ensoli et al
1999; Jeohn et al 1998; Van der Meide and Schellekens
1996; Yeung et al 1998). As part of the signaling
mech-anism of the cytokines, cytosolic calcium concentrations
can be increased, with potentially neurotoxic
conse-quences (see below). At present it remains unclear the
extent to which gp120 effects on cytokine and interleukin
release in the CNS contributes to neuotoxicity.
A second route of damage may involve a potent
excitatory neurotransmitter system involving the
N
-meth-yl-
D-aspartate (NMDA) receptor (Diop et al 1994; Lipton
1994; Lipton et al 1991; Pittaluga et al 1996; Sweetnam et
al 1993). Excitatory amino acids (EAA), such as
gluta-mate, as well as its agonists kainic acid and NMDA can be
neurotoxic. Glutamate, in addition to its use in its
tradi-tional role for metabolism and protein synthesis within the
cell is used in the CNS for synaptic transmission. Under
normal circumstances, glutamate may be involved in
several synaptic functions that result in an influx of
calcium into the cell, most notably the synaptic plasticity,
which is thought to underlie learning. Overstimulation at
the synapse by EAAs results in excess calcium entering
the cell, however, initiating a cascade of oxygen radical
production, lipid peroxidation, and promiscuous
stimula-tion of proteases and nucleases; all of these factors can
ultimately culminate in neuron death (reviewed in Choi
1992). Several aspects of the EAA cascade are manifested
by gp120 toxicity: it can increase cytosolic calcium in
cells (Ciardo and Meldolesi 1993; Codazzi et al 1995;
Diop et al 1994; Dreyer et al 1990; Lannuzel et al 1995;
Lo et al 1992; Nath et al 1995), its neurotoxicity is
inhibited by NMDA receptor antagonists (Barks et al
1997; Lipton et al 1991; Pittaluga et al 1996; Toggas et al
1996) and it increases oxygen radicals levels and lipid
peroxidation (Corasaniti et al 1998b; Foga et al 1997).
Because gp120 itself does not act directly on NMDA
receptors, it has been hypothesized that gp120 causes the
release from macrophages and glia of an as yet
unidenti-fied NMDA receptor agonist, causing EAA neurotoxicity.
This unknown substance has been speculated to be
quino-linic acid (Kerr et al 1997), arachidonic acid (Dreyer and
Lipton 1995; Genis et al 1992; Schroder et al 1998), nitric
oxide (Adamson et al 1996; Bagetta et al 1997; Mollace et
al 1994) or perhaps even glutamate because gp120 appears
to increase release and block its reuptake (Dreyer and
Lipton 1995; Vesce et al 1997).
There may also be some directly endangering effects of
gp120 on neurons. The glycoprotein can mimic the amino
acid glycine (which has a neuromodulatory function at
NMDA receptors), enhancing their activity (Pattarini et al
1998; Pittaluga et al 1996). In addition, gp120 may also
act on neuronal chemokine receptors, initiating a calcium
cascade leading to neuron death, as previously described
(Blanco et al 1999; Herbein et al 1998; Hesselgesser et al
1998; Kaul and Lipton 1999; Madani et al 1998; Meucci et
al 1998).
Glia perform primarily a supporting role for neurons such
as uptake of toxic substances, regulation of extracellular
pH, acting as a sink for potassium, releasing neuroactive
substances necessary for repair, supplying nutrition and
responding to injury. Glia also appear susceptible to gp120
toxicity, possibly via the chemokine receptor (Benos et al
1994; Bernardo et al 1994; Ciardo and Meldolesi 1993;
Codazzi et al 1995, 1996; Kort 1998; Pulliam et al 1993;
Shrikant et al 1996). Given the key supporting role of glia,
their loss or dysfunction could result in increased neuron
death.
Other routes by which gp120 alters cell activities are
increased expression of nitric oxide synthase (Bagetta et al
1997) decreased release of the neurotransmitter
noradren-aline (Pittaluga et al 1996), decreased expression of nerve
growth factor (Bagetta et al 1996a), and decreased glucose
utilization (Kimes et al 1991), any of which could
com-promise neuronal function or viability.
Based on the gp120’s putative mechanism of action, a
number of categories of agents have been proposed to
prevent gp120 neurotoxicity, such as NMDA and
chemo-kine receptor blockers, antioxidants, calcium binding
com-pounds, and neuroprotective peptides and cytokines
(Dib-bern et al 1997; Diop et al 1995, 1996; Foga et al 1997;
Meucci and Miller 1996; Miller and Meucci 1999; Uchida
et al 1996). The first clinical trials aimed at relieving ADC
symptoms without using antiviral medication also have
been reported (Navia et al 1998). The calcium channel
blocker nimodipine was given with encouraging results to
AIDS patients who were exhibiting signs of ADC and
neuropathy. For more detailed account of possible
mech-anisms and treatments for gp120 neurotoxicity and ADC
the reader is referred to a recent review (Lipton 1998).
Along with the knowledge of the basic mechanisms by
which gp120 causes neurotoxicity, and perhaps ADC, has
come an appreciation of extrinsic factors that can
modu-late the neurotoxicity of gp120. We now review the
relatively recent findings regarding two of these
modula-tors, both of which may have some clinical significance.
The two hormones to be considered are glucocorticoids
(GCs), which appear to exacerbate neurotoxicity, and
estrogen, which may be neuroprotective.
Glucocorticoids and Neurotoxicity
Glucocorticoids are steroid hormones secreted by the
adrenal glands primarily in response to physical and
psychologic stress. The GCs include natural hormones,
such as cortisol (hydrocortisone) in humans and other
primates and corticosterone (CORT) in rats, as well as
synthetic analogues such as dexamethasone, prednisone,
and triamcinolone. The release and regulation of naturally
occurring steroids are controlled by the hypothalamic–
pituitary–adrenal axis (HPA). In the short term and
pri-marily in the peripheral systems, GCs can be beneficial to
surviving a major physical stressor. They mobilize energy
(primarily to muscle), help increase cardiovascular tone,
and enhance cognition, all functions necessary for an
organism to cope with a stressful crisis. To conserve
energy for these tasks, unessential activities such growth,
digestion, reproduction, and immunity are turned off. If
the stressful situation is prolonged, however, as with many
human psychologic stressors, GCs can have adverse
ef-fects throughout the body, including hypertension,
repro-ductive impairment, ulceration, increased infectious
dis-ease risk, and cognitive impairment (Munck et al 1984).
We will first briefly examine how GCs can effect neuron
survival in general within the CNS before considering how
they exacerbate gp120-induced neurotoxicity.
Although GCs can endanger a number of brain regions,
the hippocampus, a brain region rich in corticosteroid
receptors (Jacobson et al 1993; Joels and de Kloet 1994),
is most susceptible to GCs exacerbation of neurotoxicity.
This region, which plays a key role in learning and
memory, is vulnerable to a number of neurotoxic
condi-tions that are exacerbated by GCs, including toxins such as
glutamate, kainic acid, the antimetabolite 3NP, cyanide,
ischemia, and hypoglycemia, and oxygen radical
genera-tors (reviewed in Chang et al 1998; Sapolsky 1996). Under
conditions in which GCs themselves are not toxic, they
appear to prime the system so that exposure to another
insult results in increased neuronal death.
1996). Moreover, energy supplementation blunts these
effects and results in decreased neuron death (Sapolsky
1996).
Glucocorticoids can also inhibit glucose transport in
glia (Horner et al 1990). In their supportive role, glia also
require energy, especially during an insult. If the glia are
compromised, they cannot provide adequate maintenance
for the neurons, resulting in a greater likelihood of death of
the latter.
