www.elsevier.nlrlocateraqua-online
Effects of DHA-enriched live food on growth,
survival and incidence of opercular deformities in
ž
/
milkfish Chanos chanos
R.S.J. Gapasin
), M.N. Duray
( )
Aquaculture Department, Southeast Asian Fisheries DeÕelopment Center SEAFDECrAQD ,
5021 Tigbauan, Iloilo, Philippines
Received 28 February 2000; received in revised form 27 June 2000; accepted 27 June 2000
Abstract
The use of commercial enrichers to improve the nutritional quality of live food in larviculture of milkfish was investigated. Fish were either fed rotifers cultured on Chlorella sp. and newly
Ž .
hatched Artemia nauplii Control, Trt I or rotifers and Artemia given DHA enrichment diets
ŽDHA-treated, Trt II ..
Ž .
Results showed survival was significantly better P-0.05 in the DHA-treated fish than in the untreated fish after 25-day culture period. Although growth was not statistically different
ŽP)0.05 between the control and DHA-treated fish during the hatchery phase, extensive rearing. Ž .
of the postlarvae fry in nursery ponds for another 60 days showed that DHA-treated fish
Ž .
exhibited significantly better P-0.05 growth than the untreated fish. Opercular deformities in
Ž .
85-day old milkfish juveniles were also significantly lower P-0.05 in the DHA-treated fish than the control. Survival after nursery culture, however, was high for both treatments but not
Ž .
significantly different P)0.05 .
The lack of a viable and reliable method of mass culturing copepods as live food in the hatchery makes the use of off-the-shelf commercial enrichment diets for rotifers and Artemia a practical option in the larval culture of milkfish.q2001 Elsevier Science B.V. All rights reserved.
Keywords: Milkfish; Essential fatty acids; Larval food; Growth; Survival; Opercular deformity
)Corresponding author. Tel.:q63-33-336-2965; fax:q63-33-335-1008.
Ž .
E-mail address: [email protected] R.S.J. Gapasin .
0044-8486r01r$ - see front matterq2001 Elsevier Science B.V. All rights reserved. Ž .
1. Introduction
Ž .
The long-chain highly unsaturated fatty acids HUFAs , particularly eicosapentaenoic
Ž . Ž .
acid EPA, 20:5ny3 and docosahexaenoic acid DHA, 22:6 ny3 , are important in
Ž .
the nutrition of young marine fish Kanazawa, 1985; Watanabe et al., 1989 . Various investigators have used DHArEPA ratios as an index of the optimal level required for
Ž
normal growth and development in fish larvae Koven et al., 1993; Mourente et al., 1993; Rainuzzo et al., 1994; Reitan et al., 1994; Tocher et al., 1997; Rodriguez et al.,
.
1998 . This is based on the proposition that optimum DHA and EPA levels are
Ž .
determined not on total amounts per se as excess can be harmful but rather on the
Ž . Ž
relative proportions of these essential fatty acids EFAs in the diet Watanabe and
.
Kiron, 1994; Rodriguez et al., 1997, 1998 . Apart from DHA and EPA, arachidonic acid
ŽARA, 20:4 ny6 has also been recognized as essential for marine fish Castell et al.,. Ž .
1994 . ARA is the main precursor of eicosanoids responsible for osmoregulation,
Ž
cardiovascular functions, neural control and reproduction Mustafa and Srivastava,
. Ž .
1989 . Sargent et al. 1997, 1999 have suggested that desirable ratios of 22:6 ny
3r20:5ny3r20:4 ny6 can be useful in determining optimal requirements in fish larval nutrition.
Ž .
Opercular abnormalities in fish affect its morphology Koumoundouros et al., 1997
Ž .
and biological performance Andrades et al., 1996; Sumagaysay et al., 1999 . Shortened operculum and distortion of the support cartilage have been described to be signs of
Ž .
nutritional deficiency e.g., vitamin C in fish caused by impaired collagen formation
Ž .
and support cartilage formation Halver et al., 1975 . Some researchers have theorized
Ž .
that opercular deformities e.g., milkfish are caused by mechanical stress, especially
Ž
during egg collection, transport or routine hatchery operations Toledo et al., 1996;
. Ž .
Hilomen-Garcia, 1997, 1998 . Toledo et al. 1996 even recommended transporting milkfish eggs at C-shaped embryonic stage to improve viability. Other causes such as
Ž .
genetic variations or factors, however, cannot be discounted Sargent, 1995 . Recently,
Ž .
