Langlet, A. 1973. Effets de la se´cheresse sur la croissance et la produc- and Y.V. Srirama. 1998. Response to fertilizer nitrogen and water of post-rainy season sorghum on vertisol. 1. Biomass and light tion du sorgho grain. Ann. Agron. 24:307–338.
Lemaire, G., X. Charrier, and Y. He´bert. 1996. Nitrogen uptake capac- interception. J. Agric. Sci. (Cambridge) 131:417–428.
Singh, P., J.L. Monteith, K.K. Lee, J. Rego, and S.P. Wani. 1998. ities of maize and sorghum crops in different nitrogen and water
supply conditions. Agronomie 16:231–246. Response to fertilizer nitrogen and water of post-rainy season sor-ghum on vertisol. 2. Biomass and water extraction. J. Agric. Sci. Lemaire, G., and F. Gastal. 1997. N uptake and distribution in plant
canopies. p. 3–43.InG. Lemaire (ed.) Diagnosis of the nitrogen (Cambridge) 131:429–438.
Siqueira, J.O., M.G. Nair, R. Hammerschmidt, and G.R. Safir. 1991. status in crops. Springer–Verlag, New York.
Lemaire, G., F. Gastal, P. Cruz, and D.J. Greenwood. 1990. Relation- Significance of phenolic compounds in plant-soil-microbial systems. Crit. Rev. Plant Sci. 10:63–121.
ships between plant-N, plant mass and relative growth rate for C3
and C4 crops.InA. Scaiffe (ed.) Proc. Congr. Eur. Soc. Agron., Tempel, A.S. 1981. Field studies of the relationship between herbivore damage and tannin content in bracken (Pteridium aquilinum 1st, Paris, France. 5–7 Dec. 1990. ESA, Colmar, France.
Patrick, Z.A., T.A. Toussoum, and J.C. Snyder. 1963. Phytotoxic sub- Kuhn.). Oecologia 51:97–106.
Vanderlip, R.L., and H.E. Reeves. 1972. Growth stages of sorghum. stances in arable soils associated with decomposition of plant
resi-dues. Phytopathology 53:152–161. Agron. J. 64:13–16.
Weston, L.A., C.I. Nimbal, and M.A. Czarnota. 1997. Activity and Paul, J.W., J.A. Covert, and E.G. Beauchamp. 1994. Influence of soil
temperature and moisture on water-soluble phenolic compounds persistance of sorgoleone, a long-chain hydroquinone produced by Sorghum bicolor. p. 509–516.In Brighton Crop Conf.—Weeds, in manured soil. Can. J. Soil Sci. 74:111–114.
Ple´net, D. 1995. Fonctionnement des cultures de maı¨s sous contrainte United Kingdom. 17–20 Nov. 1997. Br. Crop Protection Counc., Farnham, United Kingdom.
azote´e; de´termination et application d’un indice de nutrition. The`se
de doctorat. Instit. Natl. Polytechnique de Lorraine, Univ. de Woodhead, S. 1981. Environmental and biotic factors affecting the phenolic content of different cultivars ofSorghum bicolor. J. Chem. Nancy, France.
Ple´net, D., and P. Cruz. 1997. Maize and sorghum. p. 93–106.InG. Ecol. 7:1035–1047.
Woodhead, S., and E.A. Bernays. 1978. The chemical basis of resis-Lemaire (ed.) Diagnosis of the nitrogen status in crops. Springer–
Verlag, New York. tance ofSorghum bicolorto attack byLocusta migratoria. Entomol. Exp. Appl. 24:123–144.
Ramu, S.V., S.P. Palaniappan, R.M. Panchanathan. 1991. Growth and
dry matter partitioning of sorghum under moisture stress condition. Woodhead, S., and G. Cooper-Driver. 1979. Phenolic acids and resis-tance to insect attack inSorghum bicolor. Biochem. Syst. Ecol. J. Agron. Crop Sci. 166:273–277.
Rego, T.J., J.L. Monteith, P. Singh, K.K. Lee, V. Nageswara Rao, 7:309–310.