Given GCs ability to exacerbate neurotoxicity insults
that act via the same EAA and calcium excess pathways as
gp120, we will now examine the literature showing that
GCs can also increase gp120 neurotoxicity.
Glucocorticoids and gp120 Neurotoxicity
The exacerbation of gp120 neurotoxicity by GCs has been
demonstrated in primary hippocampal, cortical, and
stria-tal cultures of rat (Brooke et al 1997; Iyer et al 1998).
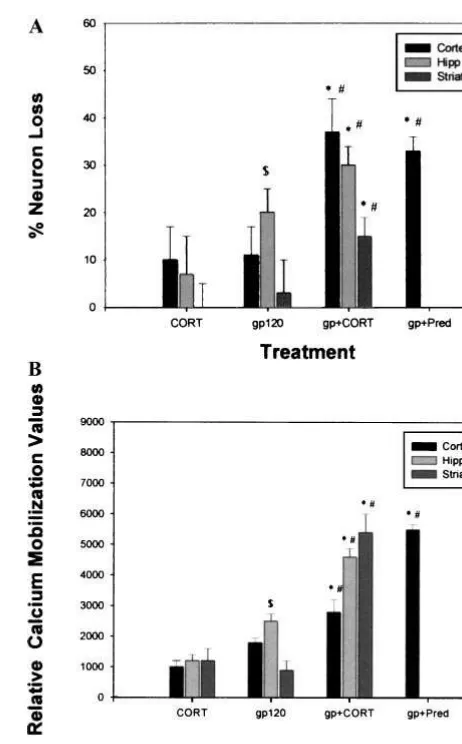
Figure 1A is a summary of these data showing that in all
three tissues there is a synergistic neurotoxicity between
gp120 and the GCs corticosterone (CORT). Increased
toxicity was shown to occur at 100 nmol/L to 1
m
mol/L
concentration of CORT (at what are considered to be high
physiologic levels). At cortisol levels of 10 and 1 nmol/L
in the normal to low range of GC levels, however, there
was no exacerbation of gp120 neurotoxicity by GCs
(Brooke et al 1997). In fact, gp120 alone is not always
toxic, but there is consistently a loss of neurons with
gp120 in the presence of CORT. Perhaps greater clinical
significance, the synthetic GCs prednisone (Figure 1A)
and dexamethasone also enhance gp120 neurotoxicity
(Brooke et al 1997).
A gp120-induced rise in cytosolic calcium, whether via
the NMDA or chemokine receptor, may in part explain its
neurotoxicity. Increased calcium mobilization with gp120
is exacerbated in the presence of CORT in cortical,
striatal, and hippocampal cultures (summarized in Figure
1B; Brooke et al 1997; Iyer et al 1998). Moreover, GCs
also worsen gp120-induced calcium mobilization in
hip-pocampal and cortical slices, particularly in the CA1 and
CA3 cell fields of the hippocampus (Yusin et al 2000).
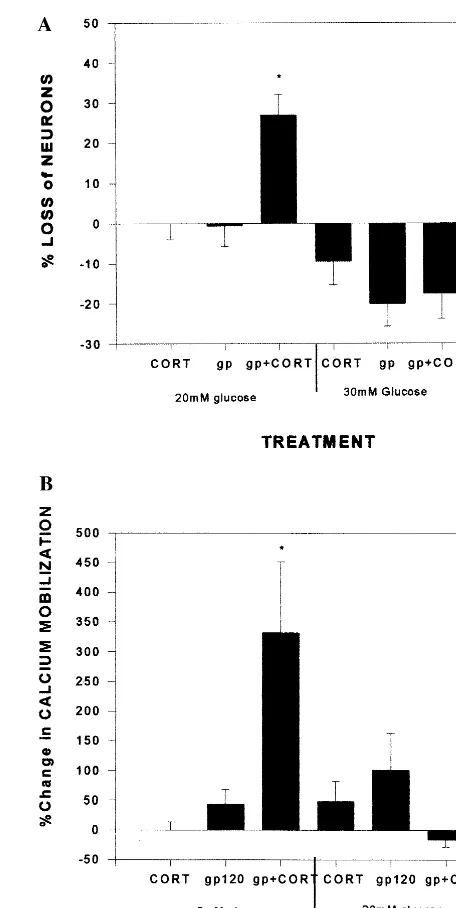
A downstream effect of increased calcium mobilization
is oxygen radical production and lipid peroxidation, and
GCs worsen this gp120 effect as well, both in hippocampal
(Figure 2) and cortical cultures (Howard et al 1999).
Nonetheless, these studies indicate that there is not a
simple GCs exacerbation of gp120 effects on oxygen
radical accumulation and subsequent lipid peroxidation. It
would appear that GCs and gp120 have different but
perhaps accumulating effects on various aspects of this
neurotoxic pathway. The reader is referred to Howard and
colleagues (Howard et al 1999) paper for more detailed
descriptions.
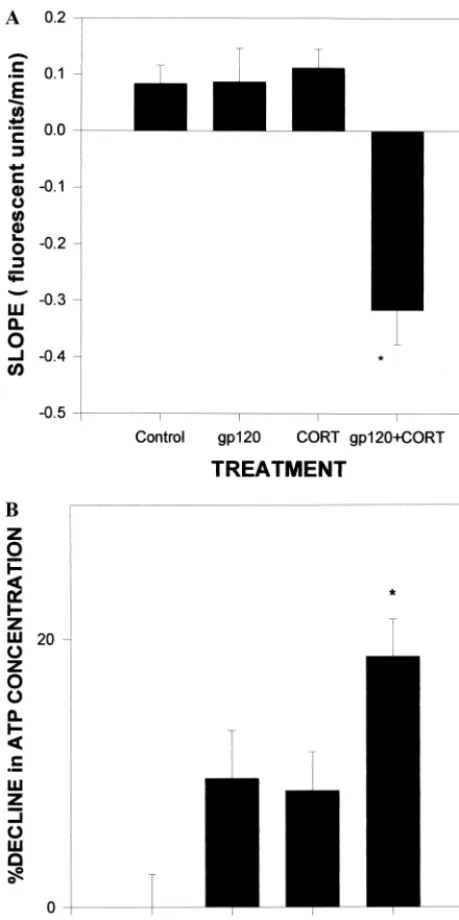
As previously mentioned, the exacerbating properties of
GCs may be caused by a disruption of energy stores, and
this also appears to be true for gp120 neurotoxicity. It has
been confirmed that there is a correlation between energy
availability and the enhanced neuron death from gp120 in
the presence of CORT. The synergistic effects of gp120
and CORT on neurotoxicity and calcium mobilization
could be reversed with glucose supplementation (Brooke
et al 1998). The rationale for excess energy requirements
Figure 1. Summary of data from Brooke et al 1997 and Iyer et al 1998 showing the relative increase in(A)neurotoxicity and
(B)calcium mobilization in cortical, hippocampal, and striatal primary mixed cultures treated with gp120 (200 pmol/L) in the presence of corticosterone (CORT; 1mmol/L), and prednisone (Pred; 1 nmol/L), compared with the agents alone. *Significantly different from CORT.#Significantly different from gp120.$
is summarized in Figure 3, in which CORT is shown to
significantly worsen the disruptive effects of gp120 on
ATP levels and mitochondrial potential (Brooke et al
1998).
