Review
A review of microbiology in swine manure odor control
Jun Zhu
∗Biosystems & Agricultural Engineering, University of Minnesota, Southern Experiment Station, 35838-120th Street, Waseca, MN 56093, USA
Received 15 April 1999; received in revised form 16 July 1999; accepted 30 July 1999
Abstract
Generation of odors is a complex process that involves many bacterial species, producing an extensive array of volatile organic compounds under different manure storage systems currently used. A lack of understanding of the basic microbiology in manure leads to a poor odor prevention and control from animal wastes. This review covers pertinent available information about the indigenous bacterial genera in swine manure and their potentials of producing odorous volatile compounds. It addresses not only the odorous compounds in swine manure but also the inherent relations between the bacterial species and the related compounds. It also discusses several odor control techniques that have been developed based on microbial activities and the limitations with these techniques. Two bacterial genera, Eubacterium and Clostridium, are most likely the major contributors to odorous volatile fatty acids. It appears that anaerobic lagoons may not be an appropriate choice for treating swine manure for odor control due to the reduced methonogenic activities resulted from the low temperatures in lagoon liquid. Also, it seems questionable that the microbial-based manure additives will work, without aeration, in a real storage system for the purpose of odor control. ©2000 Elsevier Science B.V. All rights reserved.
Keywords: Microbiology; Swine manure; Odor control
1. Introduction
The trend toward high-density, confinement rear-ing of hogs has increased tremendously in recent years. Associated with this increase is the air pol-lution problem (odors) which has become a center of public concern. This is reflected in the increased frequency of odor-related complaints in areas where swine production facilities are more intensified. Odor management is currently impacting many aspects of the swine industry and there appears a potential that the sustainability, productivity, and profitability of swine producers will be dependent upon whether they
∗Tel.:+1-507-835-3620; fax:+1-507-835-3622. E-mail address: [email protected] (J. Zhu).
can reduce the emission of offensive odorants from operating swine production units to a level which surrounding communities could tolerate. Therefore, there exists an acute need for effective methods of odor control, for if the swine industries are to coexist with their neighbors, such control measures will have to be put into operation.
Microbial activities are normally considered to be responsible for the malodor generation from the stored swine manure slurry. As a matter of fact, microbes play a major role in both production and reduction of malodors. In odor generation, the odorous volatile or-ganic compounds are the normal end products or inter-mediate products of fermentative degradation of fecal substances by anaerobic bacteria. In odor reduction, many odor control techniques that are being developed
rely on the microbial properties in the swine manure. Since the malodor originates from microbial activi-ties involving a variety of microbes, understanding the characteristics of the microflora present in swine ma-nure is essential for developing effective odor control techniques.
This paper reviews the available information in the literature related to the types of bacteria in swine ma-nure, the potential odorous compounds associated with different bacterial genera, and the corresponding tech-niques used to control odor based on microbiological principles. Areas that need further research are also recommended.
2. Microflora in swine manure and odor indicators
2.1. Bacterial genera indigenous to swine manure
Several studies have revealed the types of bacte-ria that can be isolated from fresh intestinal or fe-cal material of swine. Rall et al. (1970) reported that there were six groups of swine fecal bacteria according to metabolic functions: lactose fermenters; nonlactose fermenters; Clostridium sp.; Lactobacillus sp.; Ente-rococci; and Staphylococcus sp.. The population sizes for the identified bacterial groups in descending order are lactose fermenters, Lactobacillus sp., Clostridium sp., nonlactose fermenters, and Staphylococcus sp.. The classifications of bacterial groups based on func-tions instead of genera or species may not be of much help in determining the contributions of each single species to manure odor generation. On the other hand, it is usually difficult to clearly classify the bacterial groups only according to their metabolic characteris-tics. For example, Enterococci in the above discussion can also be grouped into lactose fermenters (Orvin, 1986).
Nuru et al. (1972) found that the fecal bacteria of pigs mainly consisted of gram-positive cocci
(Strep-tococcus, Peptostrep(Strep-tococcus, and Staphylococcus), Lactobacillus, Escherichia, and Bacillus. By using
strictly anaerobic culturing methods, Salanitro et al. (1977) concluded that the predominant fecal mi-croflora isolated from adult swine comprised several bacterial groups; namely, fecal streptococci,
Eubac-terium sp., Clostridium sp., and PropionibacEubac-terium acnes. Similar results were also reported from another
study conducted by Russell (1979) which showed that the gram-positive cocci were the predominant organisms identified in swine manure, containing
Streptococcus, Peptococcus, Peptostreptococcus, and Megasphaera. The reason that Russell (1979)
classi-fied Megasphaera as gram-positive was because most of strains in this group stained gram-positive in his study.
According to the above researchers, the swine fecal bacterial genera found can be listed in order of quantity from high to low as: Gram-positive cocci (ca. 39%),
Eubacterium (ca. 27%), Lactobacillus (ca. 20%),
Gram-negative rods (Escherichia, ca. 8%),
Clostrid-ium (ca. 4%), and some other minor groups such as Propionibacterium acnes and Bacteroides (<2%). Among these bacterial genera, Clostridium sp.,
Lac-tobacillus sp., Peptostreptococcus, Eubacterium, Pep-tococcus, Propionibacterium acnes, Bacteroides, and Megasphaera are anaerobes; Streptococcus, Staphy-lococcus, and Bacillus are facultative anaerobes; Escherichia are aerobes or facultative anaerobes.
Ob-viously, the anaerobic or facultative anaerobic bacteria account for the major portion of the bacterial species found in swine manure due to the anaerobic environ-ment in the intestinal tract of pigs. The level of aero-bic bacteria (if Escherichia can be counted as one) is low.
Identification of the bacterial genera in swine manure is important to reveal the microbial consor-tium, but it is not enough to explain the complex odor generation processes taking place in manure storage systems. In order to investigate the poten-tial of producing odorous compounds by the bacte-ria from different groups, a further examination on each individual genus of the indigenous bacteria is needed.
2.2. The characteristics of indigenous bacterial species
2.2.1. Streptococcus
Streptococcus is a group of chemoorganotrophs
addi-tional CO2 for growth and some may be strictly
anaerobic. Optimum growth temperature is usu-ally 37◦C but maximum and minimum
tempera-tures vary among species. Neutral or near neu-tral pH will favor the growth and low (<4.0) or
high (>9.6) pH will inhibit the growth. All strep-tococci ferment carbohydrates, producing predom-inantly lactic acid; minor amounts of acetic and formic acids, ethanol, and CO2 may also be
pro-duced. Many species in this genus produce ammo-nia. A total of five species were found in swine manure (Russell, 1979). However, no information regarding the identification of these species was given.
2.2.2. Peptostreptococcus
Peptostreptococcus is a group of anaerobic
chemoorganotrophs that metabolize peptone and amino acids to acetic, formic, propionic, caproic,
iso-butyric, butyric, iso-valeric, and iso-caproic acids.
Volatile amines and various alcohols may also be produced. The pH for growth ranges from 6.0 to 8.0 with the optimum being 7.0–7.5, and the temperature ranges generally from 25 to 45◦C with optimum being
35–37◦C. There is a total of three species identified
in swine manure in this genus (Russell, 1979). They are P. asaccharolyticus, P. magnus, and P. productus.
