Biochemical Systematics and Ecology 28 (2000) 933}947
Volatiles released from oak, a host tree for the
bark beetle
Scolytus intricatus
Pavl
m
H
na Vrkoc
\
ova
H
*
, Irena Valterova
H
, Jan Vrkoc
\
,
Bohum
m
H
r Koutek
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo na&me\stn&2, 166 10 Praha 6, Czech Republic
Received 15 November 1999; received in revised form 6 April 2000; accepted 10 April 2000
Abstract
Volatile compounds emitted in di!erent phases of oak (Quercus robur) development (bark,
unopened buds, young developing leaves, and blossoms) were analyzed with the aim of"nding
possible host-plant attractants for the European oak bark beetle,Scolytus intricatus. Complex
mixtures of aliphatic, aromatic, and terpenoid compounds were identi"ed in the samples. (E)-2-Hexenal and hexanal dominated in samples of bark. In buds, (Z)-3-hexenyl acetate formed
a substantial part of the mixture. In both leaves and blossoms (E,E)-a-farnesene was the main
component.
Volatiles released from oak twigs and branches during both the maturation feeding and
construction of maternal galleries byScolytus intricatuswere also analyzed. Most compounds
found in the samples from females'and males'maturation feeding were identical. High contents
of anisole, (E)-b-ocimene,a-copaene, one unidenti"ed sesquiterpenic hydrocarbon C
15H24and
b-caryophyllene were found in both samples of twigs attacked by beetles. During the construc-tion of maternal galleries by bark beetles in oak logs, monoterpene hydrocarbons such as p-cymene, (E)-b-ocimene, andc-terpinene, and sesquiterpenesa-copaene andb-caryophyllene were released in large quantities. No new compound appeared when males were added to the
log with feeding females. ( 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Quercus; Monoterpenes; Sesquiterpenes; Oak wilt; Pathogenic fungi;Scolytus intricatus; Matu-ration feeding; Brood galleries
*Corresponding author. Tel.:#4202-201-83229; fax:#4202-243-10177. E-mail address:[email protected] (P. Vrkoc\ovaH).
1. Introduction
The oak wilt, caused by pathogens of the genera Ceratocystis, Ophiostoma and others, is a disease leading to withering and death of oak trees in the eastern and central states of the USA as well as in Europe (Doganlar and Schopf, 1984). Infected trees die within a year, sometimes in less than a month. The disease is spread by root-to-root grafts and the pathogenic fungi can be transmitted by insects.
The European oak bark beetle (Scolytus intricatus; Coleoptera: Scolytidae) is re-ported as a possible vector of the fungus (Doganlar and Schopf, 1984). The adult beetles breed in cut or weakened oak branches by boring deep larval galleries in the xylem. The cut branches are often infested by pathogens.S. intricatusoverwinters in the late larva stage or as a pupa (Schwenke, 1974). In the spring time, emerging adult beetles visit healthy oaks and make twig cavities where they feed (maturation feeding). Thus, beetles contaminated with spores or parts of mycelium can transmit the fungus to the healthy trees. In the Czech Republic, the species usually has one generation per year. In exceptionally warm years or localities, a partial second generation can occur. Bark beetles feeding or breeding in trees must locate a suitable host from among the relatively few scattered widely in the forest. Usually, chemicals are the most important mediators of host-plant-searching behavior (Miller and Strickler, 1984; Visser, 1986; StaKdler, 1992). It is believed that insects have evolved behavioral responses to volatile host-plant chemicals that indicate the presence of a suitable host in which reproduc-tion can occur. Depending on the species, bark beetles "nd their host either by attraction to host volatiles from a distance or by random landing and probing (Byers, 1995). Chemical mediation of host"nding generally occurs via mixtures of chemicals, some of them stimulants and others inhibitors.
Host volatiles are attractive to a number of forest scolytids including species in the genusScolytus. In case of S. intricatus, oak (Quercusspp.) is the main host tree, but willow (Salixspp.) is also accepted by beetles for maturation feeding (Doganlar and Schopf, 1984).
The main goal of our work is to search for chemical communication of the European oak bark beetle by pheromones and for their potential primary attractants. In the present study, we have investigated (a) volatiles emitted in di!erent phases of the oak development (outer and inner bark, unopened buds, young developing leaves, and blossoms), and (b) volatiles released from oak twigs and branches during both maturation feeding and construction of maternal galleries. Biological activity was observed by preliminary electrophysiological and behavioral tests.
2. Materials and methods
2.1. Collection of plant material and insects
developing. Logs, healthy as well as infested withScolytus intricatuswere collected in the winter of 1998 in three di!erent localities within the Czech Republic. All logs were kept at 53C until used. Infested logs were then placed into net cages at laboratory temperature. Emerged beetles were collected daily, sexed and allowed to bore into young oak twigs for maturation feeding and later for making maternal galleries and eventual copulation. All plant materials studied were free of pathogenic fungi.
All samples were repeated "ve times and standard deviations are given in the results.
