Correlations of elevated levels of hexacosanoate in erythrocyte
membranes with risk factors for atherosclerosis
Yasunobu Antoku
a,*, Kohsuke Tsukamoto
b, Yuji Miyoshi
b, Hisanori Nagino
b,
Masaharu Anezaki
c, Kazuo Suwa
c, Yasushi Narabe
caDepartment of Neurology,National Chikugo Hospital,515Kurakazu,Chikugo City,Fukuoka Pref. 833-0054,Japan bMeiji Mutual Life Insurance Company,1-1,Marunouchi2-chome,Chiyoda-ku,Tokyo100-0005,Japan
cLaboratory of BML Inc.1361-1Matoba,Kawagoe-shi,Saitama350-1101,Japan
Received 21 June 1999; received in revised form 27 December 1999; accepted 19 January 2000
Abstract
We analyzed erythrocyte membrane C26:0 from 504 volunteers by high-performance liquid chromatography. The associations between the elevated levels of erythrocyte membrane C26:0 (0.20 or greater than 0.20%) and sex, obesity (body mass index`26.4), smoking (`20 cigarettes per day), present illnesses and past diseases were examined with thex2test. The correlations among age and the levels of erythrocyte membrane C26:0, plasma total cholesterol, triglycerides, LDL cholesterol and HDL cholesterol were analyzed using Spearman’s correlation coefficient. Moreover, the frequencies of high levels of erythrocyte membrane C26:0 were examined in male and female subjects divided into seven age groups. The elevated levels of erythrocyte membrane C26:0 were significantly more frequent in male subjects than in females, and were closely associated with obesity, smoking, and atherosclero-sis-related diseases of present illnesses. The levels of erythrocyte membrane C26:0 were highly correlated with age and the levels of plasma total cholesterol, triglycerides and LDL cholesterol, and inversely with those of HDL cholesterol. The frequency of high levels of erythrocyte membrane C26:0 in male subjects was greater than that in female subjects in all of the seven age groups. Elevated levels of erythrocyte membrane C26:0 may be closely related with atherosclerosis. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Erythrocyte membranes; Hexacosanoate; Atherosclerosis; Age
www.elsevier.com/locate/atherosclerosis
1. Introduction
Atherosclerosis is a serious health problem, and will become the chief cause of morbidity and mortality worldwide in the near future. The atherogenic process begins early in life, with a preclinical phase lasting many years, and leads to clinical complications later in life, such as myocardial infarction and stroke [1]. There-fore, presymptomatic detection of the disease is neces-sary to identify high-risk subjects and to apply appropriate preventive strategies.
Of the clinical tests for atherosclerosis, blood tests measuring predisposing risk factors are safe and mini-mally invasive. The effect of risk factors such as high
levels of plasma cholesterol and low levels of high-den-sity lipoprotein (HDL) cholesterol on the incidence of coronary disease in middle-aged people has been well established [2 – 6]. However, several investigators have reported that the association between risk factors and clinical coronary heart disease is weaker in the elderly [7 – 9].
For instance, young adults and middle-aged people with vascular disease commonly have higher total cholesterol levels, but the apparent effect of cholesterol on vascular disease wanes after the age of 50 and almost disappears after 65 [10].Therefore, it is crucial to identify candidates of new blood risk factors for atherosclerosis.
In the present study, we studied whether erythrocyte membrane hexacosanoate (C26:0), a saturated very long-chain fatty acid (VLFA), could be associated with well-known risk factors for atherosclerosis.
* Corresponding author. Tel.: +81-942-522195; fax: + 81-942-537053.
2. Methods
The subjects were 504 volunteers (324 men and 180 women), ranging in age from 25 to 65, working in the Meiji Mutual Life Insurance Company. Informed con-sent was obtained and information about smoking and medical histories was elicited from all of the subjects. Height and body-weight were measured to estimate the body mass index (BMI) of the subjects.
For erythrocyte membrane C26:0 assays, heparinized blood samples were obtained from fasted subjects. Ery-throcyte membranes were prepared as described
else-where [11]. The membranes were stored at−80°C until
use. Total lipids were extracted from erythrocyte mem-branes by a modification of the method of Folch et al. [12]. The lipids in the chloroform phase were dried under a stream of nitrogen gas. Butylated hydroxytoluene, an antioxidant, was added to the chloroform and methanol
used for extraction at a concentration of 5 mg/100 ml.
