The development of plants, compared with that of ani-mals, is influenced much more by the environment in which they grow, suggesting that plants have evolved mechanisms that relay environmental signals to con-trol cell division and ultimately plant growth. Most of the divisional activity in plants is localized in small groups of cells, called meristems (see Box 1), that are al-ready present in the embryo and are active during most of the life cycle of the plant. How environmental cues trigger changes in cell-division activity in meristems – that is, how the plant connects environmental changes with molecular changes in the machinery controlling the cell cycle – is largely unknown.
The cell cycle consists of the alternating phases of DNA replication (S phase) and chromosome sepa-ration (mitosis, or M phase) interrupted by gaps known as G1 (interval between M and S phases) and G2 (interval between S and M phases). Important controls operate at the transition points as cells move from G1 into S phase, and from G2 into M phase, primarily through the regulated kinase activ-ity of cyclin-dependent kinases (CDKs).
Both the G1–S and G2–M phase transitions can be controlled in plant cells in response to changing conditions. For example, during the early stages of root nodule initiation in pea and alfalfa, the root cortical cells susceptible to Rhizobium infection are in G0/G1 (Ref. 1). By contrast, before germination of seeds, the cells of the embryo are arrested in G1, or partly in G1 and partly in G2, depending on the species2. An example of G2 control is found in radish lateral root primordia, which are derived from pericycle cells arrested in G2. These cells move into M phase upon auxin stimulation3 and then continue to proliferate, producing a lateral root pri-mordium that eventually emerges from the side of the primary root.
In addition to these specific developmental con-trols, the cell cycle in plants plays an important role in growth responses to the environment. If the grass Dactylisis exposed to increased levels of CO2, cells in the shoot meristem increase their rate of prolifer-ation, resulting in faster growth. This occurs partly through an increase in the number of cells actively involved in division, and partly through a shorten-ing of the cell cycle, particularly the G1 phase4. Interestingly, both the shoot and root apical meri-stems are a mosaic of fast and slow cycling cells, and the main difference between these two populations is in the length of G1 (Refs 5 and 6), suggesting that this phase is the most responsive to signals that change cell-cycle length. The significance of G1 controls in commitment to the cell cycle has been shown in yeast, flies and mammals7, and, as excel-lent reviews covering the complete plant cell cycle have appeared recently8–10, we will limit ourselves here to a discussion of the G1–S transition in plants and how this is controlled. At the end of the review, we turn to the intriguing question of how cell divi-sion patterns are coordinated within the meristem.
The cyclin D–retinoblastoma–E2F pathway Eukaryotic cells in G1 phase have several options. The most obvious is that, in the presence of sufficient
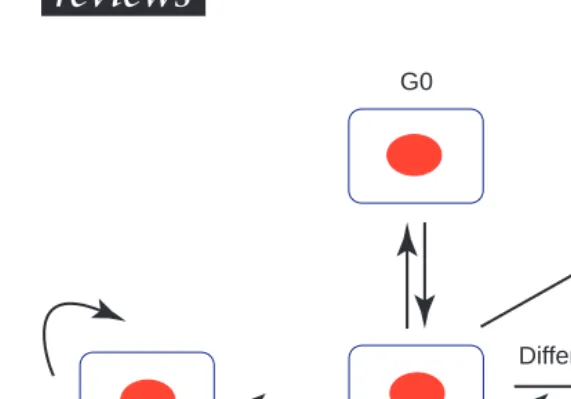
stimuli, they commit to further cell division and progress into S phase with the initiation of DNA synthesis. However, there are several other cell fates, including differentiation, programmed cell death and the adoption of a quiescent state (G0) (Fig. 1). We will first summarize the molecular events asso-ciated with the first option, the G1–S transition, and emphasize the important parallels found between mammals and plants.
In mammalian cells, the retinoblastoma protein (Rb) and its relatives, p107/p130, are important in preventing cells from progressing into S phase, by binding members of the E2F family of transcription factors that are present on promoters driving S-phase specific genes. Recently, it has been recog-nized that the active recruitment of histone deacetylase by pRb is important in keeping E2F-responsive genes switched off during G1, by creating an inactive chromatin structure11. It is only when Rb itself gets inactivated by phosphorylation in late G1 that genes under E2F control are relieved from re-pression and their subsequent exre-pression can provide the activities needed for S-phase entry12.