Rust-Enhanced Allelopathy of Perennial Ryegrass against White Clover
Scott W. Mattner* and Douglas G. Parbery
ABSTRACT Connolly, 1990). Crown rust is the most serious ryegrass fungal pathogen in these areas (Eagling and Clark, 1993),
Perennial ryegrass (Lolium perenneL.) and white clover (
Trifo-with epidemics regularly occurring between spring and lium repensL.) are important pasture components in the higher
rain-fall areas of southeastern Australia. Crown rust (Puccinia coronata autumn. A preliminary study demonstrated that rust
ac-Corda f.sp.loliiBrown) is the most serious ryegrass pathogen in these celerates senescence and reduces ryegrass yield by 56% areas. In a preliminary investigation, rust reduced ryegrass biomass (Mattner, 1998). Despite this, in ryegrass and clover mix-by 56%. Yet, interference from rusted ryegrass suppressed the yield tures, interference from rusted ryegrass suppressed clover of neighboring clover plants more than interference from healthy biomass by up to 47% compared with interference from ryegrass. The role of allelopathy in this relationship was investigated
healthy ryegrass. This suppression did not result from
in a greenhouse study using two bioassays. Soil previously growing
a direct effect of crown rust on clover, because
rust-rusted ryegrass suppressed clover biomass by 36% compared with
inoculated and non-inoculated clover monocultures
soil previously growing healthy ryegrass. Similarly, leachate from soil
yielded the same, which was expected since clover is a
surrounding rusted ryegrass suppressed clover biomass by 27%
com-pared with that from healthy ryegrass. This is the first demonstration nonhost of crown rust. Similarly, clover suppression is
that a pathogen may influence allelopathy between plants and that not explained by rust increasing ryegrass competitive-rust may enhance ryegrass allelopathy against clover. Possible implica- ness because, if this were so, the reduction in clover tions of this in pasture ecology and the evolution of mutualism are dis- yield would be greatest at high densities where resources
cussed. are most limited. Instead, clover suppression was
great-est at low densities, where competition for resources was minimal. Ryegrass allelopathy is well documented, particularly against clovers and medics (Gussin and
P
erennial ryegrass and white clover predominateLynch, 1981; Takahashi et al., 1988, 1991, 1993; Quigley improved pastures in the higher rainfall areas of
et al., 1990; Chung and Miller, 1995). For these reasons, southeastern Australia. Their growth in mixtures
with-we investigated the hypothesis that rusting increases out added nitrogen results in greater herbage yields than
ryegrass allelopathic ability. otherwise can be achieved economically (Menchaca and
Both Rice (1984) and Einhellig (1995) hypothesized that pathogens enhance their host’s allelopathic ability. S.W. Mattner, Agriculture Victoria, Institute for Horticultural
Devel-Evidence supporting this hypothesis occurs in at least opment, Private Bag 15, Scoresby Business Centre, Victoria 3176,
Australia; D.G. Parbery, Institute of Land & Food Resources, The two forms. Firstly, pathogens stimulate phytoalexin (an-Univ. of Melbourne, Parkville, Victoria 3052, Australia. Received 29 timicrobial compounds) production by their hosts Nov. 1999. *Corresponding author ([email protected]).
total there were 108 pots, half with rusted ryegrass and half
pathways as allelochemicals. For example, isoflavonoids
with nonrusted ryegrass. Plants in rusted treatments were
are important phytoalexins (Dakora and Phillips, 1996)
spray inoculated (Villalta and Clarke, 1995) 52 d after sowing
and allelochemicals (Tamura et al., 1967, 1969) from
with an aqueous solution containing 13106urediniospores/ the Leguminosae. Indeed, many phytoalexins act as
mL, 0.1% soft soap as a surfactant, and 0.5% gelatine. Plants
allelochemicals against plants, inhibiting their
germina-in nonrusted treatments were sprayed with a similar solution
tion (Chang et al., 1969), growth (Glazener and Van- containing no spores. Following inoculation all plants were Etten, 1978), and cellular metabolism and function (Gi- placed in plastic tents for 1 wk, which maintained humidity annini et al., 1990; Spessard et al., 1994). Secondly, under between 92 and 98%. Donor plants were grown for a total of some conditions, the mutualistic fungusNeotyphodium 100 d.
lolii (Latch, Christensen and Samuels) Glenn, Bacon Soil for the bioassay was gathered directly from the pots
and Hanlin, increases the allelopathic ability of some used to grow rusted and nonrusted donor ryegrass, all of which was removed by hand and then passed through a 5-mm sieve.
