Diversity of communities of arbuscular mycorrhizal (AM) fungi
present in conventional versus low-input agricultural sites in
eastern Pennsylvania, USA
q
Marlise Franke-Snyder
a, David D. Douds Jr
a,∗, Larisa Galvez
a, John G. Phillips
a,
Peggy Wagoner
b, Laurie Drinkwater
b, Joseph B. Morton
caUSDA-ARS-ERRC, 600 E. Mermaid Lane, Wyndmoor, PA 19038, USA bRodale Institute, 611 Siegfriedale Road, Kutztown, PA 19530, USA cWest Virginia University, 401 Brooks Hall, Morgantown, WV 26506, USA
Accepted 12 May 2000
Abstract
We compared the composition and structure of the communities of arbuscular mycorrhizal (AM) fungi associated with maize (Zea maysL.) and soybeans (Glycine max(L.) Merr.) in a conventional (CON) and two low-input (LI) farming systems to better understand the relationship among AM fungi present in different agricultural systems. One LI system utilized animal manure (LI-AM) and the other green manure (LI-GM) as the nitrogen source. Spores were extracted from rhizosphere soil samples by wet-sieving to perform microscopic identification of the species and to assess frequency of occurrence. These data were used to calculate species richness, Shannon and Wiener index of diversity, and indices of dominance among other ecological measures. The results indicated that 15 consecutive years of farming under the three management practices did not cause many differences among the fungal communities. The majority of the 15 fungal species found throughout the site were present in all treatments. Sporulation of particular fungal species differed among farming systems and/or among hosts, but the general structure of AM fungal communities (according to most ecological measures) was similar for all treatments. Trap cultures were set up for the different treatments and grown for three cycles to try to recover species with low or no sporulation in natural conditions. These results also supported our conclusion about the homogeneity of the communities in the different farming system/plant host combinations, because only one species (Glomus constrictum) that was not found in the field samples sporulated in trap culture pots. Given that differences in sporulation may reflect differential rates of growth, three undescribed species plusGlomus mosseaeandGlomus etunicatumwere better established, both in the field and in trap cultures, than the other 10 species present in these soils. Also,Gigaspora giganteaaccounted for more than 60% of the total volume of spores produced in each treatment, with the exception of conventional plots planted with maize where spore biovolumes were spread much more evenly among several fungal species suggesting that carbon allocation relationships were much more balanced in these plots. The focus of future studies at these sites will be a comparison of the efficacy among the communities in terms of enhancement of plant growth. © 2001 Elsevier Science B.V. All rights reserved.
Keywords:Arbuscular mycorrhizal fungus spores; Dominance; Biovolume; Sustainable agriculture
qMention of a brand or firm name does not constitute an endorsement by the US Department of Agriculture over others not mentioned. ∗Corresponding author. Fax:+215-233-6581.
E-mail address:[email protected] (D.D. Douds Jr).
1. Introduction
The role of microbial communities in the mainte-nance of soil quality and assistance of plant growth is important even in agroecosystems where human inter-ference is pronounced. They are involved in processes such as acquisition and recycling of nutrients important for plant growth, antagonism of detrimental organisms, and participation in the formation and maintenance of soil structure (Visser and Parkinson, 1992; Bever, 1994; Schimel, 1995; Pankhurst et al., 1996).
Arbuscular mycorrhizal (AM) fungi are important components of the microbiota, performing several of the tasks listed above, and especially are appreciated for their role in nutrient transfer from soil to plants (Bolan, 1991; Jakobsen et al., 1992a,b; George et al., 1995). Recently, more attention has been given to their role in soil aggregation (Bethlenfalvay, 1992; Miller and Jastrow, 1992; Schreiner and Bethlenfalvay, 1995; Wright and Upadhyaya, 1996).
We are still developing our understanding of the relationships among members of mycorrhizal fungal communities. Biodiversity has been used as a means to characterize and compare the number and relative abundance of organisms within and among commu-nities (Whittaker, 1965; Peet, 1974; Ludwig and Reynolds, 1988; Magurran, 1988; Visser and Parkin-son, 1992). Dominance weighs abundance and favors the most common species (Peet, 1974; Magurran, 1988). There are known limitations to the use of these ecolog-ical indices (O’Donnell et al., 1994; Pankhurst et al., 1996; Zak and Visser, 1996), which may become accentuated when used for characterization of AM fun-gal communities because of the unique biology of this group of fungi. Though there has been exciting recent progress in the application of molecular techniques to identify genotypic diversity of AM fungi in roots and soil (Helgason et al., 1999), mycorrhizologists presently are limited to the use of spores to identify and count the fungi to perform diversity studies (Mor-ton et al., 1995; Merryweather and Fitter, 1998). Spore morphology and numbers, however, may not necessar-ily reflect the vegetative community structure (Douds and Millner, 1999). These facts influence interpretation of such studies.
There are many questions that can be investigated by the study of microbial diversity. Some researchers have addressed the need to compare the microbial
com-munities of agroecosystems maintained with conven-tional, high chemical input practices, to those under reduced-input farming (Hassink et al., 1991; Workneh and van Bruggen, 1994; Hooker and Black, 1995; Kennedy and Smith, 1995; Buyer and Kaufman, 1996). Several studies have showed greater numbers of AM fungus spores and more inoculum of AM fungi in soils under low-input as compared to conventional, chemical-based agriculture (Limonard and Ruissen, 1989; Vivekanandan and Fixen, 1991; Douds et al., 1993; Kurle and Pfleger, 1994). There is need for compari-son of AM fungal community structure between soils under the two management regimes at the same site (Kurle and Pfleger, 1996).
We determined diversity, dominance and biovolume patterns for the communities of AM fungi from soils farmed by conventional, high-input methods and by two types of low-input agriculture based on spore pop-ulations. The conventional (CON) maize and soybean rotation incorporated inorganic fertilizers and chemi-cal control of weeds. Both low-input systems use rotary hoeing, cultivation and crop rotations for weed control. One low-input system used animal manure as a nitro-gen source (LI-AM), and a rotation of maize, soybeans, small grains and hay. The other incorporated green ma-nure as the nitrogen source (LI-GM) in a rotation of maize, soybeans, small grains and leguminous cover crops. These systems are part of the Rodale Institute’s Farming Systems Trial (Liebhardt et al., 1989). The objectives of the Farming Systems Trial when it was established in 1981 were to define yield-limiting fac-tors that occur during the transition from conventional to low-input farming, identify methods of minimizing yield reductions, and identify physical, chemical, and biological processes that occur during conversion to low-input methods. Comparing the mycorrhizal fungi in these systems gave us information about sporulation patterns associated with farming practices and about the composition of these communities.