Gapasin et al. 1998 observed that the incidence of opercular deformities among hatchery-reared milkfish larvae could be alleviated by feeding them live food supple-mented with EFAs and vitamin C. The DHArEPA ratios reported in that study ranged from 0.33–0.74 for HUFA-enriched rotifers and Artemia nauplii while values for the unenriched live food were very low ranging from 0.01–0.04.
This follow-up study was therefore conducted to determine whether increasing the
Ž .
DHA levels and the corresponding DHArEPA ratio to a value of at least G1.0 in live
Ž .
food organisms using commercially available enrichers could further improve milkfish larvae performance.
2. Materials and methods
2.1. Milkfish eggs
Ž .
whereas eggs for trial 2 came from broodstock maintained at SEAFDECrAQD Igang Marine Substation in a 10-m diameter=3-m deep sea cage. Eggs were incubated in 500-l capacity circular, flat-bottomed, fiberglass tanks following standard procedures
ŽGapasin and Marte, 1990; Gapasin et al., 1998 ..
2.2. LiÕe food enrichment
Ž . Ž
Rotifers Brachionus rotundiformis, S-type were first cultured in baker’s yeast Red
. Ž
Star brand for 3 days and then enriched with DHA Protein Selco INVE Aquaculture,
. Ž .
Dendermonde, Belgium on the 4th day as described by Lavens et al. 1994 with modification. Rotifers cultured extensively in Chlorella sp. served as a control.
Ž .
Artemia nauplii were hatched following standard practice Sorgeloos et al., 1986 and
Ž .
enriched thereafter with DHA Selco INVE Aquaculture, Dendermonde, Belgium
Ž .
following the procedures of Leger et al. 1987 . Newly hatched Artemia served as a control.
2.3. LarÕal culture
Newly hatched milkfish larvae were estimated volumetrically and stocked in 5-ton circular, concrete larval rearing tanks at a density of 30 larvaerl. Water management
Ž .
and feeding scheme followed previous protocol outlined in Gapasin et al. 1998 . The
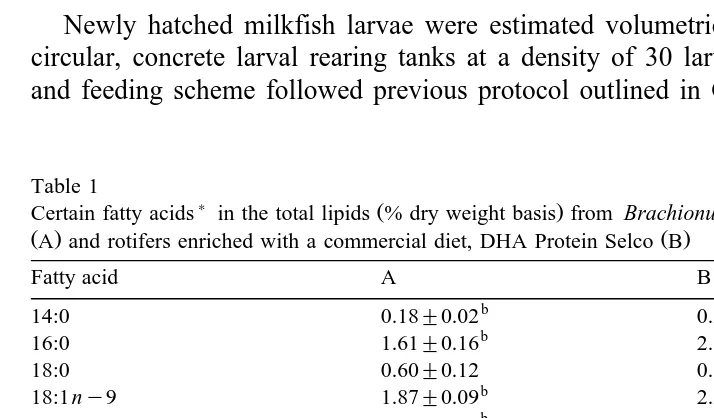
Table 1
) Ž .
Certain fatty acids in the total lipids % dry weight basis from Brachionus rotundiformis fed Chlorella sp.
Ž .A and rotifers enriched with a commercial diet, DHA Protein Selco BŽ .
Fatty acid A B
18:1ny9 1.87"0.09 2.26"0.04
b a
18:2 ny6 0.45"0.08 0.82"0.10
b a
18:3ny3 1.01"0.06 1.45"0.05
b a
18:4 ny6 0.02"0.00 0.70"0.03
b a
20:1ny9 0.10"0.00 0.25"0.01
b a
20:4 ny6 0.26"0.04 0.58"1.09
b a
20:5ny3 0.45"0.03 0.85"0.01
22:4 ny6 0.24"0.03 0.26"0.05
22:4 ny3 – 0.15"0.03
22:5ny6 – 0.09"0.01
b a
22:5ny3 0.05"0.00 0.47"0.03
b a
DHArEPA 0.07"0.00 1.50"0.04
Sny3rSny6 1.59"0.07 1.71"0.06
) Ž .
Means "S.E.M. within rows not bearing the same letter superscripts are significantly different
Ž . Ž . Ž .
treatments with three replicates were as follows: a Trt 1 control — larvae fed
Ž .
Chlorella-cultured rotifers and newly hatched Artemia nauplii and b Trt 2 — larvae fed rotifers and Artemia nauplii enriched with DHA Protein Selco and DHA Selco,
Ž .
respectively. Fish larvae ns20–25 were sampled for total length every week from
Ž .
each replicate tank. Percent survival surviving fishrinitial stock=100 were
deter-Ž .
mined at harvest day 25 . Two larviculture trials were conducted.