Phosphorylation of Rb in G1 is a two-step process involving the sequential action of cyclin D–CDK
Triggering the cell
cycle in plants
Bart G. W. den Boer and
James A. H. Murray
In essence, the mitotic cell cycle in eukaryotes involves the
duplication and separation of chromosomes, coupled to the
process of dividing one cell into two. Cytokinesis is therefore the
culmination of a series of events that were triggered during G1
phase, and brings the daughter cells back to the starting position
in G1 for another possible round of division. In all eukaryotes,
progression through the cell cycle is controlled by
cyclin-dependent kinases that bind to positive regulators called cyclins.
This review explores some of the pathways that trigger the plant
cell cycle, with emphasis on the G1 phase. Examples include
signalling pathways involving glutathione and cellular redox
potential, the possible existence of a G1 DNA-damage
checkpoint, and the plant hormones auxin and cytokinin.
Progress in understanding the link between cell proliferation, cell
differentiation and the cell-cycle machinery in a developmental
context is discussed.
Bart den Boer is at Aventis
and cyclin E–CDK complexes13. It is initiated fol-lowing stimulation by mitogens, which induce and maintain D-type cyclin expression. This is followed by a wave of cyclin E expression in late G1 (Ref. 14). The activities of these cyclin–CDK complexes is in turn constrained by CDK inhibitor proteins (CKIs). In mammals, these proteins fall into two families based on their structure and CDK targets. The INK4 family specifically inhibits the catalytic subunits of
CDK4 and CDK6 (the CDK partners of D-type cy-clins), whereas members of the CIP/KIP family act more broadly by inhibiting the kinase activity asso-ciated with cyclin D-, E- and A-dependent kinases15.
Plants
In plants, homologues of most of the key players in the Rb pathway (Fig. 2) have been identified, and most show structural and functional similarities to their animal counterparts. As yeasts do not contain direct homologues of proteins involved in the Rb pathway, the backbone of this pathway appears to be conserved between animals and plants, but not fungi16,17. There are three main classes of plant D-type cyclin (CycD) genes18, but these are not re-lated to the individual groups of mammalian D-type cyclins, indicating that the increase in gene number in the cyclin-D family might have occurred inde-pendently in animals and plants. As in animal cells, the transcription of some types of CycD is inducible during cell-cycle entry by mitogens, but generally stays relatively constant once cells are involved in continuous proliferation19.
Like animals, plants contain several types of CDK-like genes, including direct homologues of fission yeast cdc2+, which contains a consensus amino acid
sequence PSTAIRE in its cyclin-binding region. Direct homologues of cdc2 are called CDK1 or cdc2 in mammals, and cdc2a or CDK-a in plants. Plants have no direct equivalents of other mammalian CDKs, but have plant-specific CDK variants with the consensus sequence PPTALRE (CDK-b1) or PPTTLRE (CDK-b2). These are unique among CDKs in showing cell-cycle regulation of expression, being transcribed only from S until M phase9. Differences are seen in the expression timing of the PPTALRE and PPTTLRE subgroups10.
In mammalian cells, the archetypal PSTAIRE-containing CDK1 is involved mainly in the G2–M transition and al-ternative CDKs control the G1 phase (CDK4, CDK6) and the G1–S transition (CDK2). However, in plants, only CDK-a protein (the CDK1 equivalent) has been detected during the G1 phase. CDK-a, however, is presumably not specific for the G1–S transition as its levels are rela-tively constant during the cell cycle, and CDK-a-associated activity peaks at both the G1–S and the G2–M boundaries. Its activity is supplemented by CDK-b ki-nase activity during G2–M. CycD cyclins are regulatory partners of plant CDK-a during the G1–S phase transitions.