ryegrass genotypes (Sutherlund and Hoglund, 1990;
Soil from each source was bulked and thoroughly mixed prior
Quigley et al., 1990). Despite this, no one has previously
to further treatment. A fresh preparation of soil was also
observed that diseased plants suppress the growth of
included as a control. In addition, plus and minus nutrient
neighboring species (Tang et al., 1995), even though the
and steam-sterilization treatments were incorporated into the
effect of pathogens on interference between host and
design. This was done in an attempt to detect confounding
nonhost plants has been extensively investigated (Bur- effects caused by differences in soil microflora and nutrient don, 1987; Ayres and Paul, 1990). Since competition content in the soils resulting from prior rusted and nonrusted is the dominant process of plant interference (Tilman, ryegrass growth. Nutrient enrichment consisted of adding con-1988), competition effects often obscure any allelopathic centrations of the nutrients listed previously, to ensure they
effects in such experiments (Trenbath, 1974). This is were slightly in excess of growth requirements. The soils were
particularly so at high plant densities where competition then placed into 12.5-cm-diam. pots and used to grow two clover receivers. At 70 d after sowing, receivers were washed
is intense. While it is difficult to separate the processes
free of soil and dried at 808C for 4 d before total biomass was
of competition and allelopathy in the field, separation
determined. Additionally, a nodulation index was determined
can be achieved using bioassays. To date, however,
re-by bunching individual plant roots; removing three 2-cm
sec-searchers have not used bioassays to investigate the
tions from the upper, central, and lower portion of the bunch;
effects of pathogens on allelopathy.
counting the nodules captured in each section; drying each
The present investigation examined the hypothesis sample; and recording the number of nodules per gram of root. that rust enhances perennial ryegrass allelopathy against The bioassay was conducted as a randomized complete white clover using two bioassay techniques. factorial design with three blocks. There were four pots per
treatment in each block. Factors consisted of soil source (three
METHODS AND MATERIALS levels: soil previously growing rusted ryegrass, soil previously
growing nonrusted ryegrass, and freshly prepared soil as a
Pathogen and Plant Material
control), nutrient application (two levels: plus and minus) and In the following bioassays, perennial ryegrass (cv. Victo- soil sterilization (two levels: plus and minus).
rian) was used as the donor species and white clover (cv. Tamar) was used as the receiver species. The perennial
rye-grass cultivar Victorian is highly susceptible to crown rust Soil Leachate Bioassay (Critchett, 1991). Crown rust urediniospores were collected
Pots (15 cm in diam.) containing the standard soil mix were with a side-arm flask collector from naturally infected ryegrass
sown to contain eight ryegrass donor plants. Twenty days after at the Mt. Derrimut field station, 22 km west of Melbourne,
sowing, irrigation lines were placed into individual pots and Australia (378479S, 1448479E) (Mattner, 1998).
calibrated to deliver 250 mL of water per pot every day. Soil leachate was collected in plastic trays beneath the wire mesh
Growing Conditions and Soil Type
benches, bulked for each treatment, and thoroughly mixed Bioassays were conducted in temperature-controlled glass- prior to application to receiver plants. Seventy days after sow-houses (20–238C during the day and 12–158C at night) at the ing, plants in the rusted treatment were inoculated with rust Mt. Derrimut field station. Pots were drip-irrigated at the rate as described previously. One-half of the 64 pots contained of 150 mL of water twice daily, which maintained soil at near rusted ryegrass and the other half nonrusted plants.
field capacity. The soil mixture was three parts red-brown The investigation was conducted in two parts. In the first, earth topsoil to one part sand and two parts peat moss. The the pre-inoculation bioassay, the bioassay was made before following nutrients (g/L) were added to the mixture: 0.200 N, donor plant inoculation to ensure that there were no intrinsic 0.116 P, 0.140 K, 0.096 S, 0.652 Ca, 0.024 Cu, 0.012 Zn, 0.008 differences in the allelopathic potential of donor plants within Mn, 0.028 Fe, 0.012 Mo, 0.360 Mg, and 0.001 B. The mixture the rust treatments prior to inoculation. The second, the post-was then pasteurized by steam prior to planting. Following inoculation bioassay, was made after inoculation when rust pasteurization, soil used to grow white clover was inoculated symptoms had fully developed. The methodology of the bio-with the appropriate strain of Rhizobium trifoliiDangeard. assays was the same. Pots (12.5 cm in diam.) filled with the This was performed by watering soil contained in individual standard soil mix were prepared containing two clover receiv-pots with 150 mL of an inoculum solution (10 g of commercial ers. Each was hand watered daily with 150 mL of the appro-inoculum per 10 L of water).
priate leachate or with water in the case of the controls. Receivers were harvested and compared 50 d after sowing
Soil Retrieval Bioassay by determining plant biomass, leaf area (with a planimeter,
Paton Industries Pty. Ltd., South Australia), leaf number, sto-Pots (15 cm diam.) containing the standard soil mix were
Table 2. The effect of soil sterilization on the nodulation of Table 1. Summary of the levels of statistical significance for the
various treatments and their interactions on white clover bio- white clover. mass and nodulation.