2.2.3. Eubacteria
Eubacteria is a group of obligately anaerobic
chemoorganotrophs that produce mixtures of organic acids from carbohydrates or peptone. Growth usually is most rapid at 37◦C and pH near 7. Most of them
of-ten produce large amounts of butyric, acetic, formic, and lactic acids (Moore and Holdeman, 1986).
Available literature regarding the number of species in this genus identified in swine excreta is limited. According to Russell (1979), a total of five species in this genus has been identified so far (E. aerofaciens, E.
rectale, E. tenue, E. ventriosum, and E. lentum). There
were another seven species in this genus that could not be identified in his study. Since many species in this genus produce volatile fatty acids and indole and more than half of the species have not been identified, the effect of this bacterial group in general, and of individual species in particular, on swine manure odor generation should receive further research.
2.2.4. Lactobacillus
Lactobacilli are strictly fermentative, aero-tolerant or anaerobic, and aciduric or acidophilic bacte-ria. With glucose as a carbon source, lactobacilli may be either homofermentative, producing >85% lactic acid, or heterofermentative, producing lactic acid, CO2, ethanol, and/or acetic acid in equimolar
amounts.
Lactobacilli grow best in slightly acidic media with pH of 4.5–6.4. Optimal pH for growth usually ranges from 5.5 to 6.2. Growth ceases when pH 3.6–4.0 is reached, depending upon the species and strains. The growth rate is often reduced when the environment becomes neutral or alkaline. Growth temperature ranges from 2 to 53◦C with an optimum range of
30–40◦C.
There were nine species in this group found in swine manure and six of them were identified (Russell, 1979). Since the major product of this genus is lac-tic acid (only a small amount of acelac-tic acid produced by some of the species under heterofermentative con-dition), it could be assumed that the contributions to odor offensiveness by the biological activities of this bacterial group is not significant.
2.2.5. Escherichia
The typical species included in this genus is
Es-cherichia coli which is aerobic or facultatively
anaer-obic, having both respiratory and fermentative types of metabolism. Glucose and other carbohydrates are fermented by the bacteria with the production of pyru-vate, which is further converted into lactic, acetic, and formic acids. The optimum growth temperature is 37◦C. Most of the strains in this species (90–100%)
produce indole which is very odorous.
2.2.6. Clostridium
Most species in genus Clostridium are obligately anaerobic, although tolerance to oxygen varies widely and some species will grow but not sporulate in the presence of air at atmospheric pressure. For most species, growth is most rapid at pH 6.5–7 and at temperatures 30–37◦C; the range of temperature for
growth is 15–69◦C (Cato et al., 1986).
Clostridium often can ferment amino acids to
‘the Stickland reaction’ (Prescott et al., 1996). This generates ammonia, hydrogen sulfide, fatty acids, and amines during the anaerobic decomposition of pro-teins. The volatile fatty acids produced by different species in this genus in order of quantities from high to low are acetic, butyric, caproic, lactic, formic, pro-pionic, succinic, valeric, iso-butyric, iso-caproic, and
iso-valeric acids. Some of the species also produce
indoles and phenols. There were four species in this genus found in the swine excreta (Russell, 1979), but none of them were identified. The complexity of the odorous products produced by Clostridium shows a great potential that the species within this genus may be major contributors to swine manure odor. Therefore, to better understand the involvement of the bacteria in this group in producing different odorous compounds, further research is needed that should focus on the identification of the species found in swine manure.
2.2.7. Propionibacterium
Propionibacterium contains genera which are
ei-ther obligately anaerobic or aerotolerant. Bacteria included in this group can produce propionic acid and acetic acids and lesser amounts of iso-valeric, formic, succinic, and lactic acids. The optimum growth temperature is between 30 and 37◦C and pH near
neutral.
There are only two species found in swine manure, i.e., Propionibacterium acnes and Propionibacterium
granulosum. Both species have the general characters
as discussed above; however, only P. acnes has the capability of producing indole.
2.2.8. Bacteroides
Bacteroides are obligately anaerobic
chemoorgan-otrophs metabolizing carbohydrates or peptone. Fer-mentation products include a combination of suc-cinic, lactic, acetic, formic or propionic, and butyric acids. When n-butyric acid is produced, iso-butyric and iso-valeric acids are also present. Growth usually progresses most rapidly at 37◦C and pH near 7.
Two species in this genus have been found in swine manure and only one of them has been identified (B.
ruminicola). B. ruminicola has an optimum growth
temperature of 37◦C with a growth range between 25
and 45◦C (Holdeman and Moore, 1974).
2.2.9. Megasphaera
There are only two species (M. elsdenii and M.
cerevisiae) in this genus listed in Bergey’s Manual of Determinative Bacteriology (Holt et al., 1994).
Bac-teria in this genus are anaerobic chemoheterotrophs that ferment lactate to produce acetate, propionate, and volatile fatty acids with carbon numbers from 2 to 6 including 4-carbon straight- and branched-chain acids. Sulfur containing compounds are also produced by this genus. The growth temperature ranges from 25 to 40◦C and the optimal pH for growth is slightly
above neutral (pH≈7.4).
The bacterial profile discussed above may not com-pletely cover all the bacterial species in swine manure (e.g., methanogens were not reported in any of the above studies, while it is a common bacterial genus in the gastrointestinal tract of all mammals); how-ever, it does provide information on most of the in-digenous genera in the swine manure, especially those producing odorous compounds. To relate these genera to malodor production requires a basic knowledge of the major odorous compounds in swine manure. Thus, a thorough review of the past research regarding the odorous compounds in swine manure appears essen-tial to determine the relationship between the bacterial genera and these compounds.
2.3. Odorous compounds in swine manure
A considerable amount of research has been con-ducted in determining odorous compounds in swine manure (Merkel et al., 1969; Barth and Polkowski, 1974; van Gemert and Nettenbreijer, 1977; Schaefer, 1977; Lunn and van De Vyver, 1977; Spoelstra, 1977; Spoelstra, 1980; Yasuhara and Fuwa, 1980; Williams, 1984; Yasuhara et al., 1984; O’Neill and Phillips, 1992). Generally speaking, the odorous compounds are produced and accumulated in the storage systems where the mixture of feces and urine is decomposed by bacteria under the prevailing anaerobic conditions. These compounds can be divided into four different chemical classes (Mackie, 1994).