2. Materials and methods
2.1. Sampling and experimental design
was established in 1981 to monitor input and yield dif-ferences among three farming systems. The site had previously been utilized solely for conventional agri-culture (Doran et al., 1987). The dominant soil type was Comly silt loam (fine-loam, mixed, mesic Typic Fragiudalf).
Treatments consisted of three farming systems (FS), i.e. a conventional, a low-input with animal manure, and a low-input with green manure in which two crops, maize (Zea maysL.) (MZ) and soybeans (Glycine max
(L.) Merr.) (SOY), were grown that year. Each FS×crop combination was replicated in 6.1×91.5 m plots within four blocks in the 6.1 ha field. Seven rhizosphere soil sub-samples were collected at evenly-spaced intervals along one row within each plot. These were combined to yield four pooled samples (one per block) for each of the six FS×crop combinations. Soil samples were placed in zip lock plastic bags and stored at 4◦C
un-til analysis. Given the complex nature of the analyses to be performed, samples were taken at only one sam-pling date, in the fall, when populations of mycorrhizal spores were high and in good condition (Douds et al., 1993; Kirchner et al., 1993; Koske et al., 1997).
2.2. Spore extraction and taxonomic analysis
Spores were isolated from 350 cm3of soil from the pooled samples via wet sieving (Gerdemann and Nicol-son, 1963) and centrifugation (Jenkins, 1964). A very fine sieve (38mm opening) was used to collect the small spores usually present in these soils, and the coarse material remaining on the top sieve (425mm opening) also was checked for sporocarps and very large spores. Spores were separated into groups accord-ing to general morphological similarities under a Zeiss stereomicroscope and the diameter of spores was mea-sured. Permanent slides of all spores were prepared by placing them in polyvinyl alcohol-lactic acid-glycerin (PVLG) mixed with Melzer’s reagent (1:1 vol/vol) (Franke, 1992). Spores were cracked open under the cover slip to allow for observation of spore wall and inner wall characteristics. Spores were identified to species according to classical morphological analysis under a Zeiss compound microscope and their frequen-cies were recorded. Important taxonomic characters (Franke, 1992) included number and type of layers of the spore wall and their staining reaction to Melzer’s reagent; characteristics of inner walls, when present;
morphology of the subtending hypha at point of attach-ment; and color and size range of spores. Spores that could be identified were counted, even if parasitized or nonviable, given the assumption that the spores had been produced during the past growing season. Spores did not seem to persist in the soil for longer periods of time according to observations from the preliminary work performed at these sites.
2.3. Trap cultures
Greenhouse pot cultures also were established with the soil samples to recover spores from AM fungal species present in the soil, including some which may not have sporulated at the time of sampling. We re-peated these trap cultures for three consecutive cycles, modifying the culture conditions slightly each time. At first, a portion of the mix of the four-replicate compos-ite soil samples from each treatment was diluted with an equal volume of a sterilized growing mix used in our greenhouse experiments (vermiculite:soil:turface:sand at 1:3/4:3/4:1, vol/vol), potted in 15.25 cm diameter pots, and seeded with maize. These trap cultures were allowed to grow for 5 months, after which cores of growth medium were taken from each pot and spores were extracted as described above. All species present were recorded. The remaining soil mix and the plants of each pot were allowed to slowly dry at room tem-perature. The dried medium was then stored at 4◦C
for 1 month and the second generation trap culture was started, again using a 1:1 mix of the cold-stored first generation trap cultures with the sterilized growth medium described above. The host used this time was sorghum–sudangrass CV. ‘Sudax’ [Sorghum bicolor
(L.) Moench]×[Sorghum sudanense (Piper) Stapf]. These cultures grew for 3 months, and the same harvest-ing procedure was followed. A third generation of trap cultures was established by taking the center core of the second generation cultures, representing roughly half of the total pot volume, placing it into a new pot, sur-rounding the core with sterilized sand, and sowing new Sudax seeds. These cultures were grown in the green-house for 8 months and spores then were extracted from both portions of the medium.
2.4. Ecological measures of diversity
Table 1
Diversity measures used to describe communities of AM fungi
Species richness Measured as species density, i.e. the number of species in a specified collection areaa
Shannon–Wiener index of diversity (H′) H′=−6pilnpi
Evenness (E) E=H′/Hmax
Biovolume (Biovol) Biovol=4/3πr3
Simpson’s index (D) D=6[ni(ni−1)/N(N−1)]
Modified Berger–Parker index (d) d=Biovolmax/Biovoltotal
ap
iis the proportion of individuals found in theith species, estimated asni/N, whereniis the number of individuals in theith species and
Nis the total number of individuals.Hmaxis the maximum diversity and it is calculated as lnS, whereSis the number of species recorded.r
is radius calculated from the diameters of a sample of spores of a species. Biovolmaxis the biovolume of the species that presents the greatest
biovolume in a treatment and Biovoltotalis the total spore biovolume in the treatment.
species biovolume (based on spore volumes) and mod-ified Berger and Parker index of dominance (based on biovolume data) were calculated (see Table 1 for formulae). Certain species were combined into groups for calculation of biovolume: one containingGlomus
occultum Walker (isolate FST10/95), Glomus
aggregatum-like (FST10/95) andGlomus
microaggre-gatumKoske, Gemma & Olexia (FST10/95) and
an-other with theGlomus spurcum-like (FST10/95) and the Small, yellowGlomus(FST10/95) isolates (Table 2). This was necessary because these species could not be differentiated under the stereomicroscope and there-fore were measured together.Glomus microaggrega-tumproduced very small spores (from 10 to 40mm in
Table 2
Main morphological characteristics of undescribed AM fungal species found in study soils
Undescribed species designation Characteristics
Glomus spurcum-like Small hyaline spores (≈50mm), with a very thin subtending hypha (≈1–2mm diameter); spore
wall has thick and flexible laminae, which tend to form an even, thick layer (> 2.5mm) on spores
cracked under the cover slip; there seems to be an outer mucilaginous layer, but very young spores have not been seen to confirm this observation
Small yellowGlomus Small bright yellow spores (≈40–50mm) with straight subtending hypha; simple spore wall, with
an outer thin, hyaline layer attached to the thick laminated layer
Glomus aggregatum-like Spores similar toGlomus aggregatum, also appearing inside older spores; a mucilaginous layer surrounding the spores; mycorrhizal association of this type of spores has not yet been observed in a single-species pot culture
GlomusE-1 UniqueGlomus, crystalline appearance,≈95–160mm diameter (average 141mm); subtending
hy-pha very small and fragile, connected to outer layer of spore wall only; presence of inner wall layers, innermost stains mauve in Melzer’s reagent; multiple germination, through the spore wall GlomusC-1 Hyaline spores,≈(45)-55-(85)mm, with a very fragile subtending hypha which tends to break off
easily; transparent outer spore wall forming wrinkles around a more rigid ‘unit’ layer (laminations hard to distinguish) , the inner layer stains pink in Melzer’s reagent:PVLG mix; spore contents tend to form small lumps of material, which turn yellow in Melzer’s reagent:PVLG mix; mycorrhizal association of this type of spores has not yet been observed in a single species pot culture
diameter) singly in the soil or in large numbers in very tight clusters inside other empty, older mycorrhizal fun-gus spores. The calculations are made counting only the loose, individual spores, unless otherwise speci-fied. We divided the volume of the old spores by the mean volume ofG. microaggregatum spores to esti-mate the number of spores found in the clusters within old spores.