No. nodules per
Treatment gram of root LSD (p,0.05)
Source of variation Dry biomass Nodulation
Sterilized 554.55
Soil source (SS) *** NS†
191.95
Nutrient (N) NS NS
Nonsterilized 752.12
Sterilization (St) NS **
SS3N ** NS
SS3St NS NS
N3St NS NS Although soil sterilization did not affect clover
bio-SS3N3St NS NS mass, it reduced root nodulation by 26% (see Table 2).
** Significant at the 0.01 probability level.
*** Significant at the 0.001 probability level.
Soil Leachate Bioassay
† Not significant.
The pre-inoculation bioassay detected no difference
on one randomly selected plant per treatment in each block, in the growth of clover watered with leachate from rye-except for biomass where all plants were measured. grass in nonrusted or rusted treatments (see Table 3). The bioassay was conducted as a randomized complete This indicates that there was no intrinsic difference in block design. The treatments were the daily application of 150
the allelopathic ability of ryegrass plants assigned to the
mL of leachate derived from either rusted ryegrass, nonrusted
treatments prior to inoculation. Growth in the control,
ryegrass, or irrigation water. There were eight blocks
con-however, was greater than that of plants receiving soil
sisting of four pots per treatment.
leachate from ryegrass.
In the post-inoculation bioassay, the growth of clover
Statistical Analysis
receiving soil leachate from rusted ryegrass was less than
Data was analyzed using analysis of variance (ANOVA) that of plants watered with leachate from nonrusted as performed on Minitab Version 12 (Minitab, 1998). Homo- ryegrass or the control, according to each parameter geneity of variance was determined by examining plots of measured (see Table 3). Plants exposed to leachate from fitted values versus residuals, while histograms of residuals nonrusted ryegrass did not differ from the control in assessed normality of distribution.
any way. Nodulation did not vary between treatments.
RESULTS
DISCUSSION
Soil Retrieval Bioassay
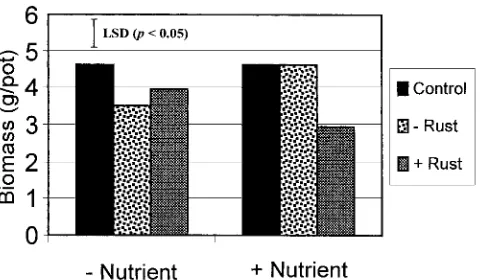
Even though several authors have suggested that Table 1 presents statistical significance levels of main pathogens increase their host’s allelopathic ability (Rice, treatments and their interactions in influencing clover 1984; Einhellig, 1995), the present study is the first to biomass and nodulation. Sterilization of the soils used evaluate the pathogen effect on allelopathy between in this bioassay had no effect on the biomass of clover plants. Furthermore, no one has previously observed growing in them. As such, sterilization treatments have that diseased plants suppress the growth of neighboring been grouped to provide a clearer demonstration of the species (Tang et al., 1995). Despite this, a preliminary effects of the different soil source and nutrient treat- investigation found that rusted ryegrass suppressed the ments (see Fig. 1). The biomass of clover grown in soil growth of neighboring clover plants by up to 47% com-from rusted ryegrass was less than that in the control pared with when grown with healthy ryegrass (Matt-or in soil from nonrusted ryegrass, with this effect being ner, 1998).
particularly marked in nutrient treated soils. In contrast, In the present study, the soil retrieval bioassay dem-clover grown in soil from nonrusted ryegrass produced onstrated that, overall, soil previously growing ryegrass less than the control only when no nutrients were added. reduced the subsequent clover growth compared with Table 3. Production of white clover in response to leachate de-rived from rusted or nonrusted perennial ryegrass or a control of irrigation water. Bioassays were performed prior to and after the inoculation of ryegrass with rust.