What is the substrate for the cyclin D–CDK activity? In mammals, Rb is the preferred substrate of G1 kinases, and Rb-like homologues have been reported in maize17 and tobacco20. In tobacco, complexes of CDK-a with CycD3 can be detected in vivo, and, when expressed in insect cells, these can phosphorylate the tobacco Rb-related protein in vitro20. trends in Cell Biology
G1 ’stem cell’
G1
Programmed cell death
Differentiate
De-differentiate
FIGURE 1
Options for G1 cells in plants. Newborn G1 cells can start another round of division (‘stem cell’) or exit the cycle (non-cycling cells). These cells die (programmed cell death), return into the cell cycle or differentiate. In contrast to animals, differentiated plant cells can more readily de-differentiate and re-enter the cell cycle,
given the appropriate signals.
trends in Cell Biology
cycD cycD
CDK-a
Sucrose
cycD3 cycD2
CDK-a
CDK-a
E2F inactive
E2F active P
G1 S
G1/S transition ICK1
ABA
Auxin
CAK ? P
E2F Rb
P
P P
P P
S-phase genes
S-phase genes
START Cytokinin
E2F ICK1
Rb
FIGURE 2
Having established that the upstream compo-nents of the cyclin D–Rb pathway are conserved in plants and animals, the next obvious question is whether conservation continues downstream: in other words, are there E2F transcription factors in plants? Until recently, the only evidence was indi-rect, as it was shown that maize Rb1 can bind to human and Drosophila E2F and inhibits the tran-scriptional activation ability of human E2F16. This changed with the identification and analysis of wheat and tobacco clones21,22. Wheat and tobacco E2F were shown to interact respectively with maize Rb1 and tobacco Rb in yeast two-hybrid assays. For wheat E2F, the interaction was also confirmed in vitroand the Rb-binding motif was mapped to the C-terminal end.
CDK activity requires activating phosphorylation by CDK-activating kinase (CAK)23, but there is little evidence that this regulates cell-cycle progression. However, inhibitory phosphorylation by wee1-like kinases is likely to play a significant regulatory role24. In addition, four CDK inhibitor (CKI) protein genes are reported in plants, but only one, ICK1, has been characterized biochemically25,26. ICK1 inter-acts with both CDK-a (cdc2a) and cyclin CycD3 in vitro, is an inhibitor of plant cdc2-like kinases and its C-terminal consensus sequence resembles part of the CDK2-binding domain of the mammalian CKI p27Kip1 (Ref. 25). Its activity throughout the cell
cycle has not been investigated, nor is it clear whether overexpression in plant cells can inhibit progression. Interestingly, ICK1 expression is in-duced by the stress hormone abscisic acid, although it is not known whether this is a direct effect25.
Thus, although several players involved in the G1–S transition in plants are structurally and func-tionally similar to their animal counterparts, differ-ences are apparent. For example, a cyclin E homo-logue has not been identified in plants. This might not come as a surprise as cyclin E and the D-type cy-clins bind to different CDKs (CDK2 and CDK4/6 re-spectively) in animals, and only one CDK activity has been reported at the G1–S transition in plants9. Perhaps plant Rb could be phosphorylated by se-quential cyclin D kinase activity as there are three main groups of plant CycD proteins with differential regulation and timing of expression (CycD1–3)18.
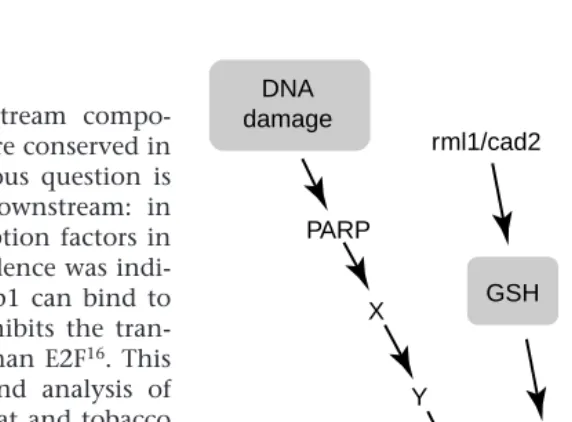
Pathways triggering the cell cycle in plants The rest of this review focuses on four examples of signalling pathways that feed into the plant cell cycle (Fig. 3). The DNA damage and glutathione pathways are not specific to plants, whereas the study of pathways relating to auxin–cytokinin and meristem function are plant-specific solutions to the problems of integrating cell division with differ-entiation and development.