Analysis of variance (ANOVA) was performed for diversity and dominance indices and Duncan’s test was applied for comparison of means when appro-priate. Percent values were transformed by arcsin√x
and spore counts were transformed by log (x+1) or
√
3. Results
Most spores retrieved possessed recognizable mor-phological characteristics which permitted their identi-fication. Spores with indistinctive features for character analysis were not considered. Data on root colonization of field plants and infectivity potential of soils under these farming systems at this site can be found in Douds et al. (1993).
3.1. Species composition
Fifteen species of AM fungi were found. Only one species belonged to the genus Gigaspora (Gerd. & Trappe) Walker & Sanders, i.e. Gigaspora gigantea
(Nicol. & Gerd.) Gerdemann & Trappe (FST10/95), and one species belonged to the genusScutellospora
Walker & Sanders, i.e.Scutellospora pellucida(Nicol. & Schenck) Walker & Sanders (FST10/95). All oth-ers belonged to the genusGlomus(Tul. & Tul.) Gerde-mann & Trappe:Glomus claroideumSchenck & Smith (FST10/95);Glomus constrictumTrappe (FST10/95);
Glomus etunicatumBecker & Gerdemann (FST10/95);
Glomus fasciculatum(Thaxter) Gerdemann & Trappe
(FST10/95); Glomus geosporum (Nicol. & Gerd.) Walker (FST10/95);Glomus microaggregatumKoske, Gemma & Olexia (FST10/95);Glomus mosseae(Nicol. & Gerd.) Gerdemann & Trappe (FST10/95);Glomus
occultumWalker (FST10/95) and five other unknown
species (see Table 2 for a brief description of charac-ters), here designated Glomus aggregatum-like (FST10/95), Glomus C-1 (FST10/95), Glomus E-1 (FST10/95), Glomus spurcum-like (FST10/95), and Small Yellow Glomus (FST10/95). Glomus C-1 (Table 2) was presumed to be a Glomalean fungus in this study, even though it could not be successfully cultured in greenhouse pots. More intense attempts to culture this specimen will be needed to confirm its mycorrhizal status, but general similarity with Glo-musspecies, for example aGlomus-like subtending hy-pha (very fragile and rarely remaining attached to the spores), and the presence of somewhat flexible spore wall layers, led us to include this putative species as part of the AM fungal community. Glomus E-1, an-other undescribed species, has sporulated in associ-ation with bahiagrass (Paspalum notatumFlugge) in two greenhouse cultures. Other attempts to culture this fungus were unsuccessful, even when pre-germinated
spores were used. All other undescribed species were successfully cultured. We also have single-species cul-tures ofG. gigantea, G. mosseae, G. claroideum, G.
etunicatumandG. geosporum.Glomus occultumand
G. microaggregatumappear in mixed-species cultures.
Glomus fasciculatumandS. pellucida, rare species in
the FST communities, were not retrieved in enough numbers to establish cultures.
3.2. Ecological measures
Species richness was ≈10 for all treatments (Table 3). Similarly, no significant differences were found among Shannon and Wiener’s diversity indices and evenness of all treatments (Table 3), reflecting the general resemblance of the proportional abundances of spores of the different species among the six com-munities. No difference was found in Simpson’s in-dex of dominance (Table 3), indicating that the gen-eral pattern of spore abundance (from the most to the least dominant) was consistent under the various farm-ing treatments. Significant differences were detected in the modified Berger and Parker indices (Table 3). This volume-based index reflects differences in domi-nance of large versus small-spored species among the six communities. Most of the spores collected were less than 80mm in diameter, and just a few were over 180mm. The biovolume of each AM fungal species as a percentage of the total volume of spores in each pooled sample was calculated in the six treatments (Table 4). Over 50% of the total spore volume belonged toG.
giganteaspores for all communities except CON/MZ
where spore biovolume was more evenly distributed among several species. This phenomenon is reflected by the Berger and Parker index, i.e. dominance is strong in all treatments except CON/MZ. Table 4 also demon-strates the rarity of some AM fungal species at this site/sampling point.
Table 4
Percentage of biovolume of AM fungal species and groups of species in conventional and low-input agricultural systems
AMF species or groups Biovolume (%)
Maize Soybean
CONa LI-CG LI-AN CON LI-CG LI-AN
Gigaspora gigantea 17.24 Bb
±5.98 68.81 A±14.37 60.86 A±13.77 63.04 A±10.39 74.23 A±9.05 58.80 A±7.34 Glomus mosseae 19.97 A±6.12 17.79 A±7.43 18.24 A±4.85 4.32 B±0.66 11.42 AB±3.86 14.16 AB±4.46 Glomus claroideum 13.93 A±9.44 2.61 A±2.37 3.74 A±2.17 6.51 A±3.45 2.90 A±1.19 2.40 A±0.99 Glomus etunicatum 20.79 A±5.98 0.93 A±0.55 4.99 A±2.80 6.81 A±3.32 2.82 A±1.06 3.34 A±1.16 Glomus geosporum 2.48 B±1.37 2.72 B±0.75 4.06 B±1.95 9.77 AB±6.11 3.19 B±2.30 13.82 A±5.30 Glomus occultum, G. aggregatum-like
andG. microaggregatum
12.57 A±9.84 0.67 A±0.2 1.38 A±0.40 1.96 A±1.16 0.86 A±0.32 2.26 A±1.59
GlomusC-1 10.36 A±4.23 5.34 A±3.84 5.86 A±4.61 5.24 A±3.28 1.12 A±0.39 4.01 A±1.48 UnivacuolarGlomus
Small, yellowGlomus
2.69 A±0.83 0.15 B±0.03 0.46 B±0.11 0.98 B±0.39 0.15 B±0.04 0.27 B±0.06
Scutellospora pellucida 0.00 A 0.74 A±0.74 0.43 A±0.43 1.39 A±1.39 3.26 A±2.38 0.85 A±0.85 Glomus fasciculatum 0.00 A 0.00 A 0.00 A 0.00 A 0.07 A±0.07 0.00 A
GlomusE-1 0.00 A 0.24 A±0.25 0.00 A 0.00 A 0.00 A 0.00 A
aFarming systems: CON, conventional; LI-GM, low-input, with green manure; LI-AM, low-input, with animal manure. bMeans of four pooled samples
±SEM. Values followed by the same letter in a row are not significantly different (Duncan’s test atp=0.05).