2.3.1. Volatile fatty acids (VFAs)
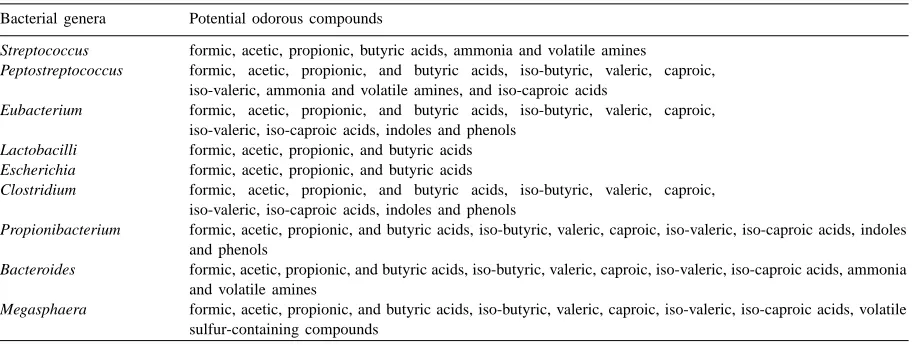
Table 1
The indigenous bacterial genera in swine manure and their odorous compounds
Bacterial genera Potential odorous compounds
Streptococcus formic, acetic, propionic, butyric acids, ammonia and volatile amines
Peptostreptococcus formic, acetic, propionic, and butyric acids, iso-butyric, valeric, caproic, iso-valeric, ammonia and volatile amines, and iso-caproic acids
Eubacterium formic, acetic, propionic, and butyric acids, iso-butyric, valeric, caproic, iso-valeric, iso-caproic acids, indoles and phenols
Lactobacilli formic, acetic, propionic, and butyric acids
Escherichia formic, acetic, propionic, and butyric acids
Clostridium formic, acetic, propionic, and butyric acids, iso-butyric, valeric, caproic, iso-valeric, iso-caproic acids, indoles and phenols
Propionibacterium formic, acetic, propionic, and butyric acids, iso-butyric, valeric, caproic, iso-valeric, iso-caproic acids, indoles and phenols
Bacteroides formic, acetic, propionic, and butyric acids, iso-butyric, valeric, caproic, iso-valeric, iso-caproic acids, ammonia and volatile amines
Megasphaera formic, acetic, propionic, and butyric acids, iso-butyric, valeric, caproic, iso-valeric, iso-caproic acids, volatile sulfur-containing compounds
the deamination of amino acids that are produced dur-ing the process of protein degradation and breakdown of carbohydrates. In the gastrointestinal tract, a neu-tral pH (6–7) normally prevails. Under this condi-tion, deamination is the major route for metabolism of amino acids, which results in the production of VFAs, CO2, H2, as well as ammonia. Bacterial
gen-era involved in this activity normally include
Eubac-teria, Peptostreptococcus, Bacteroides, Streptococcus, Escherichia, Megasphaera, Propionibacterium, Lac-tobacilli, and Clostridium.
2.3.2. Indoles and phenols
Indole, skatole, cresol, and 4-ethylphenol appear to be the major components included in this group of compounds. Phenolic compounds such as phenols and p-cresols are produced from the microbial degra-dation of tyrosine and phenylalanine in the intestinal tract of animals (Ishaque et al., 1985). Metabolism of tryptophan can result in the production of indoleac-etate which is subsequently converted into skatole (3-methylindole) and indole by a different group of bacteria (Mackie, 1994). Bacterial genera involved in these processes include Propionibacterium,
Es-cherichia, Eubacteria, and Clostridia.
2.3.3. Ammonia and volatile amines
Volatile amines include putrescine, cadaverine, methylamine, and ethylamine. Usually, aliphatic
amines (methyl- and ethyl-amine) are present at low concentrations. During the storage of fresh manure, amino acids can most likely undergo decarboxylation to produce putrescine, cadaverine, and ammonia. Bac-terial genera involved in this activity include
Strepto-coccus, PeptostreptoStrepto-coccus, and Bacteroides. Another
big source of ammonia is from urea and nitrates in addition to amino acid deamination (Spoelstra, 1980).
2.3.4. Volatile sulfur-containing compounds
Included in this group are sulfides as well as methyl- and ethyl-mercaptans. The sulfur-containing compounds are produced by bacteria through two processes, i.e., reduction of sulfate and metabolism of sulfur-containing amino acids. Sulfate reduction proceeds via either assimilatory or dissimilatory path-ways. In the first process, bacteria produce enough reduced sulfur for cell biosynthesis, while in the sec-ond process sulfate is utilized as terminal electron acceptor and large quantities of sulfide are produced (Hao et al., 1996). Bacterial genera involved in this activity include Megasphaera.
more odorous compounds and different compounds may contribute differently to the overall odor offen-siveness of the manure, it seems helpful and worth-while to find the major odorous compounds as well as the bacterial species associated with them. Once the bacterial species producing the major odorous com-pounds are determined, effective strategies and tech-niques for controlling these bacterial activities may be developed.
2.4. Major odor indicators and the related bacterial genera
Research on the major indicators for malodors of swine manure has been carried out for many years. Merkel et al. (1969) found alcohols were unimportant in determining the nature of swine confinement ma-nure odors. Barth and Polkowski (1974) reported that the volatile organic acids correlated best with the odor intensity. Ammonia was thought to be useful as an in-dicator for malodor, but in spite of the relatively high concentrations and the easy determination ammonia was proved to be a poor parameter in evaluating odor intensities (Lunn and van De Vyver, 1977). A study conducted by Spoelstra (1977) showed that indole and skatole could not be recommended as indicators for malodor because the concentrations of these two com-pounds might decline during storage. Later, Spoelstra (1980) reported in another study that both ammonia and hydrogen sulfide were not suitable indicators for the smell. The most pungent and the greatest variety of obnoxious smelling compounds originate from the de-composition of proteins. Ammonia does not reflect this degradation kinetics of the manure because the major part of ammonia in the manure originates from urea hydrolysis. Moreover, ammonia remains unchanged by methanogenesis and shows a retarded reaction to aerobic treatment compared with organic volatiles. Hydrogen sulfide formation also does not reflect ma-nure degradation kinetics because a relatively large part is derived from sulfate reduction. He concluded that the VFAs seemed to be useful indicators to test whether an effect has occurred in all odor-abatement methods. The VFAs show typical reactions for the group of accumulated volatile compounds when en-vironmental changes are made in swine manure to diminish odor. Williams (1984) found that the most
widely applicable indicator was supernatant biochem-ical oxygen demand (BOD) both during aerobic treat-ment and post-treattreat-ment storage; VFAs, total organic acids, indoles and phenols can indicate acceptable and unacceptable limits of offensiveness during aero-bic treatment and post-treatment storage; sulfide is a misleading indicator during aerobic treatment but is a useful indicator during post-treatment storage; and ammonia is of no value as an indicator. A study con-ducted by Zahn et al. (1997) reported that the volatile organic acids with carbon numbers from 2 to 9 specif-ically demonstrated the greatest potential for the de-creased air quality. Therefore, according to the above researchers, it appears that VFAs could be used as a suitable odor indicator for swine manure.
However, in recent years, it has been shown by a few studies that different VFAs will have differ-ent contribution to the odor generation. Thus, a fur-ther classification within this group is needed to deter-mine the potential of producing malodors by different VFAs so the specific bacterial species involved can be studied.