The dried total lipid extract was hydrolyzed with 0.5
mol/l HCl in a mixture of acetonitrile and water (9:1,
v/v) for 45 min at 100°C, and free fatty acids were
dissolved in chloroform [13]. The fatty acids were dried under a stream of nitrogen gas and esterified with a
fluorescent marker, 9-anthryldiazomethane
(ADAM)(Funakoshi Drug Co., Tokyo, Japan) [14]. One millilitre of ADAM solution (5 mg ADAM in 10 ml methanol) was added to the dried fatty acids and the mixture was applied to high-performance liquid chro-matography (Shimadzu LC-10AT, SCL-10A, RF-10A, DGU-3A; Shimadzu Co., Kyoto, Japan), and fatty acids were assayed as described previously [14 – 16]. The
column was a 25 cm×4.6 mm id stainless-steel tube
prepacked with Zorbax C8 (Hewlett Packard Co., USA). The column temperature was maintained at 60°C. Fatty acids were eluted by a programmed gradient elution with acetonitrile-water (v/v, 80/20 to 100/0), and
the flow rate was 1.6 ml/min. The fluorescence detector
was operated at 412 nm with excitation at 365 nm. The area percentages of fatty acids were automatically calcu-lated. We also examined the erythrocyte membrane C26:0 from nine healthy volunteers younger than 20, and all of them showed less than 0.20 in the area percentage. Therefore, erythrocyte membrane C26:0 was estimated as being high when its area percentage was 0.20 or greater.
At the same time, the plasma levels of total choles-terol, triglycerides and HDL cholesterol were measured by enzymatic procedures [17 – 19]. The low-density lipo-protein (LDL) cholesterol levels were calculated with the equation of Friedewald et al. [20]. The plasma levels of total cholesterol, triglycerides, HDL cholesterol and LDL cholesterol were estimated as abnormal when they were greater than 219 and 149, below 40, and greater than 139 mg/dl, respectively.
2.1. Statistical analysis
The associations of elevated levels of C26:0 in erythro-cyte membranes and sex, obesity (BMI: 26.4 or more), smoking (more than 19 cigarettes per day), present illnesses and past diseases were analyzed with the x2 test.
The correlations among age and the levels of erythro-cyte membrane C26:0 and four plasma risk factors for atherosclerosis (total cholesterol, triglycerides, HDL cholesterol and LDL cholesterol) were analyzed using Spearman’s correlation coefficient.
The subjects were divided into seven age groups (group 1: 25 – 29; 2: 30 – 34; 3: 35 – 39; 4: 40 – 44; 5: 45 – 49; 6: 50 – 54; 7: 55 – 65), and correlations between age and the frequency of high levels of erythrocyte membrane C26:0 in male and female subjects were analyzed using Spearman’s correlation coefficient.
3. Results
(1) Associations between erythrocyte membrane
C26:0 and sex, obesity, smoking, present illnesses and past diseases.
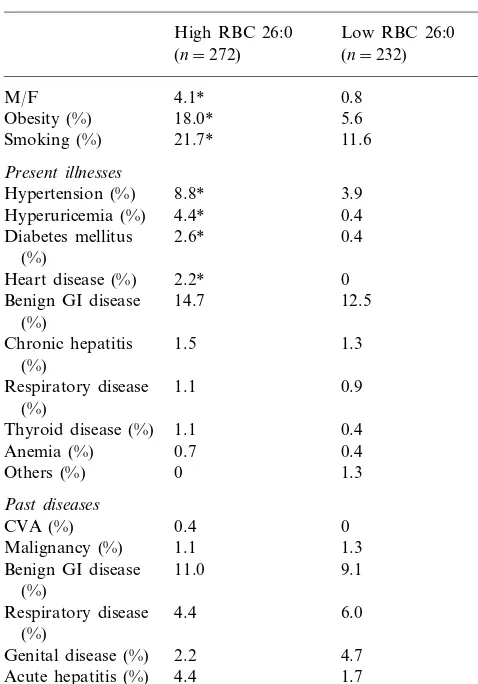
Elevated levels of erythrocyte membrane C26:0 were observed in 272 of the 504 volunteers. High erythrocyte membrane C26:0 was significantly associated with male-sex, obesity, and smoking (Table 1).
As for present illnesses, elevated levels of erythrocyte membrane C26:0 were also significantly associated with hypertension, hyperuricemia, diabetes mellitus and heart disease, but not with benign gastro-intestinal disease, chronic hepatitis, thyroid disease, anemia or respiratory disease (Table 1). While no subject with low levels of erythrocyte membrane C26:0 had heart disease, six of the 272 subjects with high levels of erythrocyte mem-brane C26:0 had heart disease (four cases of non-valvu-lar atrial fibrillation and two cases of ischemic heart disease).