3.3. Spore abundance
Though these communities appear to be similar when compared by single-value ecological indices which do not account for biovolume, these measures do not show the differences in abundance of spores of indi-vidual species of AM fungi among the six
commu-Table 5
Results of analysis of variance calculations on spore abundance data
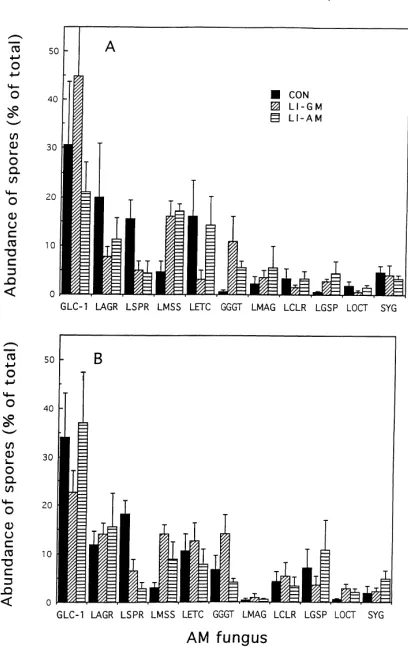
Fungi Probability >F
Source of variation
Farming system (FS) Crop FS×crop Model
G. gigantea 0.0352 0.9905 0.0170 0.0180
G. mosseae 0.0140 0.0019 0.0780 0.0002
G. claroideum 0.7973 0.6455 0.4216 0.7736
G. etunicatum 0.1480 0.0876 0.0716 0.0526
G. geosporum 0.2130 0.4891 0.3836 0.3577
G. occultum 0.8983 0.4479 0.1923 0.5098
G. aggregatum-like 0.3585 0.1910 0.3230 0.3132
GlomusC-1 0.8665 0.0924 0.6854 0.5362
G. microaggregatum 0.7247 0.0663 0.6347 0.4035
G. microaggregatum(in old spores) 0.0921 0.7390 0.4813 0.2645
G. spurcum-like 0.0028 0.0045 0.0275 0.0007
Small YellowGlomus 0.1317 0.0026 0.1271 0.0097
S. pellucida 0.6789 0.2558 0.7132 0.7209
GlomusE-1 0.3874 0.3306 0.3874 0.4457
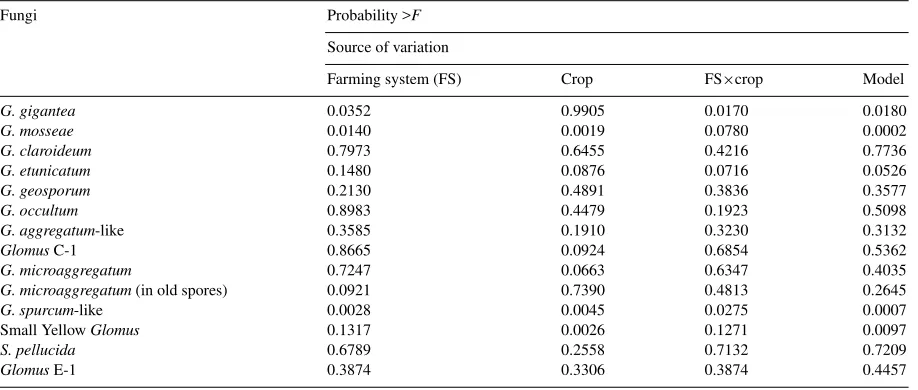
nities. Both farming system and crop had a signifi-cant (p=0.05) effect on the abundance of three species (Table 5). These differences can also be followed in the spore frequency chart (Fig. 1), comparing the six communities.
Fig. 1. Frequency of appearance of spores of 11 species of AM fungi within soils farmed via conventional (CON), low-input with green manure (LI-GM), or low-input with animal manure (LI-AM) systems following a crop of maize (A) or soybean (B). Each bar represents the mean of four replicate subplots±SEM. GLC-1,GlomusC1; LAGR, Glomus aggregatum-like; LSPR,Glomus spurcum-like; LMSS, Glo-mus mosseae; LETC,Glomus etunicatum; GGGT,Gigaspora gigan-tea; LMAG,Glomus microaggregatum; LCLR,Glomus claroideum; LGSP, Glomus geosporum; LOCT, Glomus occultumand SYG, small, yellowGlomus. See Table 2 for characteristics of GLC-1, LAGR, LSPR, and SYG. Total number of spores present in 350 cm−3
soil for the three farming systems: maize, 420±29 (CON), 228±106 (LI-GM), and 453±120 (LI-AM); soybean, 205±30 (CON), 167±42 (LI-GM), and 156±25 (LI-AM).
50 to over 7000 spores (by estimation) as clusters in-side a small number of dead spores of other species. It is unlikely that each spore in these clusters would act as an effective propagule, but rather the group would act as one propagule (therefore the presentation of data in Tables 3 and 4 without these spores). So, if these large numbers of spores were disregarded due to their
spa-tial constraints, the following would be the most com-mon species in each treatment (Fig. 1):GlomusC-1,G.
aggregatum-like,G. spurcum-like, andG. etunicatum
in CON/MZ;GlomusC-1 andGlomus spurcum-like in CON/SOY; Glomus C-1,G. mosseae, G. etunicatum
andGlomus spurcum-like in LI-AM/MZ;GlomusC-1
andG. aggregatum-like in LI-AM/SOY;GlomusC-1
andG. mosseaein LI-GM/MZ; and no specific
domi-nance in LI-GM/SOY.
3.4. Trap cultures
One new species was recovered from the trap cul-tures. Glomus constrictum Trappe sporulated in the third trap culture generation of CON/MZ and CON/ SOY, LI-GM/SOY and LI-AM/SOY. Spores of
G. mosseaeandG. claroideumtended to dominate the
soil/sand media of the first generation traps and in gen-eral, fewer species were found than in the field samples.
GlomusE-1 appeared only in CON/SOY. Second
gen-eration traps were dominated byG. mosseae, G.
etuni-catumandG. aggregatum-like spores. Species richness
varied from 9 to 11. In general, diversity was lower in the trap cultures than in the field, while dominance (Simpson’s index) was higher. The trap culture com-munities also tended to be less even. The relatively large spores ofG. mosseaedominated the total spore volume (data not shown).Glomus mosseaeandG. etunicatum
spores were the most abundant in the third generation traps, followed byG. aggregatum-like spores. Spores
ofG. constrictumwere also fairly common.