We have also observed that CORT exacerbates the
gp120 disruption of metabolism in hippocampal slices
(Figure 4). Using a microphysiometer (which indirectly
measures metabolic rate in real time in tissue), we
ascer-tained metabolic rates in slices from normal,
adrenalecto-mized, CORT-treated, or prednisone-treated rats. After
challenge with gp120, slices from CORT-treated and
prednisone-treated rats had significantly decreased
meta-bolic rates in the CA1 region and dentate nucleus of the
hippocampus and in the cortex, compared with slices from
intact and adrenalectomized rats (Yusin et al 2000).
These in vitro studies suggest that GCs augment the
neurotoxicity of gp120. If gp120 is a factor in the
development of the neuropathology of HIV infection, then
GCs may be a factor in its manifestation. Other studies
have been done in vivo to support work relating GCs to
HIV-related neuropathology.
Gendelman and colleagues have developed a model of
ADC by injecting severe combined immunodeficient
(SCID) mice with HIV-infected monocytes, producing an
HIV-encephalitis with neuropathologic features
reminis-cent of that seen in HIV patients with dementia
(Avgero-poulos et al 1998; Persidsky and Gendelman 1997;
Per-sidsky et al 1995, 1997a; Tyor et al 1993). The original
hypothesis was that these neuropathologic changes were
due to the release of inflammatory compounds in response
to the encephalitis, so the researchers attempted to reverse
the neurotoxicity with dexamethasone treatment. Synthetic
Figure 2. Increase in lipid peroxidation in mixed hippocampalcultures when insulted with gp120 (200 pmol/L) plus corticoste-rone (CORT; 1mmol/L), compared with gp120 alone. *Signifi-cantly different from control substance.#Significantly different from gp120. The data are from experiments carried out on 6 weeks of different tissue culture preparations. Within each week, experiments were performed on at least four plates of cells, each containing wells with all the experimental conditions. (Modified, and reproduced with permission, from Howard et al 1999.)
GCs such as dexamethasone and prednisone are used
extensively as antiinflammatory drugs because they
pre-vent the formation and action of cytokines. Contrary to
expectations, neuron loss was greatly enhanced by
dexa-methasone, leading the authors to conclude that the results
suggested the need for caution in administering
glucocor-ticoids for treatment of HIV encephalitis in humans and
consider nonsteroidal antiinflammatory agents
(Gendel-man et al 1998; Limoges et al 1997).
Another report (Bressler et al 1993), although not
showing increased neurotoxicity, did demonstrate that
GCs enhance the production of TNF-
a
in the brain, a
possible route of toxicity of gp120. Because of these
findings those authors likewise cautioned about the use of
GCs with AIDS patients.
In the sole relevant human study, Oberfield et al (1994)
examined HIV-infected children who had high levels of
cortisol, a condition that sometimes accompanies HIV
infection, and found that hypercortisolemia was predictive
of neurologic abnormalities (Oberfield et al 1994).
Be-cause of the clinical implications, they suggested further
studies to confirm these results. Therefore, from the data
presented, GCs exacerbation of the neurotoxic effects of
gp120 is sufficient to warrant concern.
Glucocorticoids and HIV
It has been demonstrated that gp120 raises GCs levels by
interfering with the HPA axis in rodents (Raber et al
1996). It appears that HIV also effects the HPA axis in
humans because about 40% of AIDS patients (Coodley et
al 1994; Corley 1995; Lortholary et al 1996) have
in-creased endogenous cortisol concentrations and 20% show
insufficient adrenal function and lowered cortisol levels
(Norbiato et al 1994; Piedrola et al 1996). If GCs do
indeed worsen gp120 neurotoxicity, then there is the
potential for a negative neurotoxic cascade in those
pa-tients with hypercortisolemia; however, studies to
deter-mine the clinical significance of these altered levels of
cortisol with respect to cognitive abilities have not been
done. Only one study could be mentioned here that
perhaps is relevant (Gorman et al 1991). There was a small
but significant correlation between cortisol level in HIV
patients and increased levels of depression and anxiety
that was not evident in non-HIV patients with similar
levels. Depression could have a further deleterious,
neu-rotoxic effect in HIV patients. Because GCs appear to be
a factor in increased gp120 neurotoxicity in vivo studies,
it is perhaps reasonable to at least consider that GCs may
be a factor in ADC.
Nonetheless, the use of the synthetic GCs, such as
prednisone, in the treatment of complications associated
with AIDS also would result in increased GC levels. There
are many instances of AIDS-related diseases in which the
use of GCs is recommended, including such conditions as
HIV-induced high-grade non-Hodgkins lymphoma,
wast-ing syndrome, nephropathy, optic neuropathy, and
idio-Figure 4. Decreases in(A) mitochondrial membrane potentialand(B)adenosine triphosphate (ATP) levels in mixed hippocam-pal cultures after insult with gp120 (200 pmol/L) and corticoste-rone (CORT; 1mmol/L. *gp1201CORT significantly different from control substance and gp120 and CORT alone (A, p ,
pathic colonic inflammation (Gopal et al 1997; Jager
1994; Kimmel et al 1998; Lu et al 1995; Machet et al
1996; Watterson et al 1997). The most significant use of
GCs is in the treatment of
pneumocystis carinii
pneumo-nia, the most common opportunistic infection associated
with AIDS. High doses of prednisone (60 –160 mg/day;
Bozzette et al 1990; Gagnon et al 1990; Miller et al 1996;
Pareja et al 1998; Smith et al 1996; Urakami et al 1997)
are common. There have even been reports of using 300 to
1000 mg/day of prednisone and hydrocortisone (Frey and
Speck 1992; Montaner et al 1989) for at least 7 days with
repeat treatment lasting up to 26 weeks. Experimental
administration of far lower doses of GCs to healthy human
volunteers causes significant cognitive impairments
(New-comer et al 1991, 1994, 1998; Wolkowitz et al 1990,
1997). Because it has been shown that GCs interact with
gp120 to disrupt cell function and increase neuron death in
vitro, there is a possibility that similar phenomena may be
occurring in vivo.
If, with continued research in this field, the gp120
neurotoxicity data discussed in the early part of this review
can be unequivocally shown to cause some of the neuronal
damage associated with ADC, then it should also be
recognized that GCs might adversely affect the nervous
system in individuals with HIV-infection. This conclusion
is still somewhat hypothetical because the connection
between gp120 and ADC is not absolutely confirmed, but
we feel that there are sufficient data to warrant further
study and concern. In contrast, the other recent literature to
be reviewed suggests that estrogen may prove to beneficial
because it appears to have neuroprotective properties
against gp120 neurotoxicity.
Estrogen and Neurotoxicity
Estrogen appears to promote neuron survival and function.
Its neuroprotective attributes include protection against
glutamate (Goodman and Mattson 1996; Singer et al
1996),
b
-amyloid toxicity (Green et al 1996), glucose, and
serum deprivation (Bishop and Simpkins 1994;
Faivre-Bauman et al 1981; Green et al 1997), regulation of
calcium homeostasis (Mermelstein et al 1996; Nakajima et
al 1995), decreasing the production of oxygen radicals
(Behl et al 1997; Keaney et al 1994; Lacort et al 1995;
Mooradian 1993), and increasing release of neuotrophins
and promotion of dendritic growth (Brinton et al 1997;
Chowen et al 1992; McEwen and Woolley 1994). The
exact mechanism of estrogen action is still being
deter-mined, but neuroprotection appears to involve both
tradi-tional (genomic) and nontraditradi-tional mechanisms
(Mc-Ewen 1999). Genomic mechanisms involve steroids
bind-ing to intracellular receptors, which are then translocated
to the DNA where they can serve as positive or negative
transcriptional regulators. This process is relatively slow,
requiring a number of minutes to hours. Evidence for
nonreceptor (nontraditional) mechanisms include
incre-mental increases in protection from estrogen well beyond
the
K
dof the estrogen receptor and the fact estrogen effects
occur too rapidly to be genomically mediated.