Ž
Harvested 25-day old larvae were packed in separate plastic bags according to
. Ž
treatments , transported by land and stocked in 27=38 m earthen nursery ponds three
. 2
replicates at a density of 15–20 larvaerm . Prior to stocking, ponds were fertilized and
Ž .
prepared following standard practice Lijauco et al., 1979 . Fish were reared extensively
Žon natural food present in ponds for 60 days after which the young juveniles or. Ž .
fingerlings ns100 were randomly sampled. Live fish were individually examined for
Ž .
opercular deformities following procedures in Gapasin et al. 1998 . Of the 100
Ž . Ž .
fingerlings per replicate pond checked for abnormalities, half of these ns50 were sampled for total length measurements. The rest of the fish stock were seined and
Ž .
counted to determine survival done only for trial 2 .
2.4. Lipid extraction and FAME analysis
Ž .
Random samples of rotifers Chlorella-cultured or DHA Protein Selco-enriched ,
Ž . Ž
Artemia nauplii newly hatched or DHA Selco-enriched and 25-day old fish larvae fed
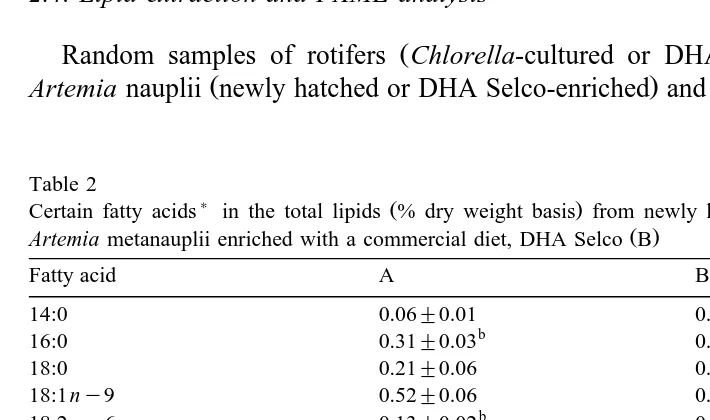
Table 2
) Ž . Ž .
Certain fatty acids in the total lipids % dry weight basis from newly hatched Artemia nauplii A and
Ž .
Artemia metanauplii enriched with a commercial diet, DHA Selco B
Fatty acid A B
14:0 0.06"0.01 0.08"0.01
b a
16:0 0.31"0.03 0.47"0.05
18:0 0.21"0.06 0.28"0.01
18:1ny9 0.52"0.06 0.66"0.07
b a
18:2 ny6 0.13"0.02 0.26"0.05
b a
18:3ny3 0.47"0.01 0.78"0.05
b a
20:5ny3 0.03"0.00 0.48"0.05
b a
Means "S.E.M. within rows not bearing the same letter superscripts are significantly different
.
unenriched or DHA-enriched live food were periodically taken and placed in
coded-Ž
plastic vials. For reference and comparison, newly spawned milkfish eggs from
.
broodstock maintained at SEAFDECrAQD Igang Marine Substation and wild milkfish
Ž
fry approximately 3-week old postlarvae seined by fry collectors along Tigbauan
. Ž .
coastal waters were also collected. All samples were kept in deep freeze y708C until
Ž .
analyzed for total lipids and fatty acid methyl esters FAME .
Ž
Total lipids were extracted from triplicated pooled samples except for the eggs and
.
wild milkfish fry which had only one pooled replicate sample after homogenization in
Ž . Ž .
chloroformrmethanol 2:1, vrv with 0.05% butylated hydroxytoluene BHT following
Ž .
the method of Folch et al. 1957 . Extracted lipid fractions were saponified with 0.5 N KOH in methanol and fatty acids were esterified in 14% boron triflouride–methanol
Ž .
complex Metcalfe et al., 1966 . FAMEs were resuspended in isooctane, flushed with
Ž .
nitrogen in glass vials and stored in deep freeze y708C before injection into the
chromatograph.
Fatty acid compositions were analyzed using a Shimadzu gas–liquid chromatograph
ŽGC-4PTF, Japan equipped with flame ionization detector initial temperature: 150. Ž 8C
.
for 5 min, program rate: 108Crmin, final temperature: 2008C , using a 30 m=0.32
Ž .