2.2. Collection ofvolatiles
An air entrainment technique was used for trapping the volatile compounds released from oak (Q. robur) buds, blossoms, young twig bark and young leaves, oak twigs (diameter 2}6 mm), logs (diameter 4}5 cm) and beetle-infested twigs and logs (40 beetles in each). Modi"ed equipment earlier described by JursmHk et al. (1991) was used
for collection of volatiles. Buds, blossoms and bark were shredded and leaves were crushed in liquid nitrogen before the entrainment. Biological material was then placed in a glass vessel (3 l). Puri"ed air (silica gel, charcoal and molecular sieve) was drawn through the vessel for 24 h at 40}50 ml/min and then through a glass tube "lter containing Porapak Q (0.1 g; mesh size 100}120). An additional"lter was placed after
the"rst one for a breakthrough check. The captured volatiles were eluted from the
sorption material with hexane (150ll).
2.3. Identixcation ofvolatile constituents
The components were identi"ed on a GC (Carlo Erba) coupled with a mass detector Fisons MD 800, using a BPX5 capillary column (SGE, 30 m]0.22 mm,"lm thickness 0.25 mm) with helium#ow 0.94 and 0.55 ml/min (measured at 503C), respectively. The temperature program was 503C for 2 min, then 43C/min up to 2603C and held for 20 min, resp. 703C for 2 min, then 43C/min up to 2603C and held for 20 min; inlet temperature was 2003C in all cases, injector working in splitless mode. The mass spectra (electron-impact) were compared to the Wiley Registry of Mass Spectral Data, 6th edition, the National Institute of Standards and Technology (NIST) Library, and Identi"cation of Essential Oil Components by Gas Chromatography/Mass Spectro-metry Library (R.P. Adams, Allured Publishing Corporation). Retention times of analyzed compounds were compared with those of authentic samples wherever possible.
The gas-phase infrared spectrum (GC-FTIR) of one of the isolated components, a sesquiterpene hydrocarbon C
15H24, was measured on an HP 5890 gas chromato-graph coupled with an HP 5695A IRD equipped with a narrow-band (4000}750 cm~1) infrared detector (mercury cadmium telluride).
equipped with split/splitless injectors and #ame ionization detectors. Deactivated fused silica capillaries were used as retention gaps and for the connection of the di!erent valves with each other and with the detectors. A DB-WAX column was used for pre-separation in the"rst chromatograph (GC-A). For chiral analysis the valve was programmed according to the retention times determined in the"rst run so as to let the chiral compounds pass to the second chromatograph (GC-B) with the Cyc-lodex B column. For further details see Borg-Karlson et al. (1993). For GC-A, helium #ow 6.2 ml/min (403C), column DB-WAX (J&W, 30 m]0.25 mm, "lm thickness 0.25 mm) and temperature program 503C for 2 min, then 43C/min up to 2103C and held for 20 min were used. For GC-B, helium#ow 9.2 ml/min (403C), column Cyclodex B (J&W, 30 m]0.25 mm,"lm thickness 0.25 mm) and temperature program 553C for 13 min, then 13C/min up to 773C were used.
3. Results and discussion
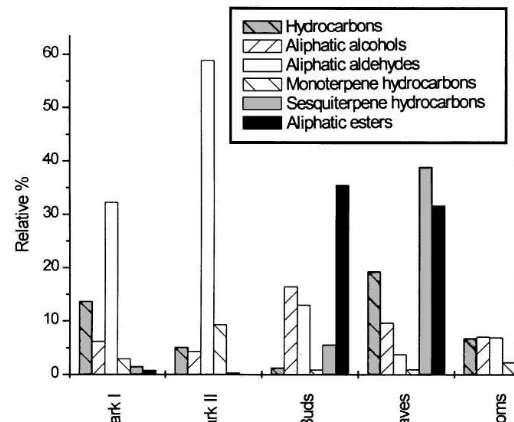
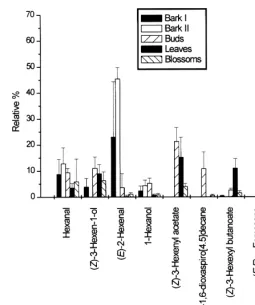
Complex mixtures of aliphatic, aromatic, and terpenoid compounds were identi"ed in the samples from oak (Table 1, Figs. 1 and 2). In the early and late bark samples, aldehydes formed the main portion of the mixture, (E)-2-hexenal and hexanal 8.7 and 12.8% resp. 23.0% and 45.4% being the main components.n-Alkanes formed 13.7% resp. 5.1% and aliphatic alcohols 6.2% resp. 4.3% of the compounds. Monoterpene and sesquiterpene hydrocarbons were present as minor components. Esters were almost absent. Proportion of compounds in early and late bark samples did not di!er signi"cantly.