Leachate source
LSD White clover Nonrusted Rusted
growth parameter Control ryegrass ryegrass p,0.05 p,0.01
Pre-inoculation bioassay
Total biomass (g) 1.10 0.76 0.78 0.16 0.22 Post-inoculation bioassay
Total biomass (g) 2.86 3.11 2.28 0.53 0.75 Leaf area (cm2) 203.88 196.2 145.4 49.9 NS†
Stolon number 4.71 5.00 4.00 0.76 NS
Leaf number 36.5 34.12 25.5 8.78 NS
Nodulation index Fig. 1. The biomass of white clover grown in soil from rusted or
(no. nodules nonrusted perennial ryegrass. The control consisted of freshly
pre-per gram root) 1222.81 1121.11 1143.78 NS NS pared soil. Nutrients were added at concentrations described in
a control of freshly prepared soil. The modification of chate only from mature ryegrass, starting at 60 d after sowing.
soil microflora by ryegrass to contain species
antagonis-In contrast to leachate from healthy ryegrass, plant tic to clover growth does not explain this result because
age did not affect the phytotoxicity of leachate from soil sterilization had no effect on the relationship. In
rusted ryegrass, where the leachate suppressed clover contrast, nutrient addition alleviated the suppressive
ef-biomass by 20% compared with the control and by 27% fect that soil previously growing healthy ryegrass had
compared with nonrusted ryegrass. This is again in simi-on clover productisimi-on. This suggests that the main effect
lar proportions to the preliminary investigation (Matt-of healthy ryegrass was to deplete soil nutrients and
ner, 1998). Similar reductions occurred in all parameters thereby diminish clover growth. This was not the case
measured in clover exposed to leachate from rusted for soil previously growing rusted ryegrass, however,
ryegrass, strongly supporting the hypothesis that rust where nutrient application markedly increased its
sup-increases ryegrass allelopathic ability against clover. The pressive effect on clover growth. Under these
condi-results also suggest that in addition to enhancing rye-tions, soil previously growing rusted ryegrass suppressed
grass allelopathic potential, rust prolongs allelopathy clover biomass by 36% compared with soil previously
well into maturity. growing healthy ryegrass. This was in similar
propor-While results from each bioassay suggest that rust can tions to that in the preliminary experiment where the
increase ryegrass allelopathy against clover, there was yield of clover grown in mixtures with rusted ryegrass
no evidence of allelopathy acting against nodulation. fell by an average of 37% (Mattner, 1998). Nutrient
Furthermore, the uniformity of size and pinkness of toxicity does not explain this result because twice the
nodules from plants in all treatments suggested that concentration of nutrients applied to soil in the rusted
allelopathy did not impair nodule nitrogen-fixing capac-treatment had no effect on clover growth in the control.
ity, although no measurement of this was made. The Instead, the result provides strong evidence that rust
only treatment that affected nodulation was soil steril-increases ryegrass allelopathic ability against clover.
ization, which reduced it by 26%. This was probably Furthermore, the above results demonstrate a potential
due to sterilization reducing the resident rhizobial popu-for rusted ryegrass to release allelochemicals into soil
lation in the soil prior to planting. at concentrations phytotoxic to clover.
The ability of rust to increase ryegrass allelopathic Previous soil-based bioassays have not reported
en-potential is not surprising given that infection by patho-hanced allelopathy following nutrient addition
(Buch-gens can induce phytoalexin production by their hosts holtz, 1971). Despite this, there are at least three
possi-(Smith, 1996). Phytoalexins can belong to similar chemi-ble explanations of how nutrients might enhance clover
cal groups as allelochemicals. For example, Mayama suppression by soil previously growing rusted ryegrass:
(1981, 1982) discovered that rust resistant oat (Avena
(i) The presence of abundant nutrients in some way sativaL.) produced three phytoalexins belonging to the facilitates allelochemical uptake. phenolic acid group when challenged by crown rust. These phenolic acids inhibited rust spore germination (ii) Nutrient addition may initiate cationic exchange.
at concentrations as low as 200 mg/L. Although these Here, nutrient addition in the form of cations
chemicals were not shown to suppress plant growth, releases allelochemicals bound to anionic
colloi-they have a great potential to do so given that many dal or organic material into the soil solution,
allelochemicals are phenolic acids. where they are absorbed by receiver plants.