4. Discussion
host plant roots. The morphology of vegetative hy-phae cannot efficiently and precisely be used to dis-tinguish among AM fungal species. Their spores, on the other hand, are discrete units. Spores have com-plex and conserved morphological traits that are de-velopmentally controlled, which enable us to group them into species (Franke and Morton, 1994; Morton, 1995). Therefore, unlike with plants, it is the reproduc-tive phase of AM fungi which is used to quantify diver-sity. Spores and vegetative mycelium, however, behave as quite independent entities (Morton, 1993), making it difficult to relate the meaning of diversity patterns (based on spores) to functional ones (allocated to the mycelia). However, some inferences are appropriate (see below).
Taxonomy of AM fungi is in its early stages, and there is still some confusion on how to recognize and analyze characters, especially among the morpholog-ically simpler spores and undescribed species as they are found (Morton, 1993; Allen et al., 1995). There are two tools that are valuable in overcoming the chal-lenges in studies such as the present one. One tool is the comparison of spores found in field samples with those obtained in controlled conditions in pot and trap cul-tures. This allows for the appreciation of the complete set of characters present in well preserved and abun-dant specimens. In our case, spores from pot cultures established during other studies from the Rodale sites (Douds et al., 1993; Galvez et al., 1995) were avail-able for examination prior to analysis of field-collected spores in this study. Another tool is the examination of characters of known species. Spore characteristics can be viewed either through the mycorrhizal collections of individual scientists or through the main interna-tional culture collections, such as Internainterna-tional Cul-ture Collection of Arbuscular and Vesicular-arbuscular Mycorrhizae (INVAM) or European Bank of Gloma-les (BEG) to gain experience in distinguishing the im-portant characters of AM fungal spores. Also, when previously undescribed species are found, these tools promote characterization by their unique features, even if a formal name is not conferred to them (a sound characterization is more valuable than the inaccurate placement of a specimen into a pre-existing taxon). We were able to overcome the greatest barriers in deal-ing with field-collected material by usdeal-ing the above guidelines and made taxonomic decisions with confidence.
An objective of the Farming Systems Trial was to identify physical, chemical, and biological processes that occur during conversion from conventional to low-input farming methods (Liebhardt et al., 1989). Our ob-jective was to investigate whether the 15 consecutive years of these three farming systems induced changes in the AM fungal communities. Differences were not as pronounced as anticipated. Sporulation of particular species differed among farming systems and/or among hosts, but the general structure of AM fungal communi-ties was similar for all treatments. Wander et al. (1995) sampled the soil at this site in 1991, 10 years after the implementation of the three different farming systems. They investigated the phospholipid fatty acid profiles of soil samples from management treatment replicates, and did not find differences in the structure of the mi-crobial communities among the management systems. Buyer and Kaufman (1996) examined fast-growing, aerobic, culturable soil bacteria and fungi associated with maize rhizosphere at this site. Bacteria were iden-tified by phospholipid fatty acid analysis and fungi by traditional taxonomical keys. Diversity, evenness and total counts for these microorganisms also were not significantly different among the conventional and the two low-input systems. A seasonal effect was detected, however, from June to August. Similar conclusions re-sulted from principal component analysis of the rela-tive abundance of these bacteria and fungi. Buyer and Drinkwater (1997) analyzed structure and function of microbial species in composite soil samples (by fatty acid methyl ester analysis and Biolog plates, respec-tively) in a later study at the same location, and derived principle components which were then compared by analysis of variance and canonical discriminant anal-ysis. This time they found that the conventional treat-ment differed from the low-input system with green manure in regard to the sampled microbial commu-nities, but mentioned that treatment effects remained generally smaller than temporal ones. Kirchner et al. (1993) also found that microbial populations and ac-tivities in the Piedmont of North Carolina were similar between CON and LI systems, even though they had significant seasonal variations. These studies arrived at fairly similar conclusions, but noted that large variabil-ity within treatments may have caused the observed homogeneity.
richness values reflected not only quantitative, but also qualitative similarities among treatments. Prior to the separation of the field into the farming systems experi-ment, the land was used for the production of maize and wheat with conventional practices (Doran et al., 1987). The fungal communities that once inhabited these fields were probably different from the ones present after cultivation, but the fungal species that survived and adapted to agricultural practices have persisted under conventional and low-input farming with either host. This may indicate that these farming systems are not so different when it comes to the ability of the fungi to survive and occupy the root niches available. All three systems have high amounts of available phospho-rus (≈160–185 mg kg−1) whether naturally occurring or accumulated from past applications, and nitrogen in-puts are about 91 kg ha−1per year for CON, 87 kg ha−1 per year for LI-AM and less for LI-GM (51 kg ha−1per year). All systems are moldboard plowed, more fre-quently for LI-GM and less for LI-AM. Even though the LI systems have greater crop diversity and longer periods of coverage with living plants than CON (Douds et al., 1993), and mechanical weed control is used in contrast to herbicides, it is possible that the elevated nutrient status and disturbance of the soil in all plots played a more influential role in homogenizing fungal diversity than any differences due to farming system. Other factors may also contribute to the system-wide occurrence of AM fungal species. For example, propag-ules might disseminate from one narrow plot to another in this 6.1 ha field, via wind-blown disturbed soil after tillage.
Trap cultures can be very helpful in unveiling community members that are undetected in the initial extraction of spores from field soil (Morton et al., 1995). When three successive culture cycles of soil from the Arizona Sonoran desertscrub were ana-lyzed, up to seven species of AM fungi, not present in the first round, appeared by the third (Stutz and Morton, 1996). Our trap cultures revealed only one species not detected in the field survey (G. constric-tum). This indicates that the species composition of the field samples was an accurate reflection of the rich-ness of these communities and a good representation of the AM fungal community for the period of time and conditions sampled. Trap cultures, however, tended to encourage preferential sporulation by a few species, even when different conditions were applied, as
observed by others (Bever et al., 1996; Koske et al., 1997).