As for past diseases, none were associated with ele-vated levels of erythrocyte membrane C26:0 (Table 1). (2) Correlations among erythrocyte membrane C26:0, age and four plasma lipid markers of atherosclerosis. The levels of erythrocyte membrane C26:0 were sig-nificantly correlated with age and the levels of plasma total cholesterol, triglycerides and LDL cholesterol, and inversely correlated with those of HDL cholesterol (Table 2).
There was no significant correlation between age and the levels of plasma HDL cholesterol (Table 2). The levels of plasma total cholesterol were not correlated with those of HDL cholesterol (Table 2).
Table 1
Associations between erythrocyte membrane C26:0 and clinical featuresa
Obesity (%) 18.0* 5.6
11.6
Hyperuricemia (%) 4.4* 0.4
2.6* 0.4
Diabetes mellitus (%)
Heart disease (%) 2.2* 0
14.7 12.5
Respiratory disease 1.1 0.9 (%)
0.4 Thyroid disease (%) 1.1
0.4
Genital disease (%) 2.2 4.7 1.7
High RBC 26:0, 0.20 or more than 0.20 of area percentage of erythrocyte membrane C26:0; low RBC 26:0, less than 0.20 of area percentage of erythrocyte membrane C26:0; M/F, the ratio of males to females. Malignancy: post-operative state of malignant tumors; benign GI disease: benign gastro-intestinal disease.
Fig. 1. Frequencies of elevated levels of erythrocyte membrane C26:0 of male and female subjects in seven age groups. 2529: male n=58, femalen=24; 3034: malen=28, female n=13; 3539: male n=47, female n=35; 4044: male n=42, female n=23; 4549: male n=57, female n=38; 5054: male n=45, female n=25; 5565: malen=47, femalen=22.
more frequent in male subjects than in female subjects in all of the seven age groups (Fig. 1).
4. Discussion
Hexacosanoate is a saturated VLFA and a minor fatty acid component in human tissues. However, this fatty acid has been used as a diagnostic marker for peroxisomal disorders including adrenoleukodystrophy [21 – 23], which involves the abnormal metabolism of VLFAs resulting in an accumulation of VLFAs in tissues.
We previously investigated hexacosanoate from ery-throcyte membranes, lymphocytes and blood plasma in adrenoleukodystrophy, and found an overlapping in the level of hexacosanoate between adrenoleukodystro-phy patients and aged controls only in erythrocyte membranes, against our expectation [23]. This finding provoked us to study erythrocyte membrane hexa-cosanoate with respect to age-related pathologies.
The present study showed that the levels of erythro-cyte membrane hexacosanoate were associated with There were significant correlations between the
fre-quency of high levels of erythrocyte membrane C26:0 and age in both male and female subjects (Fig. 1). High levels of erythrocyte membrane hexacosanoate were
Table 2
Correlations among age and the levels of erythrocyte membrane C26:0 and plasma total cholesterol, triglycerides, HDL cholesterol and LDL cholesterol in 504 volunteersa
Age RBC 26:0 TC TG HDL-c LDL-c
−0.072
0.379* 0.402* 0.223*
Age – 0.393*
0.433* −0.257* 0.206*
RBC26:0 0.379* – 0.281*
0.401* 0.004 0.798*
LDL-c 0.393* 0.206* 0.798* 0.192* −0.165* –
aValues are Spearman’s correlation coefficients. *,PB0.01; RBC 26:0, erythrocyte membrane C26:0; TC, plasma total cholesterol; TG, plasma
those of the well-known risk factors for atherosclerosis, i.e. plasma total cholesterol, triglycerides, LDL choles-terol, HDL cholesterol and age. Moreover, it was shown that obesity, smoking and atherosclerosis-related diseases were associated with high levels of erythrocyte membrane hexacosanoate. These results suggest that
increased levels of erythrocyte membrane
hexa-cosanoate are closely related to atherosclerosis. High levels of erythrocyte membrane hexacosanoate were more frequent in male subjects than in female subjects, especially in younger ages. This finding sug-gests that levels of erythrocyte membrane hexa-cosanoate can be affected by sex hormones, though its mechanism remains unclear.