Mecha-nisms of nontraditional estrogen activity are primarily
antioxidant properties and perhaps some of the effects on
calcium flux and facilitation of neurite growth.
Clinically, the neuroprotective abilities of estrogen
ap-pear to be important in decreasing the severity of
Alzhei-mer’s disease and other CNS degenerative diseases.
Hor-mone replacement therapy in Alzheimer’s disease is
associated with less severe symptoms of dementia (higher
test scores on neuropsychologic exams, even if the
treat-ment is started after the disease has been diagnosed (Fillit
et al 1986; Henderson et al 1994; Honjo et al 1989;
Robinson et al 1994; Tang et al 1996). A more extensive
discussion of estrogen action and neuroprotection can be
reviewed in a recent publication (McEwen and Alves
1999). Nonetheless, it also must be understood that
estro-gen’s beneficial effects are controversial because a recent
study has contradicted previous findings showing no
significant benefit in Alzheimer’s patients with estrogen
treatment (Mulnard et al 2000).
Estrogen and gp120
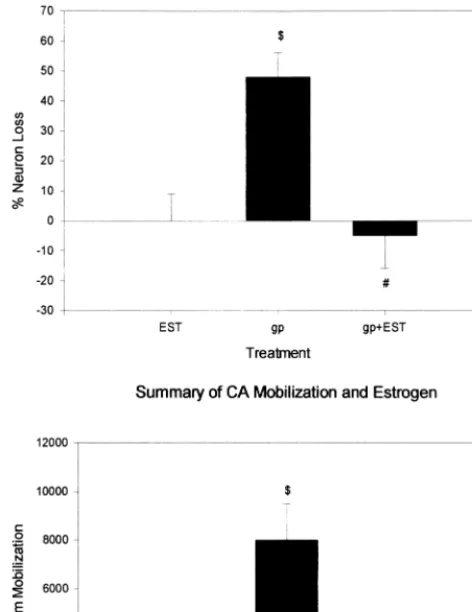
The protective effects of estrogen also extend to
decreas-ing gp120-induced neurotoxicity and calcium mobilization
(Figure 5; Brooke et al 1997). Preliminary data regarding
the interaction of gp120 and estrogen on cell lipid
peroxi-dation would indicate that perhaps the estrogen protection
in this case is via its antioxidant properties.
There are no animal studies in which estrogen
protec-tion against gp120 has been examined. Likewise there is
little literature regarding to estrogen and AIDS. One
clinical study (Clark and Bessinger 1997) reported that
among postmenopausal women with AIDS, estrogen
re-placement therapy was associated with a decreased risk of
dementia (although there are multiple confounds in this
study, and the data must be interpreted cautiously). The
authors suggested that hormone replacement therapy be
considered for HIV-infected postmenopausal women.
These results closely parallel the results, as previously
described, in which estrogen decreases severity of
Alzhei-mer’s symptoms.
Conclusions and Future Direction
gp120, a possible causative agent of ADC. Although
human and clinical implications of this data are not
known, we would hope that those working with HIV
patients would press for further studies to determine if the
implications of this data are a possible factor in the
development of AIDS-related dementia. Although the
immediate CNS effect of large doses of GC may not be
evident, the long-term neurologic effect on these patients
could be detrimental.
Certainly more study is needed to confirm these results
with other forms of gp120, with the transgenic mice that
overexpress gp120 and ultimately in primates. It would
also be informative if some human studies followed that
would show whether or not high cortisol levels or the use
of artificial GCs effect the onset and course of ADC.
On the other hand, estrogen’s apparent neuroprotective
abilities are encouraging. It would also be useful if in vivo
studies could be carried out with estrogen. Only if the
animal results continue to be positive should human
studies looking at the possibilities of protecting or
revers-ing ADC with estrogen treatment be explored.
With new treatments, AIDS patients are living longer,
and they no doubt hope to maintain normal cognitive
function. Therefore, the potential for neurologic
difficul-ties arising as a result of treatment with GCs and the
potential benefits of estrogen should be taken into
consideration.
References
Adamson DC, McArthur JC, Dawson TM, Dawson VL (1999): Rate and severity of HIV-associated dementia (HAD): Cor-relations with Gp41 and iNOS.Mol Med5:98 –109. Adamson DC, Wildemann B, Sasaki M, et al (1996):
Immuno-logic NO synthase: Elevation in severe AIDS dementia and induction by HIV-1 gp41.Science274:1917–1921.
Adle-Biassette H, Chretien F, Wingertsmann L, et al (1999): Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage.Neuropathol Appl Neurobiol25:123–133.
Aggoun-Zouaoui D, Charriaut-Marlangue C, Rivera S, et al (1996): The HIV-1 envelope protein gp120 induces neuronal apoptosis in hippocampal slices.Neuroreport7:433– 436. Altmeyer R, Mordelet E, Girard M, Vidal C (1999): Expression
and detection of macrophage-tropic HIV-1 gp120 in the brain using conformation-dependent antibodies.Virology259:314 – 323.
Annunziata P, Cioni C, Toneatto S, Paccagnini E (1998): HIV-1 gp120 increases the permeability of rat brain endothelium cultures by a mechanism involving substance P.AIDS 12: 2377–2385.
Avgeropoulos N, Kelley B, Middaugh L, et al (1998): SCID mice with HIV encephalitis develop behavioral abnormalities. J Acquir Immune Defic Syndr Hum Retrovirol18:13–20. Bacellar H, Munoz A, Miller EN, et al (1994): Temporal trends
in the incidence of HIV-1-related neurologic diseases: Mul-ticenter AIDS Cohort Study, 1985–1992. Neurology 44: 1892–1900.
Bagetta G, Corasaniti MT, Aloe L, et al (1996a): Intracerebral injection of human immunodeficiency virus type 1 coat protein gp120 differentially affects the expression of nerve growth factor and nitric oxide synthase in the hippocampus of rat.Proc Natl Acad Sci U S A93:928 –933.
Bagetta G, Corasaniti MT, Costa N, et al (1997): The human immunodeficiency virus type 1 (HIV-1) glycoprotein gp120 reduces the expression of neuronal nitric oxide synthase in the hippocampus but not in the cerebral cortex and medial septal nucleus of rat.Neurosci Lett224:75–78.
Bagetta G, Corasaniti MT, Malorni W, et al (1996b): The HIV-1
gp120 causes ultrastructural changes typical of apoptosis in the rat cerebral cortex.Neuroreport7:1722–1724.
Barks JD, Liu XH, Sun R, Silverstein FS (1997): gp120, a human immunodeficiency virus-1 coat protein, augments excitotoxic hippocampal injury in perinatal rats.Neuroscience 76:397– 409.
Bartz SR, Emerman M (1999): Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8.J Virol 73:1956 –1963.
Behl C, Skutella T, Lezoualc’h F, et al (1997): Neuroprotection against oxidative stress by estrogens: Structure-activity rela-tionship.Mol Pharmacol51:535–541.
Bell JE (1998): The neuropathology of adult HIV infection.Rev Neurol (Paris)154:816 – 829.
Benos DJ, Hahn BH, Bubien JK, et al (1994): Envelope glycoprotein gp120 of human immunodeficiency virus type 1 alters ion transport in astrocytes: implications for AIDS dementia complex.Proc Natl Acad Sci U S A91:494 – 498. Bernardo A, Patrizio M, Levi G, Petrucci TC (1994): Human
immunodeficiency virus protein gp120 interferes with beta-adrenergic receptor-mediated protein phosphorylation in cul-tured rat cortical astrocytes.Cell Mol Neurobiol14:159 –173. Bishop J, Simpkins JW (1994): Estradiol treatment increases viability of glioma and neuroblastoma cells in vitro.Mol Cell Neurosci5:303–308.