Another potential allelochemical source from rusted (iii) A reaction of allelochemicals with the added
nu-ryegrass in the present experiment is from the rust itself. trients may increase their toxicity and/or
sta-It is well established that pathogens can produce a range bility.
of phytotoxins that are important in pathogenesis (Daly Takahashi et al. (1988, 1991) established that leachate and Deverall, 1983). Despite this, a preliminary investi-from perennial ryegrass can be phytotoxic to white clo- gation demonstrated that clover monoculture inocula-ver. In a similar manner, the present study showed that tion with crown rust had no effect on its growth (Matt-soil leachate from developing ryegrass (up to 70 d after ner, 1998). This suggests that the allelochemical source sowing) suppressed clover biomass by 30% compared from rusted ryegrass in the present experiment is more with the control (i.e., in the pre-inoculation bioassay). likely to be from ryegrass than from rust. It is important This highlights the potential for ryegrass to suppress to note, however, that irrespective of the phytotoxin clover through allelopathy. As healthy ryegrass devel- source from rusted ryegrass, the relationship between oped, however, the phytotoxicity of its leachate dimin- rusted ryegrass and clover remains allelopathic. This is ished and was lost, suggesting that ryegrass allelopathic because rust is a biotrophic parasite of ryegrass and potential is greatest when it is young. Work on other because allelopathy is defined as the beneficial and det-plant species has also shown a similar decrease in allelo- rimental chemical interaction among plant organisms, pathy as plants age (Koeppe et al., 1970; Woodhead, including microorganisms (Rice, 1984).
lea-allelochemicals than healthy plants? In a related ques-
CONCLUSIONS
tion, what is the effect of disease severity on ryegrass The bioassays of the present study showed that soil allelopathic ability? The average disease severity of and leachate from rusted ryegrass suppressed clover rusted ryegrass at 42 d after inoculation in the soil re- growth compared with that from nonrusted ryegrass. trieval bioassay of the present experiment was 6.6% Together, these bioassays provide strong evidence for (as determined by a calibration method [Mattner and the hypothesis that rust enhances perennial ryegrass Parbery, 1999]). If rust enhances allelopathy between allelopathic ability against white clover. This hypothesis ryegrass and clover, heavily rusted plants should pro- would explain why rusted ryegrass suppressed the duce more allelochemicals than lightly rusted plants. growth of neighboring clover plants in a preliminary Moreover, the ryegrass cultivar used in the present ex- experiment (Mattner, 1998).
periment was highly susceptible to rust. If rust enhances ryegrass allelopathy through phytoalexin production,
REFERENCES
rust resistant cultivars might produce more
phytoalex-ins, or allelochemicals, than susceptible cultivars. Ayres, P.G., and N.D. Paul. 1990. The effects of disease on interspecific plant competition. Aspects Appl. Biol. 24:155–162.
The increased allelopathy of rusted ryegrass has
im-Buchholtz, K.P. 1971. The influence of allelopathy on mineral nutri-portant implications, both for pasture ecology and the
tion. p. 86–89.In U.S. Natl. Comm. for IBP (ed.) Biochemical evolution of mutualism. In Australia, rust epidemics interactions among plants. National Academy of Science, Washing-occur in ryegrass between late spring and early autumn. ton, DC.
Burdon, J.J. 1987. Diseases and plant population biology. Cambridge During this period, relative white clover growth is
Univ. Press, Cambridge, UK. greater than that of perennial ryegrass, due mainly to
Chang, C.F., A. Suzuki, S. Kumai, and S. Tamura. 1969. Chemical differences in temperature optima (18–218C for ryegrass studies on ‘clover sickness.’ II. Biological functions of isoflavonoids and 248C for clover) for growth (Haynes, 1980). The and their related compounds. Agric. Biol. Chem. 33:398–408. combined effects of crown rust and increased competi- Chung, I.M., and D.A. Miller. 1995. Allelopathic influence of nine
forage grass extracts on germination and seedling growth of alfalfa. tion from clover increase ryegrass mortality. For this
Agron. J. 87:767–772. reason, the increase in allelopathy by ryegrass following
Clay, K. 1988. Clavicipitaceous fungal endophytes of grasses: Coevolu-rust infection may contribute to preventing its eradica- tion and the change from parasitism to mutualism. p. 79–105.In tion from pastures during the summer–autumn period. K.A. Pirozynski and D.L. Hawksworth (ed.) Coevolution of fungi
with plants and animals. Academic Press, London. Although pathogens are largely detrimental to their
Critchett, C.I. 1991. Studies on ryegrass rusts in Victoria. Ph.D. thesis. hosts, they may also bestow some benefits (Parbery,
La Trobe University, Victoria, Australia. 1996). By conferring some benefit on its host, the
patho-Cruickshank, I.A.M. 1957. Crown rust of ryegrass. N.Z. J. Sci. Tech-gen maintains a self-advantage through increasing the nol. 38A:539–543.