mm=0.2 m film thickness SPB-PUFA capillary column Supelco, USA . The
chro-Ž
matograph was linked to a Shimadzu integratorrrecorder Chromatopac C-R7A Plus,
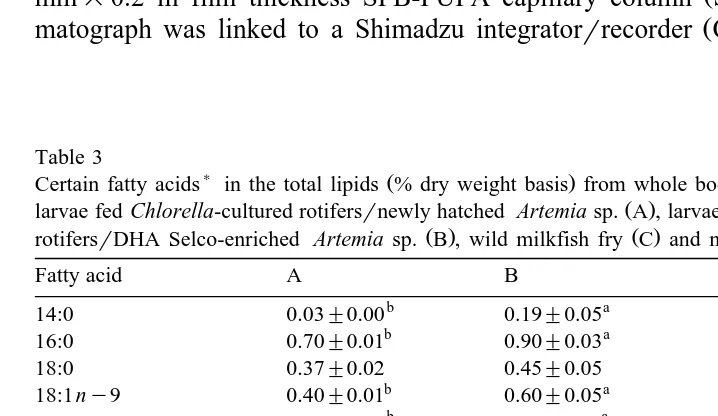
Table 3
) Ž .
Certain fatty acids in the total lipids % dry weight basis from whole body tissues of 25-day old milkfish
Ž .
larvae fed Chlorella-cultured rotifersrnewly hatched Artemia sp. A , larvae fed DHA Protein Selco-enriched
Ž . Ž . Ž .
rotifersrDHA Selco-enriched Artemia sp. B , wild milkfish fry C and newly spawned milkfish eggs D
Fatty acid A B C D
18:1ny9 0.40"0.01 0.60"0.05 0.24 2.18
b a
18:2 ny6 0.04"0.00 0.21"0.01 0.13 0.65
b a
18:3ny3 0.19"0.02 0.40"0.05 0.09 0.05
18:4 ny6 0.01"0.00 0.02"0.01 0.09 0.09
20:1ny9 0.14"0.02 0.12"0.01 0.01 0.12
20:4 ny6 0.13"0.01 0.16"0.02 0.12 0.36
b a
20:5ny3 0.30"0.02 0.39"0.02 0.42 0.29
22:4 ny6 0.03"0.00 0.03"0.00 0.01 0.02
22:4 ny3 0.14"0.02 0.12"0.01 0.01 0.01
22:5ny6 0.07"0.03 0.11"0.02 0.10 0.03
b a
22:5ny3 0.06"0.00 0.09"0.01 0.04 0.13
b a
DHArEPA 0.53"0.01 1.54"0.09 2.28 6.75
Sny3rSny6 2.93"0.11 2.98"0.13 3.41 2.14
) Ž .
Means "S.E.M. within rows not bearing the same letter superscripts are significantly different
Ž
Fig. 1. Growth of 25-day-old milkfish fed Chlorella-cultured rotifers and newly hatched Artemia nauplii Trt
. Ž .
1, control and DHA-enriched rotifers and Artemia metanauplii Trt 2, DHA-treated . Each data point
Ž .
.
Japan . Individual fatty acids were identified by comparing the retention times with
Ž .
commercially available standards Sigma-Aldrich, USA .
2.5. Statistical analysis
Ž
Total length, percent survival and deformities log- or arcsine-transformed where
.
appropriate , individual fatty acid levels, total ny3, total ny6, DHArEPA and total ny3rny6 ratios between the two treatments were subjected to Student’s t-test
Ž . Ž . Ž
analysis Ps0.05 using the Statistical Analysis System SAS software program SAS
.
Institute, 1988 for PCs.
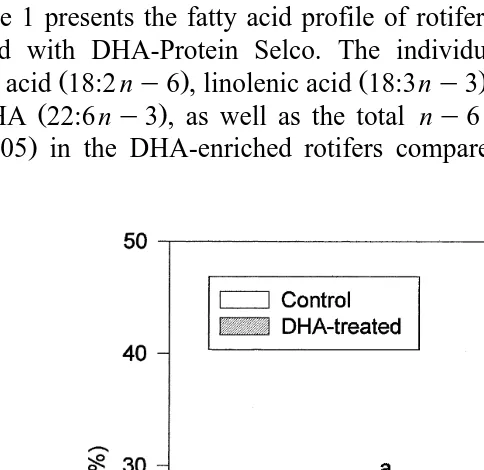
3. Results
Table 1 presents the fatty acid profile of rotifers cultured in Chlorella sp. and those enriched with DHA-Protein Selco. The individual fatty acids, including the EFAs
Ž . Ž . Ž . Ž .
linoleic acid 18:2 ny6 , linolenic acid 18:3ny3 , ARA 20:4 ny6 , EPA 20:5ny3
Ž .
and DHA 22:6 ny3 , as well as the total ny6 and ny3 were significantly higher
ŽP-0.05 in the DHA-enriched rotifers compared with the unenriched rotifers. The.
Ž .