Bjugstad KB, Flitter WD, Garland WA, et al (1998): Preventive actions of a synthetic antioxidant in a novel animal model of AIDS dementia.Brain Res795:349 –357.
Blanco J, Jacotot E, Cabrera C, et al (1999): The implication of the chemokine receptor CXCR4 in HIV-1 envelope protein-induced apoptosis is independent of the G protein-mediated signalling.AIDS13:909 –917.
Bozzette SA, Sattler FR, Chiu J, et al (1990): A controlled trial of early adjunctive treatment with corticosteroids for Pneu-mocystis carinii pneumonia in the acquired immunodefi-ciency syndrome. California Collaborative Treatment Group. N Engl J Med323:1451–1457.
Brenneman DE, Westbrook GL, Fitzgerald SP, et al (1988): Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide.Nature335:639 – 642.
Bressler P, Poli G, Justement JS, et al (1993): Glucocorticoids synergize with tumor necrosis factor alpha in the induction of HIV expression from a chronically infected promonocytic cell line.AIDS Res Hum Retroviruses9:547–551.
Brinton RD, Tran J, Proffitt P, Montoya M (1997): 17 beta-Estradiol enhances the outgrowth and survival of neocortical neurons in culture.Neurochem Res22:1339 –1351.
Brooke S, Chan R, Howard S, Sapolsky R (1997): Endocrine modulation of the neurotoxicity of gp120: implications for AIDS-related dementia complex.Proc Natl Acad Sci U S A 94:9457–9462.
Brooke SM, Howard SA, Sapolsky RM (1998): Energy depen-dency of glucocorticoid exacerbation of gp120 neurotoxicity. J Neurochem71:1187–1193.
Budka H (1991): Neuropathology of human immunodeficiency virus infection.Brain Pathol1:163–175.
Bussiere JL, Hardy LM, Peterson M, et al (1999): Lack of
developmental neurotoxicity of MN rgp 120/HIV-1 adminis-tered subcutaneously to neonatal rats.Toxicol Sci48:90 –99. Carter-Su C, Okamoto K (1985): Effect of glucocorticoids on hexose transport in rat adipocytes. Evidence for decreased transporters in the plasma membrane. J Biol Chem 260: 11091–11098.
Chang J, Jozwiak R, Wang B, et al (1998): Unique HIV type 1 V3 region sequences derived from six different regions of brain: Region-specific evolution within host-determined qua-sispecies.AIDS Res Hum Retroviruses14:25–30.
Cheng J, Nath A, Knudsen B, et al (1998): Neuronal excitatory properties of human immunodeficiency virus type 1 Tat protein.Neuroscience82:97–106.
Choi DW (1992): Excitotoxic cell death.J Neurobiol23:1261– 1276.
Chowen JA, Torres-Aleman I, Garcia-Segura LM (1992): Tro-phic effects of estradiol on fetal rat hypothalamic neurons. Neuroendocrinology56:895–901.
Ciardo A, Meldolesi J (1993): Effects of the HIV-1 envelope glycoprotein gp120 in cerebellar cultures. [Ca21]
iincreases
in a glial cell subpopulation.Eur J Neurosci5:1711–1718. Clark RA, Bessinger R (1997): Clinical manifestations and
predictors of survival in older women infected with HIV.J Acquir Immune Defic Syndr Hum Retrovirol15:341–345. Codazzi F, Menegon A, Zacchetti D, et al (1995): HIV-1 gp120
glycoprotein induces [Ca21]
iresponses not only in type-2 but
also type-1 astrocytes and oligodendrocytes of the rat cere-bellum.Eur J Neurosci7:1333–1341.
Codazzi F, Racchetti G, Grohovaz F, Meldolesi J (1996): Transduction signals induced in rat brain cortex astrocytes by the HIV-1 gp120 glycoprotein.FEBS Lett384:135–137. Coodley GO, Loveless MO, Nelson HD, Coodley MK (1994):
Endocrine function in the HIV wasting syndrome.J Acquir Immune Defic Syndr7:46 –51.
Corasaniti MT, Bagetta G, Rotiroti D, Nistico G (1998a): The HIV envelope protein gp120 in the nervous system: Interac-tions with nitric oxide, interleukin-1beta and nerve growth factor signalling, with pathological implications in vivo and in vitro.Biochem Pharmacol56:153–156.
Corasaniti MT, Navarra M, Nistico S, et al (1998b): Requirement for membrane lipid peroxidation in HIV-1 gp120-induced neuroblastoma cell death. Biochem Biophys Res Commun 246:686 – 689.
Corley PA (1995): HIV and the cortisol connection: A feasible concept of the process of AIDS. Med Hypotheses44:483– 489.
Dawson VL, Dawson TM, Uhl GR, Snyder SH (1993): Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures.Proc Natl Acad Sci U S A90:3256 –3259.
Dibbern DA Jr, Glazner GW, Gozes I, et al (1997): Inhibition of murine embryonic growth by human immunodeficiency virus envelope protein and its prevention by vasoactive intestinal peptide and activity-dependent neurotrophic factor. J Clin Invest99:2837–2841.
Diop AG, Dussartre C, Barthe D, Hugon J (1996): Neuroprotec-tive properties of calretinin against the HIV-1 gp120 toxicity. Neurosci Res Comm18:107–114.
HIV coat protein (gp120) toxicity in primary neuronal cul-tures.Neurosci Lett165:187–190.
Diop AG, Lesort M, Esclaire F, et al (1995): Calbindin D28K-containing neurons, and not HSP70-expressing neurons, are more resistant to HIV-1 envelope (gp120) toxicity in cortical cell cultures.J Neurosci Res42:252–258.
Doyle P, Rohner-Jeanrenaud F, Jeanrenaud B (1993): Local cerebral glucose utilization in brains of lean and genetically obese (fa/fa) rats.Am J Physiol264:E29 –E36.
Dreyer EB, Kaiser PK, Offermann JT, Lipton SA (1990): HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists.Science248:364 –367.
Dreyer EB, Lipton SA (1995): The coat protein gp120 of HIV-1 inhibits astrocyte uptake of excitatory amino acids via mac-rophage arachidonic acid.Eur J Neurosci7:2502–2507. Elliott EM, Mattson MP, Vanderklish P, et al (1993):
Cortico-sterone exacerbates kainate-induced alterations in hippocam-pal tau immunoreactivity and spectrin proteolysis in vivo. J Neurochem61:57– 67.
Ensoli F, Fiorelli V, DeCristofaro M, et al (1999): Inflammatory cytokines and HIV-1-associated neurodegeneration: Oncosta-tin-M produced by mononuclear cells from HIV-1-infected individuals induces apoptosis of primary neurons.J Immunol 162:6268 – 6277.
Epstein LG, Gelbard HA (1999): HIV-1-induced neuronal injury in the developing brain.J Leukoc Biol65:453– 457. Everall IP, Heaton RK, Marcotte TD, et al (1999): Cortical
synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center.Brain Pathol 9:209 –217.
Faivre-Bauman A, Rosenbaum E, Puymirat J, et al (1981): Differentiation of fetal mouse hypothalamic cells in serum-free medium.Dev Neurosci4:118 –129.
Fillit H, Weinreb H, Cholst I, et al (1986): Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer’s type.Psychoneuroendocrinology11:337–345. Foga IO, Nath A, Hasinoff BB, Geiger JD (1997): Antioxidants
and dipyridamole inhibit HIV-1 gp120-induced free radical-based oxidative damage to human monocytoid cells.J Acquir Immune Defic Syndr Hum Retrovirol16:223–229.