The odorous nature of VFAs progresses from pun-gent odors of formic and acetic acids to the distinctly unpleasant and offensive odors of valeric and caproic acids (Morrison, 1987). Although the short chain acids are present in much higher concentrations and have higher volatility, the VFAs with higher carbon num-bers have lower odor detection threshold thus are more offensive in nature (Mackie, 1994). Therefore, the high concentration of VFAs in swine manure may not nec-essarily cause high intensity of malodor as a large por-tion of the VFAs could be composed of short chain acids with less odor potential. A study conducted by Zhu et al. (1997) appeared to provide evidence sup-porting this argument. They evaluated five commer-cial pit additive products and found that some products could reduce odor threshold without significantly re-ducing the total amount of VFAs. The offensive odor potential was not directly associated with the total con-centration of VFAs in the manure. It was depending upon the types and characteristics of certain acids (not necessarily existing in high concentrations in the ma-nure). Therefore, the purview of VFAs responsible for odor generation can be narrowed down to those with long carbon chains (<C10) or branchings. The acids
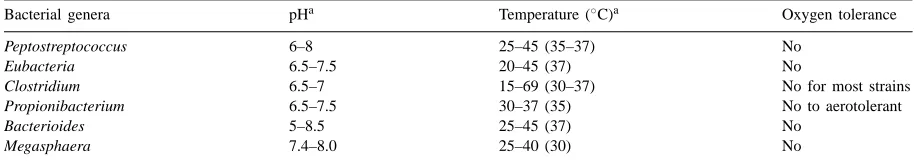
Table 2
pH and temperature ranges for growth of the related bacteria
Bacterial genera pHa Temperature (◦
C)a Oxygen tolerance
Peptostreptococcus 6–8 25–45 (35–37) No
Eubacteria 6.5–7.5 20–45 (37) No
Clostridium 6.5–7 15–69 (30–37) No for most strains
Propionibacterium 6.5–7.5 30–37 (35) No to aerotolerant
Bacterioides 5–8.5 25–45 (37) No
Megasphaera 7.4–8.0 25–40 (30) No
aNumbers in parenthesis are optimum conditions.
producing these compounds include
Peptostreptococ-cus, Clostridium, Bacteroides, Eubacteria, Propioni-bacterium, and Megasphaera (Table 1).
The bacterial genera in Table 1 are normally active in the specific environment, e.g., in the gastrointesti-nal tract of animals, where the bacteria can effectively perform their metabolic activities for growth. That en-vironment may change or even no longer exist once the feces are excreted. Since swine manure contains sufficient nutrients for bacterial growth, the limiting factors that could affect the growth of these bacterial genera are most likely pH and temperature. The suit-able ranges of these two parameters for the growth of different bacterial genera are presented in Table 2. De-viations in pH and temperature from the values listed in Table 2 may not completely stop the bacterial activ-ities in producing volatile fatty acids, but may affect the productivity of different bacterial genera in vary-ing degrees.
According to Table 2, genus Clostridium has the widest temperature range for growth among the bac-terial genera. Thus, as compared with other bacbac-terial genera, Clostridium could be more active in producing odorous acids in the real manure storage environment, especially at low temperatures. Since little appears to have been made in systematically studying the mi-crobes at the species level in swine manure under the storage environment, experimental data that estimate the contributions of this genus to the VFA produc-tion on a quantitative basis are not available. However, there is plenty of evidence showing that Clostridium, if the environment favors the growth of this group of bacteria, is a major genus that is responsible for pro-ducing all types of VFAs through amino acids fer-mentation (Gunsalus and Stanier, 1961; Mead, 1971; Gottschalk, 1985; Hill, 1986). In operating lagoons or earthen basins, the storage temperature of wastes
usually ranges from 10 to 20◦C, depending upon the
season (Spoelstra, 1980). Local lagoon waste temper-ature variation has also been reported by several other researchers (Ohio, 2–27◦C (White et al., 1977);
Okla-homa, 0–30◦C (Rice, 1977); Georgia, 3–25◦C (Smith
and Franco, 1985). The temperature range for ma-nure stored in the pits is between 2 and 18◦C
(Don-ham et al., 1985). The pH range for swine manure is between 6.5 and 7.5 (Cooper and Cornforth, 1978). Comparing these situations to the parameters listed in Table 2, plus the anaerobic environment that always exists in the liquid manure storage systems where aer-ation is not available and the availability of nutri-ents, would suggest that Clostridium very likely is a genus that can play a major role in producing odorous VFAs.
genera seems of significance in determining the types and quantities of odorous compounds produced by different species in these two groups.
As mentioned above, pH could be a factor that af-fects bacterial growth. It can be seen from Table 2 that all the bacterial genera have a neutral or near neutral pH for their growth. This offers an opportunity to reg-ulate bacterial growth by adjusting the pH in manure liquid. This can be achieved more easily than control-ling temperature that is neither practical nor effective at the farm level. There have been several studies in which alkaline materials were added into manure to increase the manure pH (Hammond and Day, 1968; Veenhuizen and Qi, 1993; Vincini et al., 1994; Bundy and Greene, 1995). These studies demonstrated odor reductions in varying degrees when manure pH was raised to a range of 8–11. However, none of these studies presented an explanation for the mechanisms. Based on previous discussions, it could be concluded that one major reason that the raised pH could reduce odor is that it inhibits the growth of those odor-causing bacteria indigenous to swine manure. Another mech-anism of reducing odor by alkaline materials is due to the precipitation of volatile fatty acids by forma-tion of salts. At high levels of pH, the formed salts will not be converted back to acids; thus, both the lev-els and volatility of the odorous acids will be reduced (Rainville and Morin, 1985; Morrison, 1987).
One problem associated with the pH adjustment is the emission of large quantities of either ammonia (at the raised pH) or hydrogen sulfide (at the lowered pH) from the treated swine manure. The emission of these two gases may cause severe problems to the environment and losses of animals and human lives under certain conditions. Therefore, to avoid the po-tential damage caused by the emission of these two gases during the pH adjustment, it would be better to treat fresh manure instead of aged manure. In fresh manure, the bacterial activity of decomposing organic substances to form ammonia and hydrogen sulfide has not fully developed, so the volatile portion of the gases is relatively low. Accordingly, these two gases may not reach a threatening level on both the environment and the properties in a short time period. The problem with this treatment is that continuous adjustment of pH has to be conducted to maintain the adjusted pH. Other-wise, due to the biological activities, pH will change and odor may return during the manure storage time.
3. Odor control techniques
In this chapter, only the major odor control tech-niques closely relevant to the bacterial properties will be discussed. Other techniques that are less related to the biological treatment in essence, such as storage tank covers, solids–liquid separation, and chemical de-odorants are not addressed here.
3.1. Aeration
The value of aeration in reducing offensive odors has been demonstrated by a number of workers us-ing olfactometric evaluation methods (Williams et al., 1984; Williams et al., 1989; Pain et al., 1990; Sneath et al., 1992). The basic principle of this treatment is to provide, by whatever means, enough dissolved oxy-gen to aerobic bacteria so they can actively decompose the odorous compounds; hence achieving odor reduc-tion. There have been some research efforts made to link aeration with specific microorganisms in terms of reducing odor (Evans et al., 1983; Evans and Baines, 1985; Evans et al., 1986; Munch et al., 1987). Ac-cording to these studies, a group of microorganisms called ‘heterotrophs’ are commonly assumed to be the most numerous and important in this biological treat-ment process. However, a complete profile of the bac-terial genera within this group seems not available. Past studies only investigated the overall performance of the aerobic bacteria, with the characteristics of each individual bacterial genera being untouched.
The importance of bacteria in aeration has not ceived specific attention since most of the papers re-lated to aeration were focused on the development and improvement of all kinds of aerators mechanically. However, even when these papers are reviewed, the inherent relation between bacteria and the aeration ef-ficiency of aerators can still be perceived. One study showed that in general, aerator performance was better at raised temperatures (Cumby, 1987a). Another study showed that if the liquid temperature was kept at 15◦C
reports, although limited, showing that several aero-bic bacterial species could effectively degrade VFAs at raised temperatures (Bourque et al., 1987; Jolicoeur and Morin, 1987). Therefore, it may not be the aera-tor that worked better at raised temperatures. It could be the microbes that enhanced their metabolic pro-cesses in decomposing organic materials at high tem-peratures. The role of microbes in improving aeration efficiency is evidently important and requires further study. While aeration alone does not destroy odors.