In the buds, esters dominated (36.5%) in the volatiles released. (Z)-3-hexenyl acetate formed 21.5% of the mixture. Substantial proportions of alcohols (16.4%) and aldehydes (13.0%) were found in the samples. Among the alcohols, 11.0% consisted of (Z)-3-hexenol. In relatively high abundance were dioxaspiroalkanes (11.5%). Homoterpene hydrocarbons were present as minor components (2.0%). Monoterpene and sesquiterpene hydrocarbons andn-alkanes were present in small quantities only. After the buds opened and small leaves appeared, the spectrum of volatiles changed again. Aldehydes (3.7%) and alcohols (9.6%) were less abundant, whilen-alkanes were moderately abundant (19.3%) constituents. Esters (31.7%) and sesquiterpene hydro-carbons (38.9%) dominated in the samples. (Z)-3-Hexenyl acetate and (Z)-3-hexenyl butanoate were the major esters while (E,E)-a-farnesene (37.5%) was the most impor-tant sesquiterpene. Also, homoterpenes were present as minor components (4.8%).
Volatiles from opened oak blossoms were included in the analytical study although the #owers open later than bark beetles start their maturation feeding. (E,E)-a -farnesene dominated in the sample (41.7%). All other types of compounds were present in moderate abundance (from 3 to 7%).
The period of maturation feeding is supposed to be critical for the transfer of fungal pathogens. The attack of beetles on young twigs for maturation feeding coincides approximately with the development of oak leaves. The knowledge of volatile pro"les of four main types of tissue may lead to better understanding of the beetle host seeking behavior.
Table 1
Compounds emitted from oak (Quercus robur) in di!erent phases of its development
Compound! RT (min)" Method of Relative % (standard deviation)
identi"cation
Bark I# Bark II$ Buds Leaves Blossoms
Toluene 3.284 MS, standard 3.37 (3.83) 1.11 (0.25) 0.61 (0.28) 0.08 (0.09) 1.59 (0.70)
Heptane 3.501 MS, standard * * 0.53 (0.20) * *
Ethyl butanoate 3.600 MS * * 1.29 (0.43) 0.46 (0.30) *
Hexanal 3.667 MS, standard 8.71 (5.78) 12.78 (6.17) 9.37 (1.60) 3.50 (1.74) 5.84 (8.73)
(Z)-3-Hexen-1-ol 4.517 MS, standard 3.87 (3.32) * 11.04 (4.34) 8.94 (3.54) 6.26 (3.40)
(E)-2-Hexenal 4.400 MS, standard 23.03 (21.29) 45.40 (4.32) 3.60 (5.35) 0.22 (0.44) 1.04 (0.62)
1-Hexanol 4.617 MS, standard 2.32 (1.91) 4.31 (2.17) 5.22 (2.02) 0.69 (0.34) 0.80 (0.58)
Dimethylbenzene, isomer I 4.717 MS 0.48 (0.45) 0.19 (0.16) * 0.03 (0.02) 0.68 (0.28)
Isopentyl acetate 4.718 MS * * 0.38 (0.13) * *
Nonane 4.984 MS, standard 0.82 (0.84) 0.38 (0.11) * 0.02 (0.01) 0.15 (0.05)
Dimethylbenzene, isomer II 5.101 MS 0.17 (0.15) * * *
Heptanal 5.201 MS, standard 0.05 (0.04) 0.14 (0.09) * * *
Pentyl acetate 5.367 MS * * 0.24 (0.09) 0.13 (0.07) *
a-Thujene 5.601 MS, standard * 0.20 (0.18) * * *
a-Pinene 5.851 MS, standard 0.27 (0.28) 0.25 (0.14) * 0.02 (0.03) 0.37 (0.21)
Ethylmethylbenzene 6.584 MS * * * 0.01 (0.00) 0.15 (0.09)
Sabinene 6.768 MS, standard * 0.08 (0.08) * * *
Myrcene 7.117 MS, standard * 1.78 (0.49) 0.33 (0.10) 0.08 (0.03) 0.68 (0.20)
Decane 7.251 MS, standard 2.83 (2.97) 0.44 (0.21) * 0.03 (0.02) 0.22 (0.11)
Trimethylbenzene 7.501 MS * * * * 0.32 (0.17)
(E)-3-Hexenyl acetate 7.534 MS, standard * * * 0.40 (0.30) *
Octanal 7.669 MS, standard * 0.18 (0.05) * * *
(Z)-3-Hexenyl acetate 7.717 MS, standard * * 21.25 (5.42) 15.22 (7.70) 3.99 (1.17)
n-Hexyl acetate 7.801 MS, standard * * 6.89 (4.17) 1.01 (0.60) 0.34 (0.14)
a-Terpinene 7.985 MS, standard * 0.09 (0.11) * * *
p-Cymene 8.317 MS, standard 1.00 (0.87) 2.93 (2.47) 0.09 (0.08) 0.02 (0.03) *