What causes an accumulation of hexacosanoate in erythrocyte membranes? Is hexacosanoate accumulated in erythrocyte membranes exogenously or endoge-nously? We cannot clearly answer these questions at present. However, we can speculate that the elevation of erythrocyte membrane hexacosanoate levels reflects the decreased functions of peroxisomes, intracellular organella. Hexacosanoate is oxidized by peroxisomal
b-oxidation [24], and age-related changes in
peroxiso-mal fatty acid oxidation activity were characterized in rodents [25,26]. Besides, it is reported that desorption of hexacosanoate from membranes is slower than that of shorter chain fatty acids [27]. Therefore, a decrement of peroxisomal functions caused by various factors, exogenous or endogenous, may result in the elevation of hexacosanoate levels of erythrocyte membranes and cell membranes of other tissues.
It can be speculated that the accumulation of hexa-cosanoate in cell membranes inevitably reduces the fluidity of the membranes. This reduced membrane fluidity can cause various abnormalities in cell mem-brane functions resulting in abnormalities of intracellu-lar metabolism. Whitcomb and colleagues reported that saturated VLFAs (C26:0 and C24:0) suppressed the adrenocorticotropin responsiveness of cultured human adrenocortical cells, which suggests that hexacosanoate can affect ACTH receptor functions [28]. Their results may suggest a close relationship between saturated VLFAs and membrane receptor functions.
Hexacosanoate is contained more in tightly-bound fatty acids than loosely-bound fatty acids in membrane proteins [29]. Tightly-bound fatty acids are not re-moved by exhaustive extraction with organic solvents, phospholipase A2 treatment, or sodium dodecyl sulfate, but are released by refluxing with hot methanolic HCl or in part by mild alkaline hydrolysis [29,30]. This fact suggests that tightly-bound fatty acids are distinguished from other membrane fatty acids (loosely-bound fatty acids) and may be covalently attached to membrane proteins [30]. Therefore, an elevation of hexacosanoate levels in the tightly-bound fatty acids can crucially affect membrane receptor functions, and may be related
to atherogenic processes in vascular endothelial cells, smooth muscle cells and so on.
If so, reducing membrane hexacosanoate may result in inhibiting the atherosclerotic processes. Several agents were reported to reduce hexacosanoate. For instance, monounsaturated long-chain fatty acids (e.g. eicosenoic acid and docosenoic acid) are known to inhibit the biosynthesis of VLFAs [31]. These fatty acids may prevent atherosclerotic diseases. Further-more, Singh et al. reported that lovastatin, a
3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor
suppressing cholesterol synthesis, markedly reduced ac-cumulated VLFAs in patients with adrenoleukodystro-phy [32]. This finding suggests that some lipid-lowering agents may inhibit atherosclerotic processes by reducing the level of membrane hexacosanoate. In conclusion, our data suggest that erythrocyte membrane hexa-cosanoate may be associated with risk factors for atherosclerosis. However, further investigations are nec-essary to clarify whether or not the elevation of ery-throcyte membrane hexacosanoate levels is a new risk factor for atherosclerosis and how the hexacosanoate accumulation in cell membranes participates in the atherosclerotic processes.
References
[1] Malcom GT, Oalmann MC, Strong JP. Risk factors for atherosclerosis in young subjects: the PDAY Study. Ann NY Acad Sci 1997;817:179.
[2] Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977;62:707.
[3] Castelli WT, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB, Kagan A, Zukel WJ. HDL cholesterol and other lipids in coronary heart disease: the cooperative lipoprotein phenotyping study. Circulation 1977;55:767.
[4] Castelli WP, Garrison RJ, Wilson PWF, Abott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipo-protein cholesterol levels: the Framingham Study. J Am Med Assoc 1986;256:2835.
[5] Stampler J, Wentworth DN, Neaton JD. Is the relationship between serum cholesterol and risk of death from coronary heart disease continuous and graded? Findings on the 356 222 primary screenees of the Multiple Risk Factor Intervention Trial (MR-FIT). J Am Med Assoc 1986;256:2823.
[6] Anderson KM, Wilson PWF, Odell PM, Kannel WB. An up-dated coronary risk profile: a statement for health professionals. Circulation 1991;83:357.
[7] Benfante R, Yano K, Hwang IJ, Curb JD, Kagan A, Ross W. Elevated serum cholesterol is a risk factor for both coronary heart disease and thromboembolic stroke in Hawaiian – Japanese men: implications of shared risk. Stroke 1994;25:814.
[8] Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease: the Framingham Study. Am J Med 1977;62:707.
[10] Kannel WB, Castelli WP. Is the serum total cholesterol an anachronism? Lancet 1979;2:950.