Since the gastrointestinal tract of pigs is strictly anaerobic, the levels of the aerobic bacteria, if any, cannot be high. Thus, under aeration, whether the ex-isting aerobic bacteria are able to compete for nutrients actively, to establish their growth firmly, and to reach dominant levels rapidly becomes critical. And the as-sumption of ‘a group of aerobic heterotrophs’ playing a major role in aeration may not hold unless dominant population levels of these bacteria have been reached. This depends largely on the bacterial species. In gen-eral, the microbial species having the fastest growth rate and the ability to utilize most of the available or-ganic matter will be the predominant species (Loehr, 1974). Since the tolerance of different bacterial gen-era or species to the living environment and the ability to effectively digest the odorous organic compounds varies, the identification of the aerobes indigenous to swine manure will be of profound significance to help find or develop good aerobic bacterial species that can be used in assisting aeration.
To date, ample research has been conducted in either improving the aeration efficiency of different aerators (Cumby, 1987b) or reducing the extent of aeration (Ginnivan, 1983; Zhang et al., 1997). However, re-search attention that has been paid to studying the other half of the story, i.e., microbes, appears meager. Due to the diversities of not only the bacterial gen-era but also their functions, it is not unreasonable to suggest that more fundamental research in completely determining the microbiological activities of different bacterial genera in aeration be needed.
3.2. Anaerobic lagoons
Anaerobic lagoons are a process in which mi-croorganisms are used under anaerobic conditions to convert biodegradable organic materials to odorless
gases, such as methane and carbon dioxide, and non-biodegradable solids. There are basically three steps involved in the process, i.e., hydrolysis, acidogene-sis, and methanogenesis. The key to preventing odor production is that the balance between the second and third step has to be maintained. In other words, the production of acids by the indigenous bacteria and the consumption of acids by the methanogens to produce methane and carbon dioxide have to be in equilibrium. Otherwise, malodor may result.
Methanogens are a group of bacteria mainly re-sponsible for methane production (Wolfe, 1971). Methanogens thrive in anaerobic environments rich in organic matter: the rumen and intestinal system of animals, fresh water and marine sediments, swamps and marshes, hot springs, and anaerobic sludge di-gesters (Prescott et al., 1996). Methanogens are very strictly anaerobic bacteria and all grow by oxidizing hydrogen. They can grow well in either mesophilic (20–45◦C) or thermophilic (40–75◦C) temperature
ranges depending upon genera. For most genera, the minimum pH for growth is ca. 6; some genera (e.g.,
Methanococcus) can grow at pH as high as 9.2. Since
the methanogens are the working force in the anaer-obic decomposition process, whether an anaeranaer-obic treatment process can function well depends largely on the performance of the methanogens.
Anaerobic lagoons are designed to employ the methanogens to decompose the organic substances in swine manure under anaerobic environments. Un-fortunately, many anaerobic lagoons do not function as properly as designed due to overloading and bad management and complaints about the odor generated from these lagoons have risen widely. As discussed early, the final products of microbial degradation of carbonaceous material in an anaerobic natural ecosys-tem are methane and carbon dioxide. However, little or a relatively low methane formation was observed in the anaerobic storage system (Stevens and Corn-forth, 1974). This may deserve an explanation from the microbiological point of view.
The major factor that influences the methanogenic process for methane production is the low temper-ature in lagoons. In operating lagoons, the storage temperature of wastes usually ranges from 10 to 20◦C, depending upon the season (Spoelstra, 1980).
Ohio; 0–30◦C for Oklahoma; 3–25◦C for
Geor-gia). These temperature ranges generally are lower than the optimum mesophilic temperature (35◦C)
for most of the methanogens to function properly. There have been three orders of methanogens found so far (Methanobacteriales, Methanococcales, and
Methanomicrobiales), consisting of 18 genera and 39
species. Genera included in Order I will not grow below 60◦C (Boone and Mah, 1989a). The growth
temperature range for genera in Order II is between 25 and 86◦C depending upon species (Whitman,
1989). In Order III, most genera have optimum growth temperatures higher than 20◦C, only three
species start growth at 15◦C (Methanogenium cariaci, Methanococcoides methylutens, and Methanogenium marisnigri) and one genera (Methanothrix) with three
species start growth at 3◦C (Boone and Mah, 1989b).
Obviously, the number of species that can grow well at the psychrophilic temperature range appears limited so the methane fermentation can not be high in this temperature range. Although methane fermentation in nature occurs below 10◦C (Svensson, 1975) and was
observed as low as 4◦C (Stevens and Schulte, 1979),
methanogens produce methane at a much lower rate and grow much slower at lower temperatures (Zeikus and Winfrey, 1976; Van den Berg, 1977). Allen and Lowery (1976) reported that the mean biogas production rate from a full-scale swine lagoon was 0.006 m3/m2-day for a mean lagoon temperature of 14◦C and 0.55 m3/m2-day for a 3-day period when
the lagoon temperature was 27.5◦C. Surprisingly, a
difference of 13.5◦C in temperature would cause a
reduction in methane production by ca. 92-fold due to the low activity of methanogens, indicating how important the temperature is in the methanogenesis. This low activity of methanogens finally has resulted in the accumulation of odorous fatty acids and the generation of odor.
The low activity of methanogens in methanogenesis due to low temperature has also caused other problems associated with this process. First, the solids retention time has increased and lagoons are liable to overload-ing. Stevens and Schulte (1979) found that low tem-perature digestion (<25◦C) required a solids retention
time approximately twice as long to achieve the same volatile solids reduction as in the mesophilic diges-tion. Safley and Westerman (1992) showed that at low temperatures (>10◦C), not only has the retention time
to be increased but the loading rates of organic wastes have to be decreased. It could be inferred from these studies that the capability of treating swine manure by anaerobic lagoons could be limited due to the low temperatures, and more critical management appears needed to avoid overloading. As a matter of fact, over-loading has become a major problem for the anaerobic lagoon systems currently used in the middle and south-ern areas in the United States due to the fast-growing swine industry producing a huge volume of manure in a short time period. Therefore, the balance between the acids produced by the indigenous bacterial groups and the acids consumed by the methanogens for methane formation can hardly be achieved. This is why there is little methane formed in, and there is strong offen-sive odor generated from, the waste storage lagoons. According to the above analysis, if no restrictions are expected to be placed on the growth of the swine industry, it might be worthwhile to reexamine the use of anaerobic lagoons for storing and treating swine manure from the standpoint of odor control.
3.3. Microbial manure additives
may affect the chemical, physiological, and biological processes of the additives.
According to Liao and Bundy (1994) and Bar-rington (1994), microbial digestive additives contain bacteria or enzymes that eliminate odors and suppress gaseous pollutants by their biochemical digestive processes. There have been only a few efforts made to investigate the bacterial decomposition of odorous compounds in swine manure by some specific bacte-rial species. Ohta and Ikeda (1978) conducted a lab-oratory study regarding the possibility of deodorizing pig feces by Streptomyces, which is a genera be-longing to a group of microbes encompassing a wide range of bacteria called Actinomycetes. They found that the optimum conditions for deodorization were as follows: pH, 8.6–10; temperature, 35–40◦C; moisture
content, 42–63%; and minimum amount of inoculum, 2 g of seed culture per 10 g of fresh feces. No aeration was introduced to the testing manure but the inocu-lated manure was incubated aerobically. Under these conditions, they reported that two bacterial genera (Streptomyces griseus and Streptomyces antibioticus) demonstrated strong ability of deodorization. Volatile fatty acids with a carbon number up to six were greatly reduced after 48-h treatment and the reduction of specific malodors of the feces was observed.