Diversity and dominance indices, with exception of Berger and Parker values, also suggested uniformity among treatments (Table 3). Shannon and Wiener and evenness values indicated that the patterns of species composition and spore distribution were similar in the six treatments, and when farming systems or crops were analyzed as main effects (data not shown). Sim-ilarly, Simpson’s index did not discriminate among the dominance patterns between the treatments, even though Table 5 shows that sporulation byG. gigantea,
G. mosseae,G. spurcum-like and Small Yellow
Glo-muswas influenced by farming system, host, and/or the interaction of both. The frequency data presented in Fig. 1 help visualize these trends. The Berger and Parker values reflected dominance patterns based on differences in biovolumes. Carbon allocated to the fun-gus for sporulation can represent a significant drain to the plant as part of the carbon cost of the sym-biotic relationship (Harris et al., 1985; Jakobsen and Rosendahl, 1990; Graham et al., 1991). Fixed carbon for sporulation may come directly from the host, or carbon may be pre-stored in hyphae before its onset (Gazey et al., 1992; Pearson and Schweiger, 1993). Sixty to seventy-five percent of the total spore volume belonged toG. giganteain all treatments but one (Ta-ble 4). The only treatment with fairly evenly distributed spore volumes was CON/MZ, which had a significantly lower Berger and Parker index. The partitioning of car-bon between extraradical hyphae and spores, and its implications for the efficacy of mycorrhizas, should be studied for members of AM fungal communities in CON and LI agriculture.Gigaspora giganteaand the community in CON/MZ are excellent candidates for these types of investigations.
The species richness at this site compares favorably with that of many agricultural soils (Douds and Millner, 1999). Though the 15 species found here is less than the 26 species found by Ellis et al. (1992) in soils farmed with a sorghum–soybean rotation in Nebraska, USA, it is greater than the 3–6 reported by Talukdar and Germida (1993) in Saskatchewan, Canada.
USA (H′=0.57–0.64, Kurle and Pfleger (1996); and
H′=0.42–1.59, Johnson et al. (1991)) and soils from
Theobroma cacaoplantations in Venezuela (H′=0.6–
0.78, Cuenca and Meneses, 1996). The community of AM fungi in Polish soils in which×Triticosecalewas grown after a variety of precrops had somewhat greater Shannon–Weiner indices (H′=1.81–2.22, Blaszkowski, 1995). Kurle and Pfleger (1996) and Vestberg et al. (1999) also noted no significant increase in diversity of AM fungi in populations due to the application of low-input agricultural management.
Variations in the patterns of spore abundance seen here, however, are liable to happen in different seasons as well as from year to year in a crop rotation. Douds et al. (1993) showed that the number of AM fungus spores in major species-groupings at this site tended to be lower in CON than in the LI systems from 1989 to 1991. Several other factors also have been previ-ously shown to influence mycorrhizal populations in general, such as duration of fallowing period (Thomp-son, 1987), age of plantations (Wacker et al., 1990), soil factors, crop species, fumigation, and others (Cuenca et al., 1990; Johnson et al., 1992a,b; An et al., 1993; Douds et al., 1995, 1997; Hendrix et al., 1995; Miller et al., 1995).
Three species were present in very low frequency and/or abundance;S. pellucida,GlomusE-1 andG.
fas-ciculatum.Scutellospora pellucida, found in all
treat-ments except CON/MZ, appeared at less than one spore per 350 cm3. However, soils collected from around maize roots at this site during the summer of 1997 con-tained more than 10 times as many spores ofS.
pel-lucida (data not shown). GlomusE-1, retrieved only
from LI-GM/MZ in the field and CON/SOY in trap culture, is common at the Compost Utilization Trial, another study site within the Rodale Institute Exper-imental Farm (data not shown).Glomus fasciculatum
was only found once (at LI-GM/SOY) in several years of research in these sites. These dynamics indicate that biotic and abiotic factors interact to induce a fungus to sporulate and that these fungi may persist without sporulating. This underscores the need for continued development of molecular methods to identify mem-bers of the AM fungal community by vegetative struc-tures.
Results from Bever et al. (1996) supported the idea that differential sporulation commonly observed in AM fungal communities results more from differential rates
of growth of the fungi than from different timing in allocation of resources to sporulation. If growth rates are reflected in the sporulation patterns seen in this experiment, then some species, namelyGlomusC-1,
G. aggregatum-like,G. spurcum-like,G. mosseaeand
G. etunicatum, are better established and spread within
the roots than others. This is supported by the fact that these were also the species most prevalent in the trap cultures (with exception ofGlomus C-1). Spores of the remaining species were few or absent in the trap cultures, probably due to more intense competition and exclusion dynamics brought about by the constricted space of the pot environment.
The taxonomic diversity found cannot necessarily be linked to the functional role of these fungi. Inter-and intraspecific variation in efficacy as symbionts has been found. Isolates of species can become locally adapted (Bethlenfalvay et al., 1989; Stahl and Chris-tensen, 1991; Allen et al., 1992; Morton and Bentivenga, 1994; Allen et al., 1995). So, even though all AM fungi occupy the same general niche, we cannot pre-dict if functional redundancy (Walker, 1992) will oc-cur among the fungi in the community. Future tests comparing symbiotic efficacy of these different fungi should indicate whether this uniformity also is reflected in the capabilities of the different communities to pro-mote plant growth, or if there is functional diversity among these communities.
This ecological assessment has described the local AM fungal community composition and structure in relation to farming system and crop host. This study and others indicate that the microbial communities of CON and LI systems can have similar structure, even though absolute numbers of individuals of different species may vary. Future work with these communities should be to assess the functional roles of AM fungi from the different farming systems and determine if trends in symbiotic efficacy correlate with CON and LI practices (Johnson, 1993). Continued sampling of spores at this site may also be valuable to monitor large changes in species composition that may occur in the future.
Acknowledgements
References
Allen, M.F., Clouse, S.D., Weinbaum, B.S., Jeakins, S., Friese, C.F., Allen, E.B., 1992. Mycorrhizae and the integration of scales: from molecules to ecosystems. In: Allen, M.F. (Ed.), Mycorrhizal Functioning an Integrative Plant–Fungal Process. Chapman and Hall, New York, pp. 488–515.
Allen, E.B., Allen, M.F., Helm, D.J., Trappe, J.M., Molina, R., Rincon, E., 1995. Patterns and regulation of mycorrhizal plant and fungal diversity. Plant Soil 170, 47–62.
An, Z.-Q., Hendrix, J.W., Hershman, D.E., Ferriss, R.S., Henson, G.T., 1993. The influence of crop rotation and soil fumigation on a mycorrhizal fungal community associated with soybean. Mycorrhiza 3, 171–182.
Bethlenfalvay, G.J., 1992. Mycorrhizae in the agricultural plant–soil system. Symbiosis 14, 413–425.
Bethlenfalvay, G.J., Franson, R.L., Brown, M.S., Mihara, K.L., 1989. The Glycine–Glomus–Bradyrhizobium symbiosis. IX. Nutritional, morphological and physiological responses of nodulated soybean to geographic isolates of the mycorrhizal fungusGlomus mosseae. Physiol. Plant. 76, 226–232.