DNA damage pathway
DNA damage occurs in all living things and our understanding of the control of cell cycle by DNA damage has made enormous progress in yeasts and mammals27. Mammalian cells are known to arrest in G1 upon DNA damage. This block is induced by the
tumour suppressor protein p53 (Ref. 28) that acti-vates transcription of p21CIP1, resulting in elevated
levels of this CKI. This inhibits the activity of G1 CDKs, leading to a G1 arrest29. The p53 protein can associate with poly(ADP-ribose) polymerase (PARP), another protein that senses DNA damage, and inhi-bition of PARP activity leads to loss of p21 upregu-lation in response to DNA damage30. PARP is cata-lytically activated by DNA strand breaks, and is responsible for the poly(ADP-ribosylation) of vari-ous nuclear proteins using NAD as substrate31.
Although there is evidence that major DNA repair mechanisms found in other species also occur in plants32, it is not known whether a G1 checkpoint control exists in plants. However, it is interesting to note that one of the Arabidopsis PARP genes is tran-scriptionally activated by the genome instability re-sulting from a mutation in the DNA ligase I gene33, suggesting that at least part of the DNA damage sig-nalling pathway might be conserved. However, with over 85% of the Arabidopsis genome sequence al-ready completed, no proteins homologous to p53 have been found. As p53 is also intimately involved in the control of apoptosis in animals34,35, its poss-ible absence in plants raises the possibility of differ-ent links between the cell cycle, DNA damage responses and programmed cell death36.
GSH-dependent control of the G1–S transition Glutathione (GSH) is an abundant and ubiquitous thiol with proposed functions in the adaptation of plants to extreme temperatures, tolerance to xeno-biotics and to biotic and abiotic environmental stresses37. Until recently, a direct link between GSH
trends in Cell Biology
G1 S
Auxin
cycD3/CDK-a Cytokinin DNA
damage
PARP
X
Y
GSH rml1/cad2
ABA
ICK1
?
FIGURE 3
levels and the cell-division cycle was lacking, but this changed when Vernoux and colleagues uncovered a role for intracellular GSH in the G1–S transition in plants38during the cloning of the ROOTMERISTEM-LESS (RML)gene. RML encodes the first enzyme of GSH biosynthesis (g-glutamylcysteine synthetase). Moreover, depletion of intracellular GSH by addition of an inhibitor of GSH biosynthesis to Arabidopsisor tobacco seedlings or tobacco BY-2 cells abolishes cell division. Interestingly, the cell-division block in seedlings affects only the root meristem, and the shoot apical meristem is unaffected.
Is there a physiological significance to the block of cell division caused when intracellular GSH concen-tration is reduced artificially? In other words, does the plant modulate its endogenous GSH levels dur-ing normal development to change the cell-division cycle? An indication that this could be the case is that, in Arabidopsisroots, high levels of GSH (meas-ured by a reporter) are associated with proliferating cells such as epidermal and cortical initials, whereas reduced levels of GSH were found in the slowly cy-cling cells of the quiescent centre that have an ex-tended G1 (Ref. 39). It would be interesting to see whether an increase in GSH levels could stimulate
Arabidopsisquiescent cells to enter the cell cycle, as was shown in maize for ascorbic acid, another molecule in-volved in the removal of active oxygen species40. Thus intracellular redox homeostasis could affect cell-cycle pro-gression by regulating key components of the G1–S transition37. It is not clear, however, how this would operate at the molecular level.
Oxidative stress and GSH-dependent transduction pathways probably also impinge on the animal cell cycle as GSH depletion leads to an arrest of cell-cycle progression in human cells41. A candidate G1–S cell-cycle factor through which the GSH pathway might have its effect in mammals could be the inhibitor p21, as mammalian cells blocked in G1 due to a low GSH level show high levels of p21 protein41.