Fig. 2. Percent survival mean"S.E.M. of 25-day-old milkfish fed Chlorella-cultured rotifers and newly
Ž . Ž
hatched Artemia nauplii Trt 1, control and DHA-enriched rotifers and Artemia metanauplii Trt 2,
.
Ž .
DHArEPA ratio in DHA-enriched rotifers 1.50 was likewise significantly higher
ŽP-0.05 than those cultured in Chlorella sp. 0.07 . Total n. Ž . y3rny6 ratio, how-ever, was not statistically significant between the control and DHA-enriched rotifers.
The fatty acid composition of the newly hatched and DHA-enriched Artemia are shown in Table 2. EPA was present in low amounts while no DHA was detected in the
Ž
newly hatched Artemia nauplii confirming earlier reports Leger et al., 1987; Tuncer
.
and Harrell, 1992 . This may perhaps explain why Artemia is an inferior live food for marine fish larvae unless enriched with HUFA-rich marine fish oils. The DHA-enriched
Ž . Ž .
Artemia contained relatively substantial amounts of DHA 0.61% and EPA 0.48% with a ratio of 1.28 compared with the control. The rest of the EFAs, as well as 16:0,
Ž .
18:4 ny6 and 22:4 ny6, were significantly elevated P-0.05 in the DHA-enriched Artemia compared with the newly hatched Artemia nauplii. In terms of total ny3rny6
Ž . Ž .
ratio, value for the DHA-enriched Artemia 2.31 was significantly higher P-0.05
Ž .
than the newly hatched Artemia 1.54 .
The whole-body tissue fatty acid pattern of 25-day old milkfish larvae fed
DHA-en-Ž .
riched rotifers and Artemia and fish fed unenriched live food control is presented in Table 3. Generally, the DHA-treated fish have increased ny3 and ny6 PUFA levels
Ž
compared with the untreated fish reflecting the fatty acid compositions of their diet see
. Ž . Ž .
Tables 1 and 2 . As expected, the DHA 0.60% and EPA 0.39% levels in the body
Ž .
tissue of the fish fed DHA-enriched live food were significantly higher P-0.05 than
Ž . Ž .
Fig. 3. Percent incidence mean"S.E.M. of opercular deformity mainly cleft branchiostegal membrane
Ž .
among 85-day-old milkfish fed Chlorella-cultured rotifers and newly hatched Artemia nauplii Trt 1, control
Ž .
Ž .
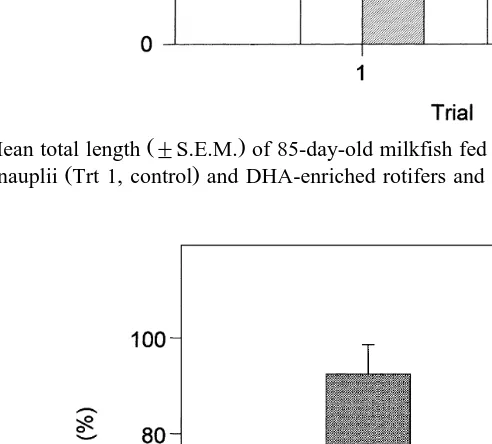
Fig. 4. Mean total length "S.E.M. of 85-day-old milkfish fed Chlorella-cultured rotifers and newly hatched
Ž . Ž .
Artemia nauplii Trt 1, control and DHA-enriched rotifers and Artemia metanauplii Trt 2, DHA-treated .
Ž .
Fig. 5. Percent survival mean"S.E.M. of 85-day-old milkfish fed Chlorella-cultured rotifers and newly
Ž . Ž
hatched Artemia nauplii Trt 1, control and DHA-enriched rotifers and Artemia metanauplii Trt 2,
.
Ž .
those of the untreated fish 0.16% and 0.30% for DHA and EPA, respectively . ARA seemed to be conserved at certain levels, i.e., 0.13% in the control, 0.16% in the DHA-treated fish and 0.12% in the wild fry. The DHArEPA ratio was significantly
Ž . Ž . Ž .
higher P-0.05 for the DHA-treated fish 1.54 than the control 0.53 . EPA and DHA levels in the tissue of wild milkfish postlarvae or fry were 0.42% and 0.96%, respectively while newly hatched milkfish eggs had 0.29% EPA, 1.99% DHA and
Ž .
0.36% ARA Table 3 .
Ž .
Growth, in terms of total length, was not significantly different P)0.05 between
Ž .
the DHA-treated and the control fish during the 25-day culture period Fig. 1 . Survival
Ž .
of 25-day old milkfish larvae, however, was significantly higher P-0.05 among the
Ž .
fish fed DHA-enriched live food 25.