Bourque et al. (1987) conducted research on mi-crobiological degradation of odorous substances of swine manure on a laboratory scale under aerobic conditions. The bacterial culture under study was in-oculated into sterilized swine manure and incubated for a maximum of six days at 29◦C. They found that
three bacterial species (Acinetobacter calcoaceticus,
Alcaligenes faecalis, and Arthrobacter flavescens)
could completely degrade all types of VFAs in swine manure while Corynebacterium glutamicum and
Mi-crococcus sp. could only degrade acetic and
propi-onic acids. Another laboratory experiment done by Jolicoeur and Morin (1987) also reported that
Acine-tobacter calcoaceticus could degrade VFAs in both
sterilized and non-sterilized swine slurry incubated at 22◦C within pH 6.2–8.6 for 21 days.
According to Grubbs (1979), the key in using bacte-rial cultures for deodorization of manure is to have the added bacteria become the predominant strain in the manure. For the added bacteria to flourish, the real en-vironment should not deviate tremendously from the optimum growth range for the bacteria. Past work was
mainly focused on determining the bacterial functions in digesting odorous compounds under optimum con-ditions. This usually does not guarantee that bacteria growing well under optimum conditions will also grow well in the field. Bourque et al. (1987) showed that none of the inoculated microorganisms became domi-nant in the non-sterilized swine manure samples. The indigenous flora (not necessarily those reducing odors) of the wastes always grew better than the inoculated microorganisms. In addition, the selected microorgan-isms may even use other organic compounds in pref-erence to the malodorous substances when inoculated in wastes. This impairs the values of the additives ac-cordingly. Goldstein et al. (1985) explained possible failure of inoculation to enhance biodegradation.
The temperature and pH of the stored manure may not favor the growth of the added bacteria. The mean temperature of stored slurry ranged between 3 and 22◦C (Patni and Jui, 1985) while the temperatures used
in laboratory studies ranged from 22 to 40◦C. In
ad-dition, most of the tests were run under pH condition higher than that of manure (6.3–7.7). Although bacte-ria can adapt to environmental changes, large devia-tions from their optimum growth condidevia-tions undoubt-edly interfere with normal metabolic activities, this results in a slow growth. The evaluation of a commer-cial product containing enzymes and selected bacteria showed no acceleration of degradation of the malodor-ous substances even at 15◦C (Bourque et al., 1987).
Since predominance of the added bacteria is criti-cal to the treatment, the quantity of bacterial material is questionable. Usually, the indigenous microorgan-isms are present in high concentration and are able to grow rapidly. Therefore, massive inoculation has to be exercised to accelerate the development of the added bacteria. Such massive inoculation can be achieved only on a laboratory scale, not at the farm level where the volumes of manure to be treated are considerable. According to Ohta and Ikeda (1978), 2 g of bacterial culture seed was needed to treat 10 g of fresh swine feces. Another study (Zhu et al., 1996) also showed that a dose of ca. 4.5 kg of bacterial material was con-sumed for odor control for each pig marketed. Obvi-ously, these numbers are not realistic in dealing with odor problems at the farm level.
discussed above are obligate aerobes while most of the storage lagoons or earthen basins (despite that some of them are claimed as aerobic) are actually anaerobic. As a result, the supplemental bacteria culture in such manure handling systems will die shortly after inocu-lation and will never achieve dominant level because of the lack of oxygen. This may explain the reason that the success of using microbial additives to control odor has been relatively limited as indicated by Ritter (1981).
It appears that the available techniques for control-ling odors are either costly (aeration) or ineffective (anaerobic lagoon and biological manure additives). The combination of aeration and microbial additives is usually more expensive than aeration alone unless the added bacteria are able to significantly reduce the aeration time to reach dominant levels. Without aer-ation, the possibility of controlling odor by any of the microbial-based manure additives that have been developed so far is questionable.
4. Summary and conclusion
1. To quantify the microbial production of odors by the bacterial genera indigenous to swine ma-nure, a complete profile of bacterial species for all the genera found in the manure is needed. According to the discussions in the paper, the odorous volatile fatty acids are mainly produced under anaerobic manure storage conditions by bacterial genera Peptostreptococcus,
Propioni-bacterium, Bacteroides, EuPropioni-bacterium, Clostrid-ium, and Megasphaera, with genera Clostridium
and Eubacterium being most likely the major contributors. More research is necessary to deter-mine the species within each genus as well as the capability of producing odorous compounds by each individual species.
2. Raising manure pH can attenuate the growth of the odor-causing bacteria, thus reducing odor emis-sion. However, caution has to be exercised in treat-ing aged manure to avoid any potential losses in lives and properties due to the emission of large quantities of either ammonia or hydrogen sulfide. The adjustment of pH to fresh manure is therefore recommended.
3. Raising temperature can enhance methanogenic process such that odor can be reduced; however, as compared with the adjustment of pH, raising temperature is less feasible in the real world. 4. Anaerobic lagoons may not be an appropriate
treatment for odor control because the low tem-perature makes methanogens unable to function properly, resulting in malodors due to the accu-mulation of volatile fatty acids.
5. Controlling odors by microbial manure additives alone needs to be restudied. At this stage, it is ac-tually questionable for any of the microbial-based manure additives to control odors without aeration in the manure storage systems currently in use. 6. Due to the complex nature of bacterial
involve-ment in swine manure odor production, research regarding how to control malodors microbiologi-cally is still in its infancy. Since the source of the odorous compounds is mainly microbial in origin, a sustained, rational research initiative is required using well-developed classical anaerobic microbi-ology technmicrobi-ology, combined with modern molec-ular techniques and the latest analytical/sensory methodology, to determine the fundamentals con-trolling the production of malodor.
References
Al-Kanani, T., Akochi, E., MacKenzie, A.F., Alli, I., Barrington, S., 1992. Odor control in liquid hog manure by added amendments and aeration. J. Environ. Qual. 21, 704–708.
Allen, J.B., Lowery, B., 1976. Methane gas production from dairy, poultry and swine anaerobic lagoons. Paper presented at the 1976 Southeast ASAE Meeting, Mobile, AL. 30pp.
Barrington, S., 1994. Odor measurement, commercial substrates and biofilters. In: Proc. of International Round Table on Swine Odor Control, 13–15 June at Ames, IA, USA, pp. 40–45. Barth, C.L., Polkowski, L.B., 1974. Identifying odorous
components of stored dairy manure. Trans. of the ASAE. 17(4), 737–741, 747.
Boone, D.R., Mah, R.A., 1989a. Order I. Methanobacteriales. In: Staley, J.T. et al. (Eds.), Bergey’s Manual of Systematic Bacteriology. Baltimore, London, Los Angeles, Sydney: Williams & Wilkins. 3, pp. 2174–2184.
Boone, D.R., Mah, R.A., 1989b. Order III. Methanomicrobiales. In: Staley, J.T. et al. (Eds.), Bergey’s Manual of Systematic Bacteriology. Baltimore, London, Los Angeles, Sydney: Williams & Wilkins. 3, pp. 2190–2211.