Limonene 8.384 MS, standard 1.33 (0.70) 1.08 (0.76) 0.18 (0.08) 0.07 (0.06) 0.43 (0.09)
Table 1. Continued
Compound! RT (min)" Method of Relative % (standard deviation)
identi"cation
Bark I# Bark II$ Buds Leaves Blossoms
1,8-Cineole 8.534 MS, standard * * 0.03 (0.03) 0.02 (0.02) 0.33 (0.29)
(E)-b-Ocimene 8.801 MS, standard * * 0.25 (0.18) 0.70 (0.84) 0.61 (0.49)
(E)-7-Methyl-1,6-dioxaspiro [4.5] decane
9.184 MS, standard * * 10.82 (6.42) 0.04 (0.03) 0.60 (0.33)
c-Terpinene 9.269 MS, standard 0.32 (0.42) 2.89 (2.84) 0.03 (0.07) * 0.16 (0.08)
1-Octanol 9.715 MS, standard * * 0.18 (0.10) * *
Linalool oxide, furanic,cis 9.717 MS, standard 0.34 (0.24) 0.22 (0.15) * 0.03 (0.03) 0.04 (0.05)
2-Ethyl-1,6-dioxaspiro [4.4] nonanMchalcogranN
10.203 MS * * 0.73 (0.25) * *
Linalool oxide, furanic,trans 10.301 MS, standard 0.88 (0.63) 0.81 (0.81) 0.09 (0.07) 0.04 (0.08) 0.04 (0.07)
Undecane 10.353 MS, standard 2.07 (1.81) 0.30 (0.34) * * 0.33 (0.19)
n-Pentyl butanoate 10.368 MS * * * 0.15 (0.06) *
(Z)-3-Hexenyl propanoate 10.551 MS * * 0.25 (0.11) 0.69 (0.37) *
Linalool 10.701 MS, standard * * * 0.01 (0.03) *
n-Hexyl propanoate 10.769 MS * * 0.14 (0.18) * *
Nonanal 10.951 MS, standard 0.47 (0.42) 0.23 (0.09) * * *
(E)-4,8-Dimethyl-1,3,7-nonatriene 11.001 MS * * 1.27 (0.78) 0.91 (0.42) 0.83 (0.84)
(Z)-3-Hexenyl isobutanoate 12.018 MS * * 0.22 (0.06) 0.89 (0.61) *
n-Hexyl isobutanoate 12.268 MS * * 0.13 (0.10) 0.03 (0.02) *
(Z)-3-Hexexyl butanoate 13.651 MS 0.36 (0.23) * 2.59 (0.72) 10.96 (3.71) 1.55 (0.79)
n-Hexyl butanoate 13.801 MS * * 0.76 (0.57) 0.15 (0.05) *
Dodecane 13.885 MS, standard 1.34 (0.66) 0.45 (0.10) * * 0.39 (0.18)
Methyl salicylate 14.401 MS, standard 1.99 (3.44) 0.22 (0.21) 0.83 (0.89) * *
Decanal 14.521 MS, standard * 0.10 (0.13) * * *
(Z)-3-Hexenyl 2-methylbutanoate 15.185 MS * * 0.50 (0.25) 0.83 (0.40) *
n-Hexyl 2-methylbutanoate 15.368 MS * * 0.65 (0.18) 0.32 (0.21) *
n-Hexyl pentanoate 15.601 MS * * 0.13 (0.04) 0.03 (0.04) *
Ethyl salicylate 17.171 MS, standard * * 0.22 (0.08) *
Tridecane 17.418 MS, standard 1.01 (0.52) 0.65 (0.20) 0.09 (0.03) 0.06 (0.07) 0.79 (1.09)
a-Cubebene 19.318 MS * * * 0.01 (0.02) *
a-Copaene 20.418 MS, standard 0.72 (0.55) 0.12 (0.07) 1.30 (0.80) 0.05 (0.04) 0.17 (0.09)
(Z)-3-Hexenyl hexanoate 20.468 MS * * * 0.25 (0.13) *
n-Hexyl hexanoate 20.635 MS * * * 0.08 (0.11) *
Tetradecane 20.868 MS, standard 1.87 (1.03) 0.67 (0.26) * 0.05 (0.02) 0.49 (0.26)
b-Caryophyllene 21.952 MS, standard * * 0.18 (0.09) 0.67 (0.72) 0.45 (0.16)
trans-a-Bergamotene 22.089 MS, standard * * 0.20 (0.24) * *
b-Gurjunene 22.390 MS * * 0.62 (0.76) * *
(Z)-b-Farnesene 22.540 MS, standard * * 0.04 (0.09) * *
(E)-b-Farnesene 22.785 MS, standard * * 0.83 (1.56) 0.03 (0.01) *
Aromadendrene 23.402 MS, standard * * 0.27 (0.19) 0.01 (0.01) *
(Z,E)-a-Farnesene 24.085 MS * * * 0.62 (0.26) *
Pentadecane 24.202 MS, standard 1.29 (0.56) 1.02 (0.24) 0.40 (0.25) * 2.19 (0.49)
(E,E)-a-Farnesene 24.702 MS, standard * 1.40 (1.01) 37.46 (10.55) 41.72 (21.40)
d-Cadinene 25.202 MS 0.76 (1.07) 0.17 (0.10) 0.73 (0.89) 0.03 (0.02) *
(E,E)} 4,8,12-Trimethyl-1,3,7,11-Tridecatetraene
26.752 MS * * 0.75 (0.78) 3.93 (3.16) *
(Z)-3-Hexenyl benzoate 27.152 MS * * * 0.03 (0.02) *
Hexadecane 27.319 MS, standard 1.26 (1.11) 0.42 (0.10) 0.07 (0.02) 0.04 (0.02) 0.56 (0.35)
Heptadecane 30.302 MS, standard 0.78 (0.48) 0.57 (0.16) 0.12 (0.03) 0.03 (0.02) 1.27 (0.31)
Octadecane 33.153 MS, standard 0.41 (0.28) 0.17 (0.03) 0.05 (0.01) 19.02 (0.01) 0.35 (0.21)
Isopropyl myristate 33.970 MS 0.42 (0.37) 0.06 (0.02) * * *
!The compounds are listed in elution order on a 30 m DB-5ms column (5% phenyl methyl silicone).