Freo U, Holloway HW, Kalogeras K, et al (1992): Adrenalec-tomy or metyrapone-pretreatment abolishes cerebral meta-bolic responses to the serotonin agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) in the hippocampus. Brain Res586:256 –264.
Frey FJ, Speck RF (1992): Glucocorticoids and infection. Schweiz Med Wochenschr122:137–146.
Gagnon S, Boota AM, Fischl MA, et al (1990): Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneu-monia in the acquired immunodeficiency syndrome. A dou-ble-blind, placebo-controlled trial.N Engl J Med323:1444 – 1450.
Gelderblom HR, Hausmann EH, Ozel M, et al (1987): Fine structure of human immunodeficiency virus (HIV) and im-munolocalization of structural proteins. Virology 156:171– 176.
Gendelman HE, Lipton SA, Tardieu M, et al (1994): The neuropathogenesis of HIV-1 infection.J Leukoc Biol56:389 – 398.
Gendelman HE, Zheng J, Coulter CL, et al (1998): Suppression of inflammatory neurotoxins by highly active antiretroviral therapy in human immunodeficiency virus-associated demen-tia.J Infect Dis178:1000 –1007.
Genis P, Jett M, Bernton EW, et al (1992): Cytokines and arachidonic metabolites produced during human immunode-ficiency virus (HIV)-infected macrophage-astroglia interac-tions: implications for the neuropathogenesis of HIV disease. J Exp Med176:1703–1718.
Giulian D, Vaca K, Noonan CA (1990): Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science 250:1593–1596.
Glass JD, Johnson RT (1996): Human immunodeficiency virus and the brain.Annu Rev Neurosci19:1–26.
Glowa JR, Panlilio LV, Brenneman DE, et al (1992): Learning impairment following intracerebral administration of the HIV envelope protein gp120 or a VIP antagonist. Brain Res 570:49 –53.
Goodman Y, Mattson MP (1996): Ceramide protects hippocam-pal neurons against excitotoxic and oxidative insults, and amyloid beta-peptide toxicity.J Neurochem66:869 – 872. Gopal DV, Hassaram S, Marcon NE, Kandel G (1997):
Idio-pathic colonic inflammation in AIDS: An open trial of prednisone.Am J Gastroenterol92:2237–2240.
Gorman JM, Kertzner R, Cooper T, et al (1991): Glucocorticoid level and neuropsychiatric symptoms in homosexual men with HIV infection.Am J Psychiatry148:41– 45.
Green PS, Bishop J, Simpkins JW (1997): 17 alpha-estradiol exerts neuroprotective effects on SK-N-SH cells.J Neurosci 17:511–515.
Green PS, Gridley KE, Simpkins JW (1996): Estradiol protects against beta-amyloid (25-35)-induced toxicity in SK-N-SH human neuroblastoma cells.Neurosci Lett218:165–168. Haase AT (1986): Pathogenesis of lentivirus infections.Nature
322:130 –136.
Henderson VW, Paganini-Hill A, Emanuel CK, et al (1994): Estrogen replacement therapy in older women. Comparisons between Alzheimer’s disease cases and nondemented control subjects.Arch Neurol51:896 –900.
Herbein G, Mahlknecht U, Batliwalla F, et al (1998): Apoptosis
of CD81 T cells is mediated by macrophages through
interaction of HIV gp120 with chemokine receptor CXCR4. Nature395:189-94.
Hesselgesser J, Taub D, Baskar P, et al (1998): Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4.Curr Biol8:595–598.
Hill JM, Mervis RF, Avidor R, et al (1993): HIV envelope protein-induced neuronal damage and retardation of behav-ioral development in rat neonates.Brain Res603:222–233. Honjo H, Ogino Y, Naitoh K, et al (1989): In vivo effects by
estrone sulfate on the central nervous system-senile dementia (Alzheimer’s type).J Steroid Biochem34:521–525. Horner HC, Packan DR, Sapolsky RM (1990): Glucocorticoids
inhibit glucose transport in cultured hippocampal neurons and glia.Neuroendocrinology52:57– 64.
Ilyin SE, Plata-Salaman CR (1997): HIV-1 envelope glycopro-tein 120 regulates brain IL-1beta system and TNF-alpha mRNAs in vivo.Brain Res Bull44:67–73.
Iyer AM, Brooke SM, Sapolsky RM (1998): Glucocorticoids interact with gp120 in causing neurotoxicity in striatal cul-tures.Brain Res808:305–309.
Jacobson L, Brooke S, Sapolsky R (1993): Corticosterone is a preferable ligand for measuring rat brain corticosteroid recep-tors: Competition by RU 28362 and RU 26752 for dexameth-asone binding in rat hippocampal cytosol.Brain Res625:84 – 92.
Jager H (1994): Adjuvant corticoid administration within the scope of HIV disease. Indications in wasting syndrome and other diseases within the scope of AIDS. Fortschr Med 112:87–91.
Jeohn GH, Kong LY, Wilson B, et al (1998): Synergistic neurotoxic effects of combined treatments with cytokines in murine primary mixed neuron/glia cultures.J Neuroimmunol 85:1–10.
Joels M, de Kloet ER (1994): Mineralocorticoid and glucocorti-coid receptors in the brain. Implications for ion permeability and transmitter systems.Prog Neurobiol43:1–36.
Kaul M, Lipton SA (1999): Chemokines and activated macro-phages in HIV gp120-induced neuronal apoptosis.Proc Natl Acad Sci U S A96:8212–216.
Keaney JF, Shwaery GT, Xu A, et al (1994): 17 beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation89:2251–2259.
Kerr SJ, Armati PJ, Pemberton LA, et al (1997): Kynurenine pathway inhibition reduces neurotoxicity of HIV-1-infected macrophages.Neurology49:1671–1681.
Kimes AS, London ED, Szabo G, et al (1991): Reduction of cerebral glucose utilization by the HIV envelope glycoprotein Gp-120.Exp Neurol112:224 –228.
Kimmel PL, Bosch JP, Vassalotti JA (1998): Treatment of human immunodeficiency virus (HIV)-associated nephropa-thy.Semin Nephrol18:446 – 458.
Koka P, He K, Camerini D, et al (1995): The mapping of HIV-1 gp160 epitopes required for interleukin-1 and tumor necrosis factor alpha production in glial cells. J Neuroimmunol57: 179 –191.
Kong LY, Wilson BC, McMillian MK, et al (1996): The effects of the HIV-1 envelope protein gp120 on the production of nitric oxide and proinflammatory cytokines in mixed glial cell cultures.Cell Immunol172:77– 83.
Kort JJ (1998): Impairment of excitatory amino acid transport in astroglial cells infected with the human immunodeficiency virus type 1.AIDS Res Hum Retroviruses14:1329 –1339. Krucker T, Toggas SM, Mucke L, Siggins GR (1998):
Trans-genic mice with cerebral expression of human immunodefi-ciency virus type-1 coat protein gp120 show divergent changes in short- and long-term potentiation in CA1 hip-pocampus.Neuroscience83:691–700.
Lacort M, Leal AM, Liza M, et al (1995): Protective effect of estrogens and catecholestrogens against peroxidative mem-brane damage in vitro.Lipids30:141–146.
Lannuzel A, Lledo PM, Lamghitnia HO, Vincent JD, Tardieu M (1995): HIV-1 envelope proteins gp120 and gp160 potentiate
NMDA-induced [Ca21
]iincrease, alter [Ca 21
]ihomeostasis
and induce neurotoxicity in human embryonic neurons.Eur J Neurosci7:2285–2293.