Bever, J.D., 1994. Feedback between plants and their soil communities in an old field community. Ecology 75, 1965–1977. Bever, J.D., Morton, J.B., Antonovics, J., Schultz, P.A., 1996. Host-dependent sporulation and species diversity of arbuscular mycorrhizal fungi in a mown grassland. J. Ecol. 84, 71–82. Blaszkowski, J., 1995. The influence of pre-crop plants on
the occurrence of arbuscular mycorrhizal fungi (Glomales) and Phialophora graminicolaassociated with roots of winter
×Triticosecale. Acta Mycol. 30, 213–222.
Bolan, N.S., 1991. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 134, 189–207. Buyer, J.S., Kaufman, D.D., 1996. Microbial diversity in the
rhizosphere of maize grown under conventional and low-input systems. Appl. Soil Ecol. 5, 21–27.
Buyer, J.S., Drinkwater, L.E., 1997. Comparison of substrate utilization assay and fatty acid analysis of soil microbial communities. J. Microb. Mets. 30, 3–11.
Clapp, J.P., Fitter, A.H., Young, J.P.W., 1999. Ribosomal small subunit sequence variation within spores of an arbuscular mycorrhizal fungus,Scutellosporasp.. Molec. Ecol. 8, 915–921. Cuenca, G., Meneses, E., 1996. Diversity patterns of arbuscular mycorrhizal fungi associated with cacao in Venezuela. Plant Soil 183, 315–322.
Cuenca, G., Herrera, R., Meneses, E., 1990. Effects of VA mycorrhiza on the growth of cacao seedlings under nursery conditions in Venezuela. Plant Soil 126, 71–78.
Doran, J.W., Fraser, D.G., Culik, M.N., Liebhardt, W.C., 1987. Influence of alternative and conventional agricultural management on soil microbial processes and nitrogen availability. Am. J. Altern. Agric. 2, 99–106.
Douds, D.D., Millner, P.D., 1999. Biodiversity of arbuscular mycorrhizal fungi in agroecosystems. Agric. Ecosyst. Environ. 74, 77–93.
Douds Jr., D.D., Janke, R.R., Peters, S.E., 1993. VAM fungus spore populations and colonization of roots of maize and soybean under conventional and low-input sustainable agriculture. Agric. Ecosyst. Environ. 43, 325–335.
Douds Jr., D.D., Galvez, L., Janke, R.R., Wagoner, P., 1995. Effect of tillage and farming system upon populations and distribution of vesicular-arbuscular mycorrhizal fungi. Agric. Ecosyst. Environ. 52, 111–118.
Douds, D.D., Galvez, L., Franke-Snyder, M., Reider, C., Drinkwater, L.E., 1997. Effect of compost addition and crop rotation upon VAM fungi. Agric. Ecosyst. Environ. 65, 257–266.
Drinkwater, L.E., Wagoner, P., Sanantonio, M., 1998. Legume-based cropping systems have reduced carbon and nitrogen loss. Nature 396, 262–265.
Ellis, J.R., Roder, W., Mason, S.C., 1992. Grain sorghum–soybean rotation and fertilization influence on vesicular-arbuscular mycorrhizal fungi. Soil Sci. Soc. Am. J. 56, 783–794.
Franke, M., 1992. Relationship among species and isolates of arbuscular mycorrhizal fungi based on morphology, ontogeny and plant–fungus interactions. Thesis, West Virginia University, Morgantown, WV.
Franke, M., Morton, J.B., 1994. Ontogenetic comparisons of arbuscular mycorrhizal fungi Scutellospora heterogama and Scutellospora pellucida: revision of taxonomic character concepts, species descriptions, and phylogeny hypotheses. Can. J. Bot. 72, 122–134.
Galvez, L., Douds Jr., D.D., Wagoner, P., Longnecker, L.R., Drinkwater, L.E., Janke, R.R., 1995. An overwintering cover crop increases inoculum of VAM fungi in agricultural soil. Am. J. Altern. Agric.10, 152–156.
Gazey, C., Abbott, L.K., Robson, A.D., 1992. The rate of development of mycorrhizas affects the onset of sporulation and production of external hyphae by two species ofAcaulospora. Mycol. Res. 96, 643–650.
Gerdemann, J.W., Nicolson, T.H., 1963. Spores of mycorrhizal Endogonespecies extracted by wet sieving and decanting. Trans. Br. Mycol. Soc. 46, 235–244.
George, E., Marschner, H., Jakobsen, I., 1995. Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil. Crit. Rev. Biotechnol. 15, 257–270.
Graham, J.H., Eissenstat, D.M., Drouillard, D.L., 1991. On the relationship between a plant’s mycorrhizal dependency and rate of vesicular-arbuscular mycorrhizal colonization. Funct. Ecol. 5, 773–779.
Harris, D., Pacovsky, R.S., Paul, E.A., 1985. Carbon economy of soybean–Rhizobium–Glomusassociations. New Phytol. 101, 427–440.
Hassink, J., Voshaar, J.H.O., Nijhuis, E.H., van Veen, J.A., 1991. Dynamics of the microbial populations of a reclaimed-polder soil under a conventional and a reduced-input farming system. Soil Biol. Biochem. 23, 515–524.
Helgason, T., Fitter, A.H., Young, J.P.W., 1999. Molecular diversity of arbuscular mycorrhizal fungi colonizing Hyacinthoides non-scripta(bluebell) in a seminatural woodland. Molec. Ecol. 8, 659–666.
Hendrix, J.W., Guo, B.Z., An, Z.-Q., 1995. Divergence of mycorrhizal fungal communities in crop production systems. Plant Soil 170, 131–140.
Jakobsen, I., Rosendahl, L., 1990. Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytol. 115, 77–83.
Jakobsen, I., Abbott, L.K., Robson, A.D., 1992a. External hyphae of vesicular-arbuscular mycorrhizal fungi associated withTrifolium subterraneumL. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol. 120, 371–380.
Jakobsen, I., Abbott, L.K., Robson, A.D., 1992b. External hyphae of vesicular-arbuscular mycorrhizal fungi associated withTrifolium subterraneumL. 2. Hyphal transport of32P over defined distances.
New Phytol. 120, 509–516.
Jenkins, W.R., 1964. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 73, 288–300. Johnson, N.C., 1993. Can fertilization of soil select less mutualistic
mycorrhizae? Ecol. Appl. 3, 749–757.
Johnson, N.C., Pfleger, F.L., Crookston, R.K., Simmons, S.R., Copeland, P.J., 1991. Vesicular-arbuscular mycorrhizas respond to corn and soybean cropping history. New Phytol. 117, 657–663. Johnson, N.C., Copeland, P.J., Crookston, R.K., Pfleger, F.L., 1992a. Mycorrhizae: possible explanation for yield decline with continuous corn and soybean. Agron. J. 84, 387–390.