"Temperature program: 503C (2 min); 43/min to 2603(20 min); helium#ow 0.94 ml/min at 503C.
#Collected at time when buds were developing.
$Collected at time when leaves were developing.
Fig. 1. Sum of relative percent of di!erent compound classes emitted from plant tissues.
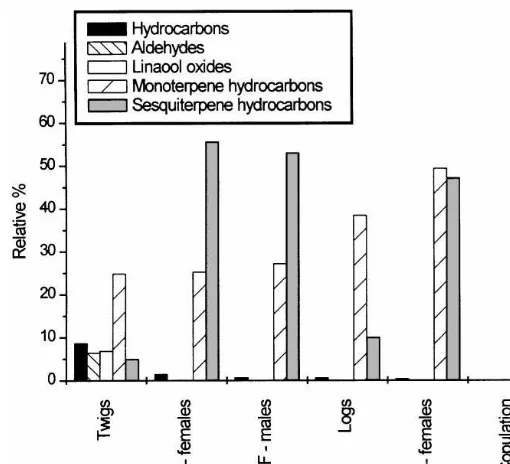
Samples obtained from oak twigs during the maturation feeding ofScolytus in-tricatusfemales and males had many components in common, though their propor-tions di!ered (Table 2, Figs. 3, 4a and b). Generally, the twigs and logs without beetles gave sample of much lower concentration. After beetles started to feed and build galleries, the amounts of emitted volatiles increased substantially.n-Alkanes formed a substantial part (8%) of the twig sample without beetles. During the maturation feeding of both females and males, more compounds were released as a consequence of the beetles'activity and the proportions of alkanes dropped to the barely detectable level. In twigs, monoterpenes formed 24.8% of the compounds. The most abundant compound was (E)-b-ocimene (22.5%). Linalool oxides were present at 6.8% and aldehydes at 6.5%. Only small amounts of sesquiterpenes (4.9%) were found.
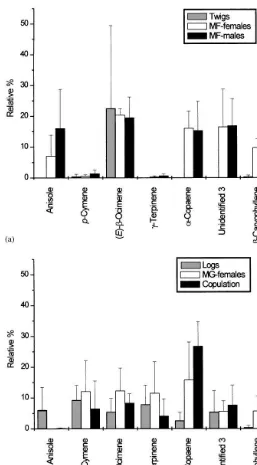
Most of the compounds found in the samples from females'and males'feeding were identical. Their proportions in the two samples were not very di!erent (see Fig. 4a). High contents of anisole, (E)-b-ocimene, a-copaene, one unidenti"ed sesquiterpenic hydrocarbon C
15H24 (unidentixed 3&in Table 2) andb-caryophyllene were found in both samples of twigs with beetles.
Fig. 2. Relative percent and standard deviation of selected compounds (over 5%) emitted from plant tissues.
CH-stretching area 3070}3080 cm~1and only a weak band around 1640}1660 cm~1 (C"C stretch) excludes the presence of an exomethylene terminal C"CH
2group in the compound (Svatos\ and Attygalle, 1997). Bands at 3052 and 3024 cm~1 (C}H stretch) suggest the presence of two di!erent types of carbon}carbon double bonds. Double bonds are probably not conjugated, as the C"C stretch (1642 cm~1) is not too intense. Comparison with IR spectra of terpenes from the literature, the CH stretch at 3052 cm~1and CH wagging 780 cm~1imply a trisubstituted double bond in a ring.