Bundy, D.S., Greene, G., 1995. Evaluation of alkalilne by-products for the control of swine odors in manure storage. In: Proc. of International Livestock Odor Conference ’95, 16–18 October 1995, at Ames, IA, USA, pp. 73–76.
Cato, E.P., Geroge, W.L., Finegold, S.M., 1986. Genus Clostridium. In: Sneath, P.H.A. et al. (Eds.), Bergey’s Manual of Systematic Bacteriology, Baltimore, London, Los Angeles, Sydney: Williams & Wilkins. 2, pp. 1141–1200.
Cole, C.A., Bartlett, H.D., Buckner, d.H., Younkin, D.E., 1975. Odor control of liquid dairy and swine manure using chemical and biological treatments. In: Proc. of 3rd International Symposium on Livestock Wastes: Managing Livestock Wastes, ASAE. St. Joseph, MI, pp. 374–377.
Cooper, P., Cornforth, I.S., 1978. Volatile fatty acids in stored animal slurry. J. Sci. of Food and Agric. 29 (1), 19–27. Cumby, T.R., 1987a. A review of slurry aeration 1. Factors
affecting oxygen transfer. J. Agric. Engng. Res. 36, 141–156. Cumby, T.R., 1987b. A review of slurry aeration 3. Performance
of aerators. J. Agric. Engng. Res. 36, 175–206.
Donham, K.J., Yeggy, J., Dague, R.R., 1985. Chemical and physical parameters of liquid manure from swine confinement facilities: health implications for workers, swine and environment. Agric. Wastes 14, 97–113.
Evans, M.R., Deans, E.A., Hissett, R., Smith, M.P.W., Svoboda, I.F., Thacker, F.E., 1983. The effect of temperature and residence time on aerobic treatment of piggery slurry — degradation of carbonaceous compounds. Agric. Wastes 5, 25– 36.
Evans, M.R., Baines, S., 1985. Aeration and odour control by heterotrophic and autotrophic microorganisms. In: Nielsen, V.C., Voorburg, J.H.,’Hermite, P.L. (Eds.), Odour Prevention and Control of Organic Sludge and Livestock Farming. Elseview Applied Science Publishers, London and New York, pp. 273–281.
Evans, M.R., Deans, E.A., Smith, M.P.W., Svoboda, I.F., Thacker, F.E., 1986. Aeration and control of slurry odours by heterotrophs. Agric. Wastes 15, 187–204.
Ginnivan, M.J., 1983. Shallow aeration of piggery waste treatment lagoons. I. removal of organic pollutants and indicator bacteria. Agric. Ecosystems and Environ. 10, 23–29.
Goldstein, R.M., Mallory, L.M., Alexander, M., 1985. Reasons for possible failure of inoculation to enhance biodegradation. Appl. and Environ. Microbiol. 50, 977–983.
Gottschalk, G., 1985. Bacterial metabolism, second ed., Springer-Verlag, New York.
Grubbs, R.B., 1979. Bacteria supplementation: what it can and cannot do. Paper presented at 9th Engineering Foundation Conference in Environmental Engineering in the Food Processing Industry. Pacific Grove, CA., USA 27 February. Gunsalus, I.C., Stanier, R.Y., 1961. The bacteria: a treatise on
structure and function, vol. 2. Metabolism. Academic Press, New York.
Hammond, W.C., Day, D.L., 1968. Can lime and chlorine suppress odors in liquid hog manure?. Agric. Engng. 6, 340–343. Hao, O.J., Chen, J.M., Huang, L., Buglass, R., 1996.
Sulfate-reducing bacteria. Critical Reviews in Environ. Sci. and Technol. 26 (1), 155–187.
Hill, M.J., 1986. Microbial betabolism in the digestive tract. CRC Press, Inc. Boca Raton, Florida, USA.
Holdeman, L.V., Moore, W.E.C., 1974. Genus I. Bacteroides. In: Buchanan, R.E., Gibbons, N.E. (Eds.), Bergey’s Manual of Determinative Bacteriology, eighth ed. Baltimore: The Williams & Wilkins Company, pp. 385–404.
Holt, J.G., Krieg, N.R., Sneath, P.H.A., Staley, J.T., Williams, S.T., 1994. Anaerobic Gram-negative cocci. In: Hensyl, W.R. et al. (Eds.), Bergey’s Manual of Determinative Bacteriology, ninth ed. Baltimore, London, Los Angeles, Sydney: Williams & Wilkins, pp. 347–349.
Ishaque, M., Bisaillon, J.G., Beaudet, R., Sylvestre, M., 1985. Degradation of phenolic compounds by microorganisms indigenous to swine waste. Agric. Wastes 13, 229–235. Jolicoeur, P., Morin, A., 1987. Isolation of Acinetobacter
calcoaceticus Strains degrading the volatile fatty acids of swine wastes. Biolog. Wastes 19, 133–140.
Liao, C.M., Bundy, D.S., 1994. Bacteria additives to the changes in gaseous mass transfer from stored swine manure. J. Environ. Sci. Health B29 (6), 1219–1249.
Loehr, R.C., 1974. Agricultural Waste Management — Problems, Processes, and Approaches. New York and London: Academic Press.
Lunn, F., van De Vyver, J., 1977. Sampling and analysis of air in pig houses. Agric. and Environ. 3, 159–169.
Mackie, R.I., 1994. Microbial production of odor components. In: Proc. of International Round Table on Swine Odor Control, 13–15 June at Ames, IA, USA, pp. 18–19.
Mead, G.C., 1971. The amino acid-fermenting clostridia. J. Gen. Microbiol. 67, 47–56.
Merkel, J.A., Hazen, T.E., Miner, J.R., 1969. Identification of gases in a swine confinement building atmosphere. Trans. of the ASAE 12, 310–316.
Moore, W.E.C., Holdeman, L.V., 1986. Genus Eubacteria. In: Sneath, P.H.A. et al. (Eds.), Bergey’s Manual of Systematic Bacteriology, Baltimore, London, Los Angeles, Sydney: Williams & Wilkins. 2, pp. 1353–1373.
Morrison, R.T., 1987. Organic Chemistry, fifth ed., Allyn and Bacon, Boston, Massachusetts.
Munch, B., Larsen, H.E., Aalbaek, B., 1987. Experimental studies on the survival of pathogenic and indicator bacteria in aerated and non-aerated cattle and pig slurry. Biolog. Wastes 22, 49–65. Nuru, S., Osbaldiston, G.W., Stowe, E.C., Walker, D., 1972. Fecal microflora of healthy cattle and pigs, The Cornell Veterinarian LXII (2), 242–253.
Ohta, Y., Ikeda, M., 1978. Deodorization of pig feces by actinomycetes. Appl. and Environ. Microbiol. 36 (3), 487–491. Orvin, M. J., 1986. Enterococci. In: Sneath, P.H.A. et al. (Eds.), Bergey’s Manual of Systematic Bacteriology, Baltimore, London, Los Angeles, Sydney: Williams & Wilkins 2, pp. 1063–1065.
O’Neill, D.H., Phillips, V.R., 1992. A review of the control of odor nuisance from livestock buildings. Part 3. properties of the odorous substances which have been identified in livestock wastes or in the air around them. J. Agric. Engng. Res. 53, 23–50.
following the spreading of aerobically treated pig slurry on grassland. Biolog. Wastes 34, 149–160.