Johnson, N.C., Tilman, D., Wedin, D., 1992b. Plant and soil controls on mycorrhizal fungal communities. Ecology 73, 2034–2042. Kennedy, A.C., Smith, K.L., 1995. Soil microbial diversity and the
sustainability of agricultural soils. Plant Soil 170, 75–86. Kirchner, M.J., Wollum II, A.G., King, L.D., 1993. Soil
microbial populations and activities in reduced chemical input agroecosystems. Soil Sci. Soc. Am. J. 57, 1289–1295. Koske, R.E., Gemma, J.N., Jackson, N., 1997. Mycorrhizal fungi
associated with three species of turfgrass. Can. J. Bot. 75, 320– 332.
Kurle, J.E., Pfleger, F.L., 1994. Arbuscular mycorrhizal fungus spore populations respond to conversions between low-input and conventional management practices in a corn–soybean rotation. Agron. J. 86, 467–475.
Kurle, J.E., Pfleger, F.L., 1996. Management influences on arbuscular mycorrhizal fungal species composition in a corn–soybean rotation. Agron. J. 88, 155–161.
Liebhardt, W.C., Andrews, R.W., Culik, M.N., Harwood, R.R., Janke, R.R., Radke, J.K., Reiger-Schwartz, S.L., 1989. Crop production during conversion from conventional to low-input methods. Agron. J. 81, 150–159.
Limonard, T., Ruissen, M.A., 1989. The significance of VA-mycorrhiza to future arable farming in the Netherlands. Neth. J. Pl. Path. 95, 129–135.
Ludwig, J.A., Reynolds, J.F., 1988. Statistical ecology. A primer on methods and computing. Wiley, New York, 337 pp.
Magurran, A.E., 1988. Ecological Diversity and its Measurement. Croom Helm Limited, London, 179 pp.
Merryweather, J., Fitter, A., 1998. The arbuscular mycorrhizal fungi ofHyacinthoides non-scripta. I. Diversity of fungal taxa. New Phytol. 138, 117–129.
Miller, R.M., Jastrow, J.D., 1992. The role of mycorrhizal fungi in soil conservation. In: Mycorrhizae in sustainable Agriculture. ASA Special Publication No. 54. American Society of Agronomy, Madison, Wisconsin, pp. 29–44.
Miller, M.H., McGonigle, T.P., Addy, H.D., 1995. Functional ecology of vesicular arbuscular mycorrhizas as influenced by phosphate fertilization and tillage in an agricultural ecosystem. Crit. Rev. Biotechnol. 15, 241–255.
Morton, J.B., 1993. Problems and solutions for the integration of glomalean taxonomy, systematic biology, and the study of endomycorrhizal phenomena. Mycorrhiza 2, 97–109.
Morton, J.B., 1995. Taxonomic and phylogenetic divergence among fiveScutellosporaspecies based on comparative developmental sequences. Mycologia 87, 127–137.
Morton, J.B., Bentivenga, S.P., 1994. Levels of diversity in endomycorrhizal fungi (Glomales, Zygomycetes) and their role in defining taxonomic and non-taxonomic groups. Plant Soil 159, 47–59.
Morton, J.B., Bentivenga, S.P., Bever, J.D., 1995. Discovery, measurement, and interpretation of diversity in arbuscular endomycorrhizal fungi (Glomales, Zygomycetes). Can. J. Bot. 73, 25–32.
O’Donnell, A.G., Goodfellow, M., Hawksworth, D.L., 1994. Theoretical and practical aspects of the quantification of biodiversity among microorganisms. Phil. Trans. R. Soc. Lond. B. 345, 65–73.
Pankhurst, C.E., Ophel-Keller, K., Doube, B.M., Gupta, V.V.S.R., 1996. Biodiversity of soil microbial communities in agricultural systems. Biodivers. Conserv. 5, 197–209.
Pearson, J.N., Schweiger, P., 1993.Scutellospora calospora(Nicol. & Gerd.) Walker & Sanders associated with subterranean clover: dynamics of colonization, sporulation and soluble carbohydrates. New Phytol. 124, 215–219.
Peet, R.K., 1974. The measurement of species diversity. Annu. Rev. Ecol. Syst. 5, 285–307.
Schimel, J., 1995. Ecosystem consequences of microbial diversity and community structure. Ecol. Studies 113, 239–254. Schreiner, R.P., Bethlenfalvay, G.J., 1995. Mycorrhizal interactions
in sustainable agriculture. Crit. Rev. Biotechnol. 15, 271–285. Stahl, P.D., Christensen, M., 1991. Population variation in the
mycorrhizal fungusGlomus mosseae: breadth of environmental tolerance. Mycol. Res. 95, 300–307.
Stutz, J.C., Morton, J.B., 1996. Successive pot cultures reveal high species richness of arbuscular endomycorrhizal fungi in arid ecosystems. Can. J. Bot. 74, 1883–1889.
Talukdar, N.C., Germida, J.J., 1993. Occurrence and isolation of vesicular-arbuscular mycorrhizae in cropped field soils of Sask., Canada. Can. J. Microbiol. 39, 567–575.
Thompson, J.P., 1987. Decline of vesicular-arbuscular mycorrhizae in long fallow disorder of field crops and its expression in phosphorus deficiency of sunflower. Aust. J. Agric. Res. 38, 847– 867.
Vestberg, M., Cardoso, M., Martensson, A., 1999. Occurrence of arbuscular mycorrhizal fungi in different cropping systems at Cochabamba, Bolivia. Agric. Food Sci. Finland 8, 309–318. Visser, S., Parkinson, D., 1992. Soil biological criteria as indicators
of soil quality: soil microorganisms. Am. J. Altern. Agric. 7, 33– 37.
Wacker, T.L., Safir, G.R., Stephenson, S.N., 1990. Evidence for success of mycorrhizal fungi in Michigan aspargus fields. Acta Hortic. 271, 273–278.
Walker, B.H., 1992. Biodiversity and ecological redundancy. Conserv. Biol. 6, 18–23.
Wander, M.M., Hedrick, D.S., Kaufman, D., Traina, S.J., Stinner, B.R., Kehrmeyer, S.R., White, D.C., 1995. The functional significance of the microbial biomass in organic and conventionally managed soils. Plant Soil 170, 87–97.
Whittaker, R.H., 1965. Dominance and diversity in land communities. Science 147, 250–260.
Workneh, F., van Bruggen, A.H.C., 1994. Microbial density, composition, and diversity in organically and conventionally managed rhizosphere soil in relation to suppression of corky root of tomatoes. Appl. Soil Ecol. 1, 219–230.
Wright, S.F., Upadhyaya, A., 1996. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 161, 575–586.