Auxin and cytokinin action
In higher plants, only two groups of hormones, the auxins and cytokinins, are generally stimulatory to the proliferation of most cell types42. Many plant tissues, such as leaf, root or stem pieces, can be ex-planted into culture, where dedifferentiation and proliferation occur to form a callus of largely un-differentiated cells. This process normally occurs if both auxin and cytokinin are present. Conceptually, the link between auxin–cytokinin ac-tion and cell division has been around for more than 40 years43, but the progress in understanding the molecular basis of their action in cell prolifer-ation has been very slow until recently. Auxin alone increases the level of a CDK protein in cultured to-bacco cells and stem pith explants, but addition of a cytokinin was required for activation of this ki-nase44. The b-glucuronidase reporter gene under control of the ArabidopsisCDK-a (cdc2a) promoter is inducible by auxins and, to a lesser degree, by cy-tokinins45. However, it is often difficult to separate the direct control of cell-cycle genes by hormones from their indirect induction as a consequence of the cell-cycle progress that the hormones provoke. Progress in analysing modes of action has also been hampered by the different types of interaction that both hormones show, depending on the plant species or tissue type46.
At what point of the cell cycle do auxins and cytokinins act? Somewhat confusingly, both hor-mones have been associated with progression through the G1–S and the G2–M control points. Suspension cells arrest in both G1 and G2 phases after they are transferred to medium containing cytokinin but lacking auxin44,47. However, most progress has been made in linking cytokinin to the cell cycle. First, cells of certain suspension cultures arrest in G2 when deprived of cytokinin, and these cells have inactive CDK complexes. Addition of cytokinin or tyrosine dephosphorylation restores kinase activity47, suggesting that cytokinin-depleted cells accumulate CDK–cyclin complexes that are
trends in Cell Biology
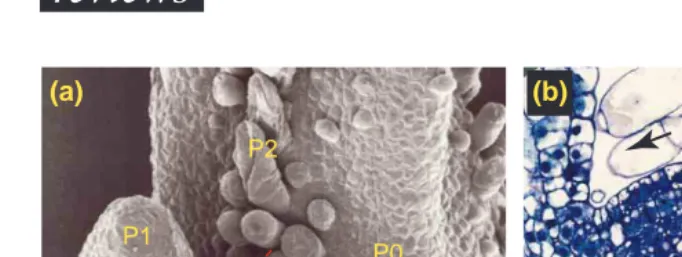
P1
P2
P0
(a)
(b)
L1
L2
FIGURE 4
Cell proliferation in the shoot apex. (a) Scanning electron micrograph of a tobacco shoot apical meristem (SAM). Leaf primordia are initiated on the flanks of the apical meristem. P0 is the youngest primordium, P1 the next oldest primordium, and P2 the oldest. Hatched line indicates the plane of section in (b). Bar, 100mm. (b) Cross-section through the SAM and flanking leaf primordia. Note the layered configuration of the dome (L1 and L2). Proliferating cells in the peripheral zone are displaced towards the leaf primordia where eventually they will exit the cell cycle and differentiate. Black arrows indicate the direction of cell displacement. Bar, 100 mm.
BOX 1 – GLOSSARY
Primordium – A group of cells that will develop into a new organ.
Meristem – group of cells acting as a source of cells for all main plant organs.
Pericycle – layer between the endodermis and the con-ducting tissue, from which lateral roots arise.
Cortex – bulk tissue in stem and root that lies between the epidermis and the central tissues.
inactive due to phosphorylation of the threonine 14 and/or tyrosine 15 regulatory residues of the CDK subunit. Second, in tobacco BY-2 cells, the cytokinin biosynthesis inhibitor lovastatin is able to block cells in G2, and this can be reversed by addition of exogenous cytokinin48. However, proof that cy-tokinin operates through CDK dephosphorylation in vivois still lacking.
Evidence has emerged that cytokinin also regu-lates the G1–S transition. Cytokinin application to shoot meristems reduces the size of chromosomal DNA replication units, resulting in the closer spac-ing of sites of DNA replication initiation. This leads to faster DNA synthesis, thereby providing a mecha-nism for the shortening of S phase that was ob-served49. More recently, CycD3 has been shown to be induced by cytokinin in both cultured cells and intact plants50, suggesting a role in the cytokinin control of cell division. If CycD3 is a primary target of cytokinin action in G1–S control, constitutive ex-pression of this cyclin should be able to bypass the requirement for cytokinin. In a key experiment, CycD3 was expressed in stable Arabidopsis transfor-mants, and was found to remove the requirement for exogenous cytokinin during callus initiation and growth from leaf pieces50. The timing of CycD3 in-duction occurs slightly before the expression of an S-phase marker gene histone H4 in partially syn-chronized Arabidopsis cells, pointing to its action during the G1 phase50. It is attractive to speculate that the increase in the number of DNA replication origins caused by cytokinin application might also be due to increased CycD3-associated kinase activ-ity. The different steps in the signalling pathway from cytokinin to CycD3 are unknown but might involve a phosphorelay as used in bacterial two-component signalling51 as CycD3 induction was found to be independent of protein synthesis50.