Table 2
Compounds captured from oak twigs and logs during maturation feeding and making maternal galleries ofScolytus intricatus
Compound! RT (min)" Method of Relative % (standard deviation)
identi"cation
6.317 MS 0.77 (0.57) 0.08 (0.06) 0.06 (0.09) 1.22 (1.60) 0.10 (0.12) 0.01 (0.02)
Nonane 6.634 MS, standard 1.25 (1.01) 0.28 (0.24) 0.22 (0.21) 0.61 (0.46) 0.14 (0.14) 0.03 (0.02)
Dimethylbenzene, Isomer II
6.900 MS 0.25 (0.49) * 0.02 (0.03) 0.46 (0.67) * *
Heptanal 7.034 MS, standard 0.14 (0.28) * * * * *
a-Thujene 7.534 MS, standard * * * 0.92 (0.95) 4.22 (3.74) 0.94 (1.23)
Anisole 7.567 MS, standard * 7.01 (6.83) 15.99 (12.74) 6.03 (7.39) 0.03 (0.06) 0.13 (0.14)
a-Pinene (!/#: 96.2/3.8)#
7.801 MS, standard 0.50 (0.52) 0.90 (0.39) 1.43 (0.68) 2.45 (2.48) 2.17 (1.85) 0.34 (0.27)
Camphene (!/#: 100/0)#
8.367 MS, standard 0.42 (0.85) 1.48 (0.90) 1.96 (1.40) 2.03 (2.38) 0.52 (0.38) 0.13 (0.08)
Ethylmethylbenzene 8.734 MS 0.78 (1.41) * * 0.40 (0.40) * *
Sabinene 8.967 MS, standard * 0.17 (0.05) 0.37 (0.14) 1.88 (1.31) 0.67 (0.59) 0.07 (0.09)
Benzaldehyde 9.134 MS, standard 0.20 (0.39) * * * * *
b-Pinene (!/#: 100/0)# 9.217 MS, standard * 0.23 (0.11) 0.30 (0.24) 0.65 (0.81) 0.16 (0.13) 0.01 (0.02)
Myrcene 9.317 MS, standard * 0.47 (0.14) 0.61 (0.34) 2.43 (1.36) 1.00 (0.49) 0.39 (0.17)
Decane 9.434 MS, standard * 0.39 (0.36) 0.21 (0.25) * 0.10 (0.11) 0.02 (0.03)
Trimethylbenzene 9.817 MS 1.26 (2.51) * * * * *
Octanal 9.984 MS, standard 1.83 (0.68) * * * * *
a-Phellandrene 10.084 MS, standard * * * * 0.08 (0.07) 0.02 (0.03)
a-Terpinene 10.434 MS, standard * * * 0.25 (0.31) 0.70 (0.62) 0.21 (0.28)
p-Cymene 10.767 MS, standard 0.38 (0.77) 0.54 (0.46) 1.34 (1.16) 9.20 (4.87) 12.01 (10.01) 6.42 (9.07) Limonene (!/#:
83.1/16.9)#
10.884 MS, standard 0.37 (0.45) 0.71 (0.36) 0.52 (0.78) 4.78 (2.91) 3.41 (2.19) 0.64 (1.29)
(Z)-b-Ocimene 10.934 MS, standard 0.65 (1.31) * 0.49 (0.43) * * 0.59 (0.77)
1,8-Cineole 11.034 MS, standard 0.06 (0.11) 0.05 (0.06) 0.34 (0.23) 3.56 (2.13) 0.19 (0.20) 0.03 (0.03)
(E)-b-Ocimene 11.317 MS, standard 22.49 (26.91) 20.34 (2.12) 19.45 (6.77) 5.38 (4.48) 12.23 (7.43) 8.26 (3.04)
(E)-7-Methyl-1,6-dioxaspiro[4,5]decane
11.751 MS, standard 0.89 (1.15) 0.37 (0.17) 0.38 (0.35) * 0.10 (0.15) 0.10 (0.13)
c-Terpinene 11.867 MS, standard * 0.31 (0.31) 0.63 (0.60) 7.80 (6.35) 11.46 (10.25) 4.05 (5.53)
Terpinolene 12.834 MS, standard * 0.09 (0.11) 0.02 (0.04) 0.66 (0.41) 0.71 (0.59) 0.29 (0.37)
Undecane 12.951 MS, standard * 0.40 (0.37) 0.16 (0.26) * 0.06 (0.12) *
Linalool oxide, furanic,
13.668 MS 2.10 (3.18) 0.18 (0.06) 0.74 (0.55) 3.27 (2.70) 0.31 (0.30) 0.04 (0.05)
Dodecane 16.568 MS, standard 1.74 (1.04) 0.25 (0.23) 0.09 (0.14) * 0.03 (0.05) *
Dodecanal 17.368 MS, standard 1.90 (2.56) * * * * *
Thymol, methyl ether 18.451 MS * 0.01 (0.02) 0.07 (0.09) * 0.01 (0.02) 0.03 (0.04)
Unidenti"ed 1$ 19.118 MS * 0.11 (0.06) * * 0.26 (0.32) 0.37 (0.30)
Tridecane 20.302 MS, standard 2.87 (1.26) 0.09 (0.13) * * 0.02 (0.03) *
Unidenti"ed 2% 21.535 MS * * * * 0.04 (0.05) 0.21 (0.12)
a-Ylangene 22.368 MS * 0.15 (0.09) 0.12 (0.05) * 0.20 (0.22) 0.33 (0.21)
a-Copaene 23.552 MS, standard * 16.02 (5.54) 15.25 (9.60) 2.59 (2.68) 15.81 (12.25) 26.73 (7.96)
Unidenti"ed 3& 23.768 MS * 16.46 (12.36) 16.86 (8.79) 5.37 (6.88) 5.61 (3.36) 7.57 (6.52)
Tetradecane 23.935 MS, standard 0.83 (1.02) * * * * *