Limoges J, Persidsky Y, Bock P, Gendelman HE (1997): Dexamethasone therapy worsens the neuropathology of hu-man immunodeficiency virus type 1 encephalitis in SCID mice.J Infect Dis175:1368 –1381.
Lipton SA (1994): HIV-related neuronal injury. Potential thera-peutic intervention with calcium channel antagonists and NMDA antagonists.Mol Neurobiol8:181–196.
Lipton SA (1998): Neuronal injury associated with HIV-1: Approaches to treatment. Annu Rev Pharmacol Toxicol38: 159 –177.
Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB (1991): Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity.Neuron7:111–118.
Lo TM, Fallert CJ, Piser TM, Thayer SA (1992): HIV-1 envelope protein evokes intracellular calcium oscillations in rat hip-pocampal neurons.Brain Res594:189 –196.
Lortholary O, Christeff N, Casassus P, et al (1996): Hypo-thalamo-pituitary-adrenal function in human immunodefi-ciency virus-infected men.J Clin Endocrinol Metab81:791– 796.
Lowy MT, Wittenberg L, Yamamoto BK (1995): Effect of acute stress on hippocampal glutamate levels and spectrin proteol-ysis in young and aged rats.J Neurochem65:268 –274. Lu W, Salerno-Goncalves R, Yuan J, et al (1995):
Glucocorti-coids rescue CD41T lymphocytes from activation-induced apoptosis triggered by HIV-1: Implications for pathogenesis and therapy.AIDS9:35– 42.
Machet L, Jan V, Machet MC, et al (1996): Cutaneous alterna-riosis: Role of corticosteroid-induced cutaneous fragility. Dermatology193:342–344.
Madani N, Kozak SL, Kavanaugh MP, Kabat D (1998): gp120 envelope glycoproteins of human immunodeficiency viruses competitively antagonize signaling by coreceptors CXCR4 and CCR5.Proc Natl Acad Sci U S A95:8005– 8010. Masliah E, Achim CL, Ge N, et al (1992): Spectrum of human
immunodeficiency virus-associated neocortical damage.Ann Neurol32:321–329.
McEwen BS (1999): Clinical review 108: The molecular and neuroanatomical basis for estrogen effects in the central nervous system.J Clin Endocrinol Metab84:1790 –1797. McEwen BS, Alves SE (1999): Estrogen actions in the central
nervous system.Endocr Rev20:279 –307.
McEwen BS, Woolley CS (1994): Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain.Exp Gerontol29:431– 436. McIntosh LJ, Sapolsky RM (1996): Glucocorticoids increase the
accumulation of reactive oxygen species and enhance adria-mycin-induced toxicity in neuronal culture.Exp Neurol141: 201–206.
Mermelstein PG, Becker JB, Surmeier DJ (1996): Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor.J Neurosci16:595– 604.
hippocampal pyramidal neuron cultures: protective action of TGF-beta1.J Neurosci16:4080 – 4088.
Michaels J, Sharer LR, Epstein LG (1988): Human immunode-ficiency virus type 1 (HIV-1) infection of the nervous system: A review.Immunodefic Rev1:71–104.
Miller RF, Le Noury J, Corbett EL, et al (1996): Pneumocystis carinii infection: Current treatment and prevention.J Antimi-crob Chemother37(suppl B):33–53.
Miller RJ, Meucci O (1999): AIDS and the brain: Is there a chemokine connection?Trends Neurosci22:471– 479. Mollace V, Colasanti M, Rodino P, et al (1994): HIV coating gp
120 glycoprotein-dependent prostaglandin E2 release by hu-man cultured astrocytoma cells is regulated by nitric oxide formation.Biochem Biophys Res Commun203:87–92. Montaner JS, Russell JA, Lawson L, Ruedy J (1989): Acute
respiratory failure secondary to Pneumocystis carinii pneu-monia in the acquired immunodeficiency syndrome. A poten-tial role for systemic corticosteroids.Chest95:881– 884. Mooradian AD (1993): Antioxidant properties of steroids. J
Steroid Biochem Mol Biol45:509 –511.
Mulnard RA, Cotman CW, Kawas C, et al (2000): Estrogen replacement therapy for treatment of mild to moderate Alz-heimer disease: a randomized controlled trial. AlzAlz-heimer’s Disease Cooperative Study.JAMA283:1007–1015. Munck A, Guyre PM, Holbrook NJ (1984): Physiological
func-tions of glucocorticoids in stress and their relation to phar-macological actions.Endocr Rev5:25– 44.
Nakajima T, Kitazawa T, Hamada E, et al (1995): 17beta-Estradiol inhibits the voltage-dependent L-type Ca21 cur-rents in aortic smooth muscle cells. Eur J Pharmacol 294: 625– 635.
Nath A, Padua RA, Geiger JD (1995): HIV-1 coat protein gp120-induced increases in levels of intrasynaptosomal cal-cium.Brain Res678:200 –206.
Navia BA, Dafni U, Simpson D, et al (1998): A phase I/II trial of nimodipine for HIV-related neurologic complications. Neurology51:221–228.
Newcomer JW, Craft S, Askins K, et al (1998): Glucocorticoid interactions with memory function in schizophrenia. Psycho-neuroendocrinology23:65–72.
Newcomer JW, Craft S, Hershey T, et al (1994): Glucocorticoid-induced impairment in declarative memory performance in adult humans.J Neurosci14:2047–2053.
Newcomer JW, Faustman WO, Whiteford HA, et al (1991): Symptomatology and cognitive impairment associate inde-pendently with post-dexamethasone cortisol concentrations in unmedicated schizophrenic patients.Biol Psychiatry29:855– 864.
Norbiato G, Galli M, Righini V, Moroni M (1994): The syn-drome of acquired glucocorticoid resistance in HIV infection. Baillieres Clin Endocrinol Metab8:777–787.
Oberfield SE, Cowan L, Levine LS, et al (1994): Altered cortisol response and hippocampal atrophy in pediatric HIV disease. J Acquir Immune Defic Syndr7:57– 62.
Ohagen A, Ghosh S, He J, et al (1999): Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: Evidence for a role of the envelope.J Virol73:897–906.
Pahwa S, Pahwa R, Saxinger C, et al (1985): Influence of the
human T-lymphotropic virus/lymphadenopathy-associated virus on functions of human lymphocytes: Evidence for immunosuppressive effects and polyclonal B-cell activation by banded viral preparations. Proc Natl Acad Sci U S A 82:8198 – 8202.
Pareja JG, Garland R, Koziel H (1998): Use of adjunctive corticosteroids in severe adult non-HIV Pneumocystis carinii pneumonia.Chest113:1215–1224.
Pattarini R, Pittaluga A, Raiteri M (1998): The human immuno-deficiency virus-1 envelope protein gp120 binds through its V3 sequence to the glycine site of N-methyl-D-aspartate receptors mediating noradrenaline release in the hippocam-pus.Neuroscience87:147–157.
Persidsky Y, Buttini M, Limoges J, et al (1997a): An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis.J Neurovi-rol3:401– 416.
Persidsky Y, Gendelman HE (1997): Development of laboratory and animal model systems for HIV-1 encephalitis and its associated dementia.J Leukoc Biol62:100 –106.
Persidsky Y, Nottet HS, Sasseville VG, et al (1995): The development of animal model systems for HIV-1 encephalitis and its associated dementia.J Neurovirol1:229 –243. Persidsky Y, Stins M, Way D, et al (1997b): A model for
monocyte migration through the blood-brain barrier during HIV-1 encephalitis.J Immunol158:3499 –3510.