Coordination of cell division in intact plants Understanding plant growth and development re-quires an understanding of how plant cells grow and divide and how positional information is integrated with cell division. As meristems provide the cells that eventually give rise to the root, leaves, stem and flowers, it is essential to understand the molecular controls underlying cell division in the primary root meristems and shoot apical meristems52. Here we take the divisional activity of cells present in the shoot apical meristem (SAM) as an example (Fig. 4a). SAMs of higher plants are not homogeneous, as there is a gradient of cell division and growth rate within the apex53. At the summit (central zone) cells have long cell-cycle times, whereas displaced cells that end up at the flank in emerging primordia di-vide faster. This reduction in cell-cycle time is due mainly to a shortening of G1 phase, although the G2 phase is reduced in some species5. This picture of divisional activity is superimposed on the layered structure of the SAM. Cells in the two outermost layers (L1 and L2, Fig. 4b) divide primarily with cell walls normal to the surface of the meristem, so cells in these sheets rarely move from one layer to another54. It is not known how the pattern of cell
division activity, which seems to bear no resem-blance to the layered structure of the apex, is coor-dinated in these different layers, particularly because there are cells with faster and slower cell-cycle times in all meristem zones.
A little later, when groups of cells develop into or-gans, individual cells exit the cell cycle and differ-entiate into specific cell types. During maize leaf de-velopment, the exit from G1 occurs from tip to base, and is correlated with Rb expression and loss of cell-division activity16.
Somehow, the fate of cells that are in G1, and therefore could exit the cell cycle, is influenced by signalling molecules originating from neighbouring cells or cells even further away55. Little is known about the connections between signalling pathways operating at type level in organs and the cell-division cycle, although several mutations have been identified that disrupt patterned cell division operat-ing in meristems, and some of the affected genes have been cloned. This should allow the identification of molecular pathways describing the connection between known signalling cascades and the cell-cycle machinery, – that is, the activity of cyclin–CDK complexes. One might have expected to identify cell-cycle components such as cyclins or CDKs by this route, but this was not the case. Most of the mutations are present in genes that encode homeodomain proteins, components of a common signal-transduction pathway or evolutionary con-served members of the piwifamily56,57. Such genes act upstream in cell division control, in the sense that they are probably involved both in the correct patterning of cell division within the meristem and in ensuring the continued proliferation of meristem cells.
Recent work on the AINTEGUMENTA (ANT) gene suggests that it might exert a more direct regulation on the proliferation of meristem cells as overex-pression of ANT results in larger organs containing more cells without changes to the morphology of the final organ58,59. Conversely, a loss-of-function ant mutation decreases floral meristem size and floral organ size by reducing cell number59.
Conclusions and prospects
Yeasts, plants and mammals control progression through their cell cycles by using CDKs that bind to cyclins. During evolution, plants and mammals have evolved into multicellular organisms with many different cell types, a change that is marked by the appearance of the cyclin D–Rb–E2F pathway. Perhaps this is important in allowing the differenti-ation of multiple cell types in complex tissues.
the molecular details of a plant hormone in cell-cycle control are starting to be understood, and the importance of oxidative stress is apparent from the cell-cycle arrest brought about by inhibition of glutathione biosynthesis. With the complete genome sequence of Arabidopsis within reach, a complete inventory of the cell-cycle genes in a higher multicellular eukaryote is conceivable.