b-Elemene 23.952 MS * 0.48 (0.29) 0.66 (0.65) * 1.03 (0.81) 1.58 (0.68)
a-Gurjunene 24.652 MS * 0.09 (0.06) 0.31 (0.26) * 1.99 (1.81) 1.37 (1.70)
b-Caryophyllene 25.219 MS, standard 0.27 (0.54) 9.72 (2.90) 5.98 (5.24) 0.33 (0.67) 5.65 (4.79) 8.87 (3.69)
trans-a-Bergamotene 25.352 MS, standard 0.66 (1.33) 1.48 (0.91) 1.51 (0.67) 0.73 (1.02) 0.52 (0.16) 0.55 (0.44)
a-Guaiene 25.535 MS * 1.06 (0.53) 0.98 (0.49) * 2.12 (2.31) 2.96 (1.87)
(E)-b-Farnesene 25.635 MS, standard * 1.02 (0.66) 0.96 (0.69) 0.38 (0.46) 0.29 (0.37) 0.36 (0.39)
a-Humulene 26.452 MS * 1.87 (0.65) 1.56 (1.44) * 2.17 (1.90) 3.84 (1.85)
Aromadendrene 26.602 MS, standard 1.02 (0.94) 0.46 (0.12) 0.64 (0.57) 0.37 (0.74) 0.46 (0.48) 0.76 (0.16)
a-Amorphene 27.035 MS * 0.00 (0.00) 0.67 (0.44) * 0.82 (0.33) 1.07 (0.12)
a-Curcumene 27.085 MS 0.04 (0.07) * * * * *
Pentadecane 27.219 MS, standard 1.96 (1.24) * * * * *
Germacrene D 27.285 MS * 0.57 (0.29) 1.03 (1.08) * 1.28 (1.09) 2.63 (1.38)
b-Selinene 27.635 MS * 2.29 (0.85) 1.13 (0.62) * 1.50 (1.73) 2.67 (1.77)
a-Muurolene 27.752 MS 1.59 (1.05) 1.99 (0.68) 2.06 (1.40) 0.14 (0.28) 1.00 (0.99) 0.97 (1.24)
b-Bisabolene 27.819 MS * * * * 3.89 (4.82) 6.87 (5.44)
d-Cadinene 28.352 MS 1.35 (2.04) 1.98 (0.70) 3.23 (3.22) 0.10 (0.20) 2.80 (1.03) 3.90 (0.34)
!The compounds are listed in elution order on a 30 m BPX-5 column (5% phenyl methyl silicone).
"Temperature program: 703C (2 min); 43/min to 2603(20 min); helium#ow 0.55 ml/min at 503C.
#Proportion of enantiomers determined on 2D-GC system.
$Mass spectrum*m/z%: 77(57), 79(56), 91(100), 105(52), 119(25), 133(14), 147(34), 162(29).
%Mass spectrum*m/z%: 41(79), 91(100), 105(53), 115(47), 117(44), 119(74), 131(56), 145(44), 159(86), 187(19), 202(16).
&Mass spectrum*m/z%: 41(100), 77(63), 91(79), 93(90), 105(25), 119(75), 161(4), 189(1), 204(0.6).
IR spectrum: 3052, 3024, 2969, 2925, 2876, 2741, 1642, 1452, 1381, 1349, 1159, 1111, 1027, 943, 845, 779 cm~1.
Fig. 3. Sum of the relative percent of di!erent compound classes released from host branches and beetle-attacked branches.
(E)-b-ocimene (12.2 and 8.3%, respectively),c-terpinene (11.5 and 4.0%, respectively), a-copaene (15.8 and 26.7%, respectively), unidenti"ed sesquiterpene (5.6 and 7.4%, respectively) andb-caryophyllene (5.7 and 8.9%, respectively) were the most abundant compounds in logs attacked by beetles. Only relative proportion of anisole,a-copaene andb-caryophyllene changed signi"cantly with the beetle attack (see Fig. 4b).
Terpenes and homoterpenes are known to be produced by plants in response to herbivore feeding. (E)-b-Ocimene and (E)-4,8-dimethyl-1,3,7-nonatriene were released in large amounts from infested cucumber plants (Takabayashi et al., 1994). It is not clear whether the biosynthesis of the volatiles released from infested plants is induced by herbivore feeding or if they are stored in the plant and released at the time of infestation (PareH and Tumlinson, 1996). Infested cotton plants released high amounts of a-pinene, b-caryophyllene, (E,E)-a-farnesene, (E)-b-farnesene, (E)-b-ocimene, and (E)-4,8-dimethyl-1,3,7-nonatriene (Loughrin et al., 1994). Loughrin suggested from the released timing and rate thata-pinene andb-caryophyllene come from the constitut-ive compounds (stored in the plant) while the other compounds were biosynthesized de novo in response to insect feeding.