Piedrola G, Casado JL, Lopez E, et al (1996): Clinical features of adrenal insufficiency in patients with acquired immunodefi-ciency syndrome.Clin Endocrinol (Oxf)45:97–101. Pittaluga A, Pattarini R, Severi P, Raiteri M (1996): Human brain
N-methyl-D-aspartate receptors regulating noradrenaline re-lease are positively modulated by HIV-1 coat protein gp120. AIDS10:463– 468.
Price RW, Brew B, Sidtis J, et al (1988): The brain in AIDS: Central nervous system HIV-1 infection and AIDS dementia complex.Science239:586 –592.
Pulliam L, Herndier BG, Tang NM, McGrath MS (1991): Human immunodeficiency virus-infected macrophages produce solu-ble factors that cause histological and neurochemical alter-ations in cultured human brains.J Clin Invest87:503–512. Pulliam L, West D, Haigwood N, Swanson RA (1993): HIV-1
envelope gp120 alters astrocytes in human brain cultures. AIDS Res Hum Retroviruses9:439 – 444.
Raber J, Toggas SM, Lee S, et al (1996): Central nervous system expression of HIV-1 Gp120 activates the hypothalamic-pituitary-adrenal axis: Evidence for involvement of NMDA receptors and nitric oxide synthase.Virology226:362–373. Rafalowska J (1998): HIV-1-infection in the CNS. A
pathogen-esis of some neurological syndromes in the light of recent investigations.Folia Neuropathol36:211–216.
Robinson D, Friedman L, Marcus R, et al (1994): Estrogen
replacement therapy and memory in older women. J Am
Geriatr Soc42:919 –922.
Saksena NK, Jozwiak R, Wang B (1998): Molecular and biolog-ical mechansims in the development of AIDS dementia complex (ADC).Bull Inst Pasteur96:171–188.
apoptosis by calmodulin-dependent intracellular Ca21 eleva-tion in CD41 cells expressing gp 160 of HIV. Virology 224:18 –24.
Savio T, Levi G (1993): Neurotoxicity of HIV coat protein gp120, NMDA receptors, and protein kinase C: A study with rat cerebellar granule cell cultures.J Neurosci Res34:265– 272.
Schneider J, Kaaden O, Copeland TD, et al (1986): Shedding and interspecies type sero-reactivity of the envelope glyco-polypeptide gp120 of the human immunodeficiency virus. J Gen Virol67:2533–2538.
Schroder HC, Perovic S, Kavsan V, et al (1998): Mechanisms of prionSc- and HIV-1 gp120 induced neuronal cell death. Neurotoxicology19:683– 688.
Sharer LR (1992): Pathology of HIV-1 infection of the central nervous system. A review.J Neuropathol Exp Neurol51:3– 11.
Shrikant P, Benos DJ, Tang LP, Benveniste EN (1996): HIV glycoprotein 120 enhances intercellular adhesion molecule-1 gene expression in glial cells. Involvement of Janus kinase/ signal transducer and activator of transcription and protein kinase C signaling pathways.J Immunol156:1307–1314. Singer CA, Rogers KL, Strickland TM, Dorsa DM (1996):
Estrogen protects primary cortical neurons from glutamate toxicity.Neurosci Lett212:13–16.
Smith MC, Austen JL, Carey JT, et al (1996): Prednisone improves renal function and proteinuria in human immuno-deficiency virus-associated nephropathy.Am J Med101:41– 48.
Stein-Behrens BA, Elliott EM, Miller CA, et al (1992): Glu-cocorticoids exacerbate kainic acid-induced extracellular ac-cumulation of excitatory amino acids in the rat hippocampus. J Neurochem58:1730 –1735.
Stein-Behrens BA, Lin WJ, Sapolsky RM (1994): Physiological elevations of glucocorticoids potentiate glutamate accumula-tion in the hippocampus.J Neurochem63:596 – 602. Sweetnam PM, Saab OH, Wroblewski JT, et al (1993): The
envelope glycoprotein of HIV-1 alters NMDA receptor func-tion.Eur J Neurosci5:276 –283.
Tan SV, Guiloff RJ (1998): Hypothesis on the pathogenesis of vacuolar myelopathy, dementia, and peripheral neuropathy in AIDS.J Neurol Neurosurg Psychiatry65:23–28.
Tang MX, Jacobs D, Stern Y, et al (1996): Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease.Lancet348:429 – 432.
Toggas SM, Masliah E, Mucke L (1996): Prevention of HIV-1 gp120-induced neuronal damage in the central nervous sys-tem of transgenic mice by the NMDA receptor antagonist memantine.Brain Res706:303–307.
Toggas SM, Masliah E, Rockenstein EM, et al (1994): Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice.Nature367:188 –193.
Tyor WR, Power C, Gendelman HE, Markham RB (1993): A model of human immunodeficiency virus encephalitis in scid mice.Proc Natl Acad Sci U S A90:8658 – 8662.
Uchida D, Arimura A, Somogyvari-Vigh A, et al (1996): Prevention of ischemia-induced death of hippocampal neu-rons by pituitary adenylate cyclase activating polypeptide. Brain Res736:280 –286.
Urakami R, Nagasaka Y, Tohda Y, et al (1997): Clinical features of eight cases of opportunistic fungal pneumonias. Kansen-shogaku Zasshi71:65–71.
Van der Meide PH, Schellekens H (1996): Cytokines and the immune response.Biotherapy8:243–249.
Vesce S, Bezzi P, Rossi D, et al (1997): HIV-1 gp120 glycop-rotein affects the astrocyte control of extracellular glutamate by both inhibiting the uptake and stimulating the release of the amino acid.FEBS Lett411:107–109.
Virgin CE Jr, Ha TP, Packan DR, et al (1991): Glucocorticoids inhibit glucose transport and glutamate uptake in hippocam-pal astrocytes: Implications for glucocorticoid neurotoxicity. J Neurochem57:1422–1428.
Vitkovic L, Tardieu M (1998): Neuropathogenesis of HIV-1 infection. Outstanding questions.C R Acad Sci III321:1015– 1021.
Wang P, Barks JD, Silverstein FS (1999): Tat, a human immu-nodeficiency virus-1-derived protein, augments excitotoxic hippocampal injury in neonatal rats. Neuroscience 88:585– 597.
Watterson MK, Detwiler RK, Bolin P Jr (1997): Clinical re-sponse to prolonged corticosteroids in a patient with human immunodeficiency virus-associated nephropathy. Am J Kid-ney Dis29:624 – 626.
Wiley CA, Masliah E, Achim CL (1994): Measurement of CNS HIV burden and its association with neurologic damage.Adv Neuroimmunol4:319 –325.
Wolkowitz OM, Reus VI, Canick J, et al (1997): Glucocorticoid medication, memory and steroid psychosis in medical illness. Ann N Y Acad Sci823:81–96.
Wolkowitz OM, Reus VI, Weingartner H, et al (1990): Cognitive effects of corticosteroids.Am J Psychiatry147:1297–1303. Wyss-Coray T, Masliah E, Toggas SM, et al (1996):
Dysregu-lation of signal transduction pathways as a potential mecha-nism of nervous system alterations in HIV-1 gp120 transgenic mice and humans with HIV-1 encephalitis. J Clin Invest 97:789 –798.
Yeung MC, Geertsma F, Liu J, Lau AS (1998): Inhibition of HIV-1 gp120-induced apoptosis in neuroblastoma SK-N-SH cells by an antisense oligodeoxynucleotide against p53.AIDS 12:349 –354.