References
1 Yang, W-C. et al.(1994) RhizobiumNod factors reactivate the cell cycle during infection and nodule primordium formation, but the cycle is only completed in primordium formation. Plant Cell6, 1415–1426
2 Bewley, J.D. and Black, M. (1994) Seeds. Physiology of Development and Germination(2nd edn), Plenum Press
3 Blakely, L.M. and Evans, T.A. (1979) Cell dynamics studies on the pericycle of radish seedling roots. Plant Sci. Lett.14, 79–83
4 Kinsman, E.A. et al.(1997) Elevated CO2stimulates cells to divide in grass meristems: a differential effect in two natural populations of Dactylis glomerata. Plant Cell Environ.20, 1309–1316
5 Lyndon, R.F. (1973) in The Cell Cycle in Development and Differentiation (Balls, M. and Billett, F.S., eds), pp. 167–183, Cambridge University Press
6 Clowes, F.A.L. (1976) in Cell Division in Higher Plants(Yeoman, M.M., ed.), pp. 253–284, Academic Press
7 Neufeld, T.P. and Edgar, B.A. (1998) Connections between growth and the cell cycle. Curr. Opin. Cell Biol.10, 784–790
8 Fowler, M.R. et al.(1998) The plant cell cycle in context. Mol. Biotechnol. 10, 123–153
9 Mironov, V. et al.(1999) Cyclin-dependent kinases and cell division in plants – the nexus. Plant Cell11, 509–521
10 Huntley, R.P. and Murray, J.A.H. (1999) The plant cell cycle. Curr. Opin. Plant Biol.2, 440–446
11 Lipinski, M.M. and Jacks, T. (1999) The retinoblastoma gene family in differentiation and development. Oncogene18, 7873–7882
12 Lavia, P. and Jansen-Dürr, P. (1999) E2F target genes and cell-cycle checkpoint control. BioEssays21, 221–230
13 Lundberg, A.S. and Weinberg, R.A. (1998) Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin–cdk complexes. Mol. Cell. Biol. 18, 753–761
14 Sherr, C.J. (1993) Mammalian G1cyclins. Cell73, 1059–1065
15 Sherr, C.J. and Roberts, J.M. (1999) CDK inhibitors: positive and negative regulators of G1phase progression. Genes Dev.13, 1501–1512
16 Huntley, R.P. et al.(1998) The maize retinoblastoma protein homologue ZmRb-1 is regulated during leaf development and displays conserved interactions with G1/S regulators and plant cyclin D (cycD) proteins. Plant Mol. Biol.37, 155–169
17 Gutiérrez, C. (1998) The retinoblastoma pathway in plant cell cycle and development. Curr. Opin. Plant Biol.1, 492–497
18 Meijer, M. and Murray, J.A.H. The role and regulation of D-type cyclins in the plant cell cycle. Plant Mol. Biol.(in press)
19 Murray, J.A.H. et al.(1998) in Plant Cell Division(Francis, D. et al., eds), pp. 99–127, Portland Press
20 Nakagami, H. et al.(1999) Tobacco retinoblastoma-related protein phosphorylation by a distinct cyclin-dependent kinase complex with Cdc2/cyclin D in vitro. Plant J.18, 243–252
21 Ramírez-Parra, E. et al.(1999) The cloning of plant E2F, a retinoblastoma-binding protein, reveals unique and conserved features with animal G(1)/S regulators. Nucleic Acids Res.27, 3527–3533
22 Sekine, M. et al. (1999) Isolation and characterization of the E2F-like gene in plants. FEBS Lett.460, 117–122
23 Umeda, M. et al.(1998) A distinct cyclin-dependent kinase-activating kinase of Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 95, 5021–5026
24 Sun, Y. et al.(1999) Characterization of maize (Zea mays L.) Wee1 and its activity in developing endosperm. Proc. Natl. Acad. Sci. U. S. A. 96, 4180–4185
25 Wang, H. et al.(1998) ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thalianainteracts with both cdc2a and CycD3, and its expression is induced by abscisic acid. Plant J.15, 501–510
26 Inzé, D. et al.(1999) Trends in plant cell cycle research. Plant Cell11, 991–994
27 Paulovich, A.G. et al.(1997) When checkpoints fail. Cell88, 315–321
28 Levine, A.J. (1997) p53, the cellular gatekeeper for growth and division. Cell88, 323–331
29 El-Deiry, W.S. et al.(1993) WAF1, a potential mediator of p53 tumour suppression. Cell75, 817–825