[11] Mawatari S, Antoku Y, Kuroiwa Y. Erythrocyte membrane cation-stimulated ATPase activities in myotonic muscular dys-trophy. J Neurol Sci 1982;53:23.
[12] Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497.
[13] Aveldano M, Horrocks LA. Quantitative release of fatty acids from lipids by a simple hydrolysis procedure. J Lipid Res 1983;24:1101.
[14] Antoku Y, Sakai T, Tsukamoto K, Imanishi K, Ohtsuka Y, Iwashita H, Goto I. A simple diagnostic method of adrenoleukodystrophy: total fatty acid analysis of erythrocyte membranes. Clin Chim Acta 1987;169:121.
[15] Antoku Y, Sakai T, Goto I, Iwashita H, Kuroiwa Y. Adrenoleukodystrophy: abnormality of very long-chain fatty acids in erythrocyte membrane phospholipids. Neurology (Cleve-land) 1984;14:1499.
[16] Antoku Y, Sakai T, Goto I, Iwashita H, Kuroiwa Y. Fatty acid analysis of total glycerophospholipids in erythrocyte membranes. Acta Neurol Scand 1985;72:193.
[17] Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974;20:470.
[18] Sugiura M, Oikawa T, Hirano K, Maeda H, Yoshimura H, Sugiyama M, Kuratsu T. A simple colorimetric method for determination of serum triglycerides with lipoprotein lipase and glycerol dehydrogenase. Clin Chim Acta 1977;81:125.
[19] Shiraik, Nema T, Hiroh Y, Itoh Y, Miyashita Y, Watanabe H. Clinical efficacy of the direct assay method using polymers for serum high density lipoprotein cholesterol. J Clin Lab Anal 1997;11:82.
[20] Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499.
[21] Moser HW, Moser AB, Frayer KK, Chen W, Schulman JD, O’Neill BP, Kishimoto Y. Adrenoleukodystrophy: increased plasma content of saturated very long-chain fatty acids. Neurol-ogy (NY) 1981;31:1241.
[22] Tsuji S, Suzuki T, Ariga M, Sekine M, Kuriyama M, Miyatake T. Abnormality of long-chain fatty acids in erythrocyte mem-brane sphingomyelin from patients with adrenoleukodystrophy. J Neurochem 1981;36:1046.
[23] Antoku Y, Ohtsuka Y, Nagara H, Sakai T, Tsukamoto K, Iwashita H, Goto I. A comparison of erythrocytes, lymphocytes and blood plasma as samples in fatty acid analysis for the diagnosis of adrenoleukodystrophy. J Neurol Sci 1989;94:193. [24] Pe´richon R, Bourre JM, Kelly JF, Roth GS. The role of
perox-isomes in aging. CMLS Cell Mol Life Sci 1998;54:641. [25] Pe´richon R, Bourre JM. Peroxisomal boxidation activity and
catalase activity during development and aging in mouse liver. Biochimie 1995;77:288.
[26] Pe´richon R, Bourre JM. Aging-related decrease in liver peroxiso-mal fatty acid oxidation in control and clofibrate-treated mice: a biochemical study and mechanistic approach. Mech Ageing Dev 1996;87:115.
[27] Ho JK, Moser H, Kishimoto Y, Hamilton JA. Interactions of a very long chain fatty acid with model membranes and serum albumin: implications for the pathogenesis of adrenoleuko-dys-trophy. J Clin Invest 1995;96:1455.
[28] Whitcomb RW, Linehan WM, Knazek RA. Effects of long-chain fatty acids on membrane microviscosity and adrenocorti-cotropin responsiveness of human adrenocortical cells in vitro. J Clin Invest 1988;81:185.
[29] Antoku Y, Sakai T, Goto I, Iwashita H, Kuroiwa Y. Tightly bound fatty acids in the erythrocyte membrane proteins in myotonic dystrophy. Exp Neurol 1985;87(2):206.
[30] Marinetti GV, Cattieu K. Tightly (covalently) bound fatty acids in cell membrane proteins. Biochim Biophys Acta 1982;685:109. [31] Koike R, Tsuji S, Ohno T, Suzuki Y, Orii T, Miyatake T. Physiological significance of fatty acid elongation system in adrenoleukodystrophy. J Neurol Sci 1991;103(2):188.
[32] Singh I, Khan M, Key L, Pai S. Lovastatin for X-linked adrenoleukodystrophy. N Engl J Med 1998;339(10):702.