survival chances of its host and ultimately itself. Further- Dakora, F.D., and D.A. Phillips. 1996. Diverse function of isoflavo-noids in legumes transcend anti-microbial definitions of phytoalex-more, Clay (1988) suggested that pathogens evolve
to-ins. Physiol. Mol. Plant Pathol. 49:1–20. ward a mutualistic relationship with their hosts through
Daly, J.M., and B.T. Deverall. 1983. Toxins and plant pathogenesis. the acquisition of beneficial characteristics or “new Academic Press, Sydney, Australia.
functions.” Since biotrophic parasites such as the rusts Eagling, D.R., and R.G. Clarke. 1993. The effect of pathogens on are heavily dependent on the continuity of their host perennial pasture grasses. p. 49–55.InProc. of the First National Perennial Grass Workshop. Oct. 1993. Pastoral and Veterinary genotype into succeeding generations, the evolution of
Inst., Hamilton, Australia. interactions that enhance the chances of host survival
Einhellig, F.A. 1995. Allelopathy: Current status and future goals. p. are important. It is well established that infections of 1–24.InInderjit et al. (ed.) Allelopathy: Organisms, processes, and fodder species by biotrophs can create conditions that applications. Am. Chem. Soc., Washington, DC.
either limit grazing of their hosts, limit the number of Fletcher, L.R., and B.L. Sutherland. 1993. Liveweight changes in lambs grazing perennial ryegrasses with different endophytes. p. 115–120. grazing animals, or both. Morgan and Parbery (1980)
In Proc. of the 2nd Int. Symp. on Acremonium/Grass Interac-established that infection byPseudopeziza medicagnis
tions. Feb. 1993. Grasslands Res. Centre, Palmerston North, New (Lib.) Sacc. lowered protein content, digestibility, and Zealand.
palatability of lucerne (Medicago sativa L.) as well as Giannini, J.L., J.S. Holt, and D.P. Briskin. 1990. The effect of glyceollin increasing its oestrogenic activity. Similarly, Critchett on proton leakage in Phytophthora megasperma f.sp. glycinea plasma membrane and red beet tonoplast vesicles. Plant Sci. 68: (1991) determined that crown rust reduces ryegrass
di-39–45. gestibility and quality. For this reason, ruminants
prefer-Glazener, J.A., and H.D. VanEtten. 1978. Phytotoxicity of phaseollin entially graze healthy ryegrass rather than rusted rye- to, and alteration of phaseollin by, cell suspension cultures of grass (Cruickshank, 1957), indirectly benefiting rusted Phaseolus vulgaris. Phytopathology 68:111–117.
Gussin, E.J., and J.M. Lynch. 1981. Microbial fermentation of grass ryegrass. Evidence from the present study suggests that
residues to organic acids as a factor in the establishment of new rust adds a further benefit to ryegrass, that of increased
grass swards. New Phytol. 89:449–457. allelopathy with neighboring plants. In a similar manner
Haynes, R.J. 1980. Competitive aspects of the grass–legume associa-to crown rust, amongst other benefits, the mutualistic tion. Adv. Agron. 33:227–261.
endophyteN. loliireduces ryegrass palatability to rumi- Koeppe, D.E., L.M. Rohrbaurgh, E.L. Rice, and S.H. Wender. 1970. Tissue age and caffeoylquinic acid concentration in sunflower. Phy-nants (Fletcher and Sutherland, 1993) and reportedly
tochemistry 9:297–301. increases its allelopathic ability (Sutherland and
Hog-Mattner, S.W. 1998. The impact of crown rust on interference between lund, 1990; Quigley et al., 1990). Is the pathogenic rela- perennial ryegrass and white clover. Ph.D. thesis. The University tionship between crown rust and ryegrass evolving to- of Melbourne, Victoria, Australia.
and rust in perennial ryegrass. p. 95.InProc. of the 12th Austral- Takahashi, Y., T. Otani, and K. Hagino. 1993. Studies on interactions asian Plant Pathology Soc. Conf., Rydges, Canberra, Australia. among some grassland species. V. Collection and isolation of
allelo-Sept. 1999. pathic hydrophobic compounds from the root exudates ofLolium
Mayama, S., Y. Matsuura, H. Iida, and T. Tani. 1982. The role of perenneL. J. Jap. Soc. Grassl. Sci. 39:236–245.