30 Vaziri, H. et al.(1997) ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J.16, 6018–6033
31 De Murcia, G. and Ménissier-de Murcia, J. (1994) Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem. Sci.19, 172–176
32 Britt, A.B. (1999) Molecular genetics of DNA repair in higher plants. Trends Plant Sci. 4, 20–25
33 Babiychuk, E. et al.(1998) Higher plants possess two structurally different poly(ADP-ribose) polymerases. Plant J. 15, 635–645
34 Sherr, C.J. (1998) Tumor surveillance via the ARF–p53 pathway. Genes Dev. 12, 2984–2991
35 Sionov, R.V. and Haupt, Y. (1999) The cellular response to p53: the decision between life and death. Oncogene18, 6145–6157
36 Pennel, P.I. and Lamb, C. (1997) Programmed cell death in plants. Plant Cell9, 1157–1168
37 May, M.J. et al.(1998) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J. Exp. Bot.49, 649–667
38 Vernoux, T. et al.(2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell12, 97–110
39 Sánchez-Fernández, R. et al.(1997) Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc. Natl. Acad. Sci. U. S. A.94, 2745–2750
40 Kerk, N.M. and Feldmann, L.J. (1995) A biochemical model for the initiation and maintenance of the quiescent center: implications for organisation of root meristems. Development121, 2825–2833
41 Russo, T. et al.(1995) A p53-independent pathway for activation of WAF1/CIP1 expression following oxidative stress. J. Biol. Chem. 270, 29386–29391
42 Davies, P.J. (1995) in Plant Hormones(Davies, P.J., ed.), pp. 1–12, Kluwer Academic
43 Skoog, F. et al.(1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol.11, 118–131
44 John, P.C.L. et al.(1993) p34cdc2related proteins in control of cell cycle progression, the switch between division and differentiation in tissue development, and stimulation of division by auxin and cytokinin. Aust. J. Plant Physiol. 20, 503–526
45 Hemerly, A.S. et al.(1993) Cdc2aexpression in Arabidopsisis linked with competence for cell division. Plant Cell5, 1711–1723
46 Coenen, C. and Lomax, T.L. (1997) Auxin–cytokinin interactions in higher plants: old problems and new tools. Trends Plant Sci.2, 351–356
47 Zhang, K. et al.(1996) Cytokinin controls the cell cycle at mitosis by stimulating the tyrosine dephosphorylation and activation of p34cdc2 -like H1 histone kinase. Planta200, 2–12
48 Laureys, F. et al.(1998) Zeatin is indispensable for the G2–M transition in tobacco BY-2 cells. FEBS Lett.426, 29–32
49 Houssa, C. et al.(1994) Activation of latent DNA-replication origins: a universal effect of cytokinins. Planta193, 247–250
50 Riou-Khamlichi, C. et al.(1999) Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science283, 1541–1544
51 D’Agostino, I.B. and Kieber, J.J. (1999) Molecular mechanisms of cytokinin action. Curr. Opin. Plant Biol.2, 359–364
52 Meyerowitz, E.M. (1997) Genetic control of cell division patterns in developing plants. Cell88, 299–308
53 Lyndon, R.F. (1999) in The Shoot Apical Meristem: its Growth and Development(Lyndon, R.F., ed.), pp. 43–69
54 Satina, S. et al.(1940) Demonstration of the three germ layers in the shoot apex of Daturaby means of induced polyploidy in periclinal chimeras. Am. J. Bot. 27, 895–905
55 Szymkowiak, E.J. and Sussex, I.M. (1996) What chimeras can tell us about plant development. Annu. Rev. Plant Physiol. Plant Mol. Biol.47, 351–376
56 Lenhard, M. and Laux, T. (1999) Shoot meristem formation and maintenance. Curr. Opin. Plant Biol.2, 44–50
57 Cox, D.N. et al.(1998) A novel class of evolutionary conserved genes defined by piwiare essential for stem cell self-renewal. Genes Dev.12, 3715–3727
58 Mizukami, Y. and Fischer, R.L. (2000) Plant organ size control: AINTEGUMENTAregulates growth and cell numbers during organogenesis. Proc. Natl. Acad. Sci. U. S. A.97, 942–947