Fig. 4. (a) and (b). Relative percent and standard deviation of selected compounds (over 5%) released from host branches and beetle-attacked branches.
development. Electrophysiological testing of potential host-plant attractants in-dicated the activity of several components of our samples (dimethylbenzene, nonane, a-thujene, sabinene, p-cymene, c-terpinene, terpinolene, dodecane, tridecane, and a-gurjunene).
Many forest bark beetles are attracted by host volatiles. Some of them, possessing a multicomponent aggregation pheromone, are attracted by host volatiles at the beginning of their mass attack. Another smaller group of scolytids may not use an aggregation pheromone, but generally they are strongly attracted either to the host monoterpenes, ethanol, or a combination of both. The compounds released from a host tree in the particular development state are not only important for primary attraction to plants but they may play a role in enhancing the bark beetles'response to the aggregation pheromone, if any.
The production of an aggregation or sex pheromone byS. intricatusis still uncertain despite the evidence from behavioral trials that seems to indicate its presence. The situation could be similar to that inScolytusventraliswhere, according to latest data, the attack dynamics can be explained solely by its sensitive primary attraction response to host volatiles (MarcmHas-SaHmano et al., 1998) or toTomicus piniperdawhere
the pheromone exists, but it remains elusive due to a strong masking e!ect of tree odors (Czokajlo, 1998).
Acknowledgements
The"nancial support of this work by the Grant Agency of the Czech Republic
(grants nos. 203/97/0037 and 203/00/0219) and by COST E16.10. is gratefully acknow-ledged. The authors also wish to thank Dr. A.-K. Borg-Karlson (KTH, Stockholm, Sweden) for 2D-GC instrument time, Dr. A.B. Attygalle (Cornell University, Ithaca, USA) for GC-FTIR instrument time, Dr. S. Vas\mHc\kovaH and Dr. A. Svatos\ (Institute of
Organic Chemistry and Biochemistry, Prague, Czech Republic) for the GC-IR con-sulting, and Prof. R.M. Coates (University of Illinois, Urbana, USA) for sending a sample oftrans-a-bergamotene.
References
Borg-Karlson, A.K., LindstroKm, M., Norin, T., Persson, M., ValterovaH, I., 1993. Enantiomeric composition of monoterpene hydrocarbons in di!erent tissues of Norway Spruce,Picea abies(L.) Karst. A multi-dimensional gas chromatography study. Acta Chem. Scand. 47, 138}144.
Byers, J.A., 1995. In: Bell, W.J., CardeH, T.T. (Eds.), Chemical Ecology of Insects 2. Chapman & Hall, New York, pp. 154.
Czokajlo, D., 1998. Ph.D. Thesis, State University of New York, Syracuse.
Doganlar, M., Schopf, R., 1984. Some biological aspects of the European oak bark beetle,Scolytus intricatus(Ratz.) (Col., Scolytidae) in northern parts of Germany (BRD). Z. Angew. Entomol. 97, 153}162.
Hovorka, O., Kindl, J., Vrkoc\ovaH, P., KalinovaH, B., Hoskovec, M., DoubskyH J., Koutek, B., 2000. Chemically mediated aggregation behaviour in the European oak bark beetle,Scolytus intricatus(Ratz.) (Coleoptera, Scolytidae). Physiol. Entomol., submitted for publication.
JursmHk, T., StraHnskyH, K., Ubik, K., 1991. Trapping system for trace organic volatiles. J. Chromatogr. 586,
315}322.
Loughrin, J.H., Manukian, A., Heath, R.R., Turlings, T.C.J., Tumlinson, J.H., 1994. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plants. Proc. Natl. Acad. Sci. U.S.A. 91, 11836.
MarcmHas-SaHmano, J.E., Borden, J.H., Gries, R., Pierce Jr., H.D., Gries, G., King, G.G.S., 1998. Primary
attraction of the"r engraver,Scolytusventralis. J. Chem. Ecol. 24, 1049}1075.
Miller, J.R., Strickler, K.L., 1984. In: Bell, W.J., CardeH, T.T. (Eds.), Chemical Ecology of Insects 2. Chapman & Hall, New York, pp. 127.
PareH, P.W., Tumlinson, J.H., 1996. Plant volatile signals in response to herbivore feeding. Fla. Entomol. 79, 93}103.
Schwenke, W., 1974. In: Parey, P., Die ForstschaKdlinge Mitteleuropas, Vol. 2. Hamburg. StaKdler, E., 1992. In: Rosentahl, G.A., Berenbaum, M.R. (Eds.), Herbivores: their Interaction with Secondary Plant Metabolites. Academic Press, New York, p. 45.
Svatos\, A., Attygalle, A.B., 1997. Characterization of vinyl-substituted, carbon-carbon double bonds by Gc/Ft-Ir analysis. Anal. Chem. 69, 1827}1836.
Takabayashi, J., Dicke, M., Takahashi, S., Posthumus, M.A., Van Beek, T.A., 1994. Leaf age a!ects composition of herbivore induced synomones and attraction of predatory mites. J. Chem. Ecol. 20, 373}386.