avenalumin in the resistance of oat to crown rust,Puccinia coronata Takahashi, Y., T. Otani, S. Uozumi, Y. Yoden, and R. Igarashi. 1988. f.sp.avenae. Physiol. Plant Pathol. 20:189–199. Studies on the allelopathic interactions among some grassland spe-Mayama, S., T. Tani, Y. Matsuura, T. Ueno, and H. Fukami. 1981. cies. I. Effects of root exudates from some grass and legume species The production of phytoalexins by oat in response to crown rust, on the growth of their own species and other species. J. Jap. Soc. Puccinia coronataf.sp.avenae. Physiol. Plant Pathol. 19:217–226. Grassl. Sci. 33:334–338.
Menchaca, L., and J. Connolly. 1990. Species interference in white Takahashi, Y., T. Otani, S. Uozumi, Y. Yoden, and R. Igarashi. 1991. clover–ryegrass mixtures. J. Ecol. 78:223–232. Studies on the allelopathic interactions among some grassland spe-Minitab. 1998. spe-Minitab. Version 12. Minitab, State College, PA. cies. II. Assessment of the allelopathic interactions between peren-Morgan, W.C., and D.G. Parbery. 1980. Depressed fodder quality and nial ryegrass (Lolium perenneL.) and white clover (Trifolium re-increased oestrogenic activity of lucerne infected withPseudopez- pensL.) using a root exudate recirculating system. J. Jap. Soc. iza medicagnis. Aust. J. Agric. Res. 28:1029–1040. Grassl. Sci. 37:274–282.
Newman, E.I., and M.H. Miller. 1977. Allelopathy among some British Tamura, S., C. Chang, A. Suzuki, and S. Kymai. 1967. Isolation and grasslands species. II. Influence of root exudates on phosphorus structure of a novel isoflavone derivative in red clover. Agric. Biol.
uptake. J. Ecol. 65:399–411. Chem. 31:1108–1109.
Newman, E.I., and A.D. Rovira. 1975. Allelopathy among some Brit- Tamura, S., C. Chang, A. Suzuki, and S. Kymai. 1969. Chemical studies ish grasslands species. J. Ecol. 63:727–737. on ‘clover sickness.’ I. Isolation and structural elucidation of two Parbery, D.G. 1996. Trophism and the ecology of fungi associated new isoflavonoids in red clover. Agric. Biol. Chem. 33:391–397.
with plants. Biol. Rev. 71:473–527.
Tang, C.S., W.F. Cai, K. Kohl, and R.K. Nishimoto. 1995. Plant stress Quigley, P.E., F.J. Snell, P.J. Cunningham, and W. Frost. 1990. The and allelopathy. p. 142–157.InInderjit et al. (ed.) Allelopathy:
effects of endophyte infected ryegrass on the establishment,
persis-Organisms, processes, and applications. Am. Chem. Soc., Washing-tence and production of mixed pastures in Australia. p. 49–50.In
ton, DC. Proc. of the Int. Symp. on Acremonium/Grass Interactions. Nov.
Tilman, D. 1988. Plant strategies and the dynamics and structure of 1993. Louisiana Agric. Exp. Stn., Baton Rouge.
plant communities. Princeton Univ. Press, Princeton, NJ. Rice, E.L. 1984. Allelopathy. 2nd ed. Academic Press, Orlando, FL.
Trenbath, B.R. 1974. Biomass productivity of mixtures. Adv. Smith, C.J. 1996. Accumulation of phytoalexins: Defence mechanism
Agron. 26:177–210. and stimulus response system. New Phytol. 132:1–45.
Villalta, O.N., and R.G. Clarke. 1995. Evaluation of genotypes of Spessard, G.O., C. Hanson, J.S. Halvorson, and J.L. Giannini. 1994.
tall fescue for resistance toPuccinia graminissubsp.graminisin Effects of phaseollin on membrane leakage in red beet vacuoles
controlled conditions. Aust. Plant Pathol. 24:82–87. and tonoplast vesicles. Phytochemistry 35:43–47.
Woodhead, S. 1981. Environmental and biotic factors affecting the Sutherland, B.L., and J.H. Hoglund. 1990. Effect of ryegrass
con-phenolic content of different cultivars ofSorghum bicolor. J. Chem. taining the endophyteAcremonium loliion associated white clover.
Ecol. 7:1035–1047. p. 67–71.InProc. of the Int. Symp. on Acremonium/Grass