Patni, N.K., Jui, P.Y., 1985. Volatile fatty acids in stored dairy-cattle slurry. Agric. Wastes 13, 159–178.
Prescott, L.M., Harley, J.P., Klein, D.A., 1996. Microbiology, Chicago: Wm. C. Brown Publishers.
Rainville, N., Morin, A., 1985. Change in the volatile fatty acids content of laboratory stored sterilized and non-sterilized swine wastes. Microbios 42, 175–182.
Rall, G.D., Wood, A.J., Westcott, R.B., Dommert, A.R., 1970. Distribution of bacteria in feces of swine. Appl. Microbiol. 20, 789–792.
Rice, C.E., 1977. The effect of mixing on an anaerobic lagoon. Trans. of the ASAE, 1119–1122, 1128.
Ritter, F.W., 1981. Chemical and biochemical odor control of livestock wastes: a review. Can. Agric. Engng. 23, 1–4. Ritter, W.F., Eastburn, R.P., 1980. Odor control in liquid swine and
dairy manure with commercial products. Can. Agric. Engng. 22 (2), 117–118.
Russell, E.G., 1979. Types and distribution of anaerobic bacteria in the large intestine of pigs. Appl. and Environ. Microbiol. 37 (2), 187–193.
Salanitro, J.P., Blake, I.G., Muirhead, P.A., 1977. Isolation and identification of fecal bacteria from adult swine. Appl. and Environ. Microbiol. 33 (1), 79–84.
Safley, L.M., Westerman, P.W., 1992. Performance of a low temperature lagoon digester. Bioresource Technol. 41, 67–175. Schaefer, J., 1977. Sampling, characterization , characterization
and analysis of malodors. Agric. and Environ. 3, 121–127. Smith, M.P.W., Evans, M.R., 1982. The effects of low dissolved
oxygen tension during the aerobic treatment of piggery slurry in completely mixed reactors. J. Appl. Bacteriol. 53 (1), 117–126. Smith, R.E., Franco, t.L., 1985. Predicting anaerobic lagoon temperatures from weather data. Trans. of the ASAE 28, 551– 554.
Sneath, R.W., Burton, C.H., Williams, A.G., 1992. Continuous aerobic treatment of piggery slurry for odour control scaled up to a farm-size unit. J. Agric. Engng. Res. 53, 81–92. Spoelstra, S.F., 1977. Simple phenols and indoles in anaerobically
stored piggery wastes. J. Sci. Food Agric. 28, 415–423. Spoelstra, S.F., 1980. Origin of objectionable odorous components
in piggery wastes and the possibility of applying indicator components for studying odor development. Agric. and Environ. 5, 241–260.
Stevens, R.J., Cornforth, I.S., 1974. The effect of aeration and gases produced by slurry during storage. J. Sci. Food Agric. 25, 1249–1261.
Stevens, M.A., Schulte, D.D., 1979. Low temperature digestion of swine manure. J. Environ. Eng. Div., ASCE 105 (EE1), 33–42. Svensson, B.H., 1975. Methane production in tundra peat. In: Schlegel, H.G., Gottschalk, G., Pfenning, N., (Eds.), Microbial Production and Utilizaton of Gases, E. Goltze K.G., Göttingen, pp. 133–139.
Sweeten, J.M., Reddell, D.R., Schake, J.S., Garner, B., 1977. Odor intensities in cattle feedlots. Trans. of the ASAE 20, 502–508. Ulich, W.L., Ford, J.P., 1975. Malodor reduction in beef cattle feedlots. In: Proc. of 3rd International Symposium on Livestock Wastes: Managing Livestock Wastes ASAE. St. Joseph, MI., pp. 369–371.
Van den Berg, L., 1977. Effect of temperature on growth and activity of a methanogenic culture utilising acetate. Can. J. Microbiol. 23, 898–902.
van Gemert, L.J., Nettenbreijer, A.H., 1977. Compilation of odor threshold values in air and water. Central Institute for Nutrition and Food research, Zeist, Netherlands.
Veenhuizen, M.A., Qi, R., 1993. Manure storage pH adjustment to control gas release. ASAE Paper #: 93-4552. St. Joesph, MI. Vincini, M., Carini, F., Silva, S., 1994. Use of alkaline fly ash as an amendment for swine manure. Bioresource Technol. 49, 213–222.
Warburton, D.J., Scarborough, J.N., Day, D.L., Mueling, A.J., Curtis, S.E., Jensen, A.H., 1980. Evaluation of Commercial Products for Odor Control and Solids Reduction of Liquid Swine Manure. In: Proc. of the 4th International Symposium on Livestock Wastes, Livestock Waste: A Renewable Resource, Amarillo, Texas, pp. 201–203.
White, R.K., Curtner, R.L., Miller, R.H., 1977. Performance of an anaerobic waste treatment lagoon system. Cornell Agricultural Waste Management Conference, 659–679.
Williams, A.G., 1981. The biological control of odours emanating from piggery slurry. PhD thesis, University of Glasgow. Whitman, W.B., 1989. Order II. Methanococcales. In: Staley, J.T.
et al. (Eds.), Bergey’s Manual of Systematic Bacteriology. Baltimore, London, Los Angeles, Sydney: Williams & Wilkins. 3, pp. 2185–2190.
Williams, A.G., 1984. Indicators of piggery slurry odour offensiveness. Agric. Wastes. 10, 15–36.
Williams, A.G., Shaw, M., Adams, S.J., 1984. The biological stability of aerobically-treated piggery slurry during storage. J. Agric. Engng. Res. 29, 231–239.
Williams, A.G., Shaw, M., Selviah, C.M., Cumby, R.J., 1989. The oxygen requirements for deodorising and stabilising pig slurry by aerobic treatment. J. Agric. Engng. Res. 43, 291–311. Wolfe, R.S., 1971. Microbial formation of methane. Adv.
Microbiol. Physiol. 6, 107–145.
Yasuhara, A., Fuwa, K., 1980. Isolation and characterization of odorous components in solid swine manure. Agric. Biol. Chem. 44 (10), 2379–2385.
Yasuhara, A., Fuwa, K., Jimby, M., 1984. Identification of odorous compounds in fresh and rotten swine manure. Agric. Biol. Chem. 48 (12), 3001–3010.
Zahn, J.A., Hatfield, J.L., Do, Y.S., DiSpirito, A.A., Laird, D.A., Pfeiffer, R.L., 1997. Characterization of volatile organic emissions and wastes from a swine production facility. J. Environ. Qual. 26, 1687–1696.
Zeikus, J.G., Winfrey, M.R., 1976. Temperature limitation of methanogenesis in aquatic sediments. Appl. Environ. Microbiol. 31, 99–107.
Zhang, R.H., Dugba, P.N., Bundy, D.S., 1997. Laborotory study of surface aeration of anaerobic lagoons for odor control of swine manure. Trans. of the ASAE 40 (1), 185–190. Zhu, J., Bundy, D.S., Li, X., Rashid, N., 1996. Odor control of
livestock manure using pit additives — standardizing the test procedure. ASAE paper # : 964068. St. Joseph, MI. Zhu, J., Bundy, D.S., Li, X., Rashid, N., 1997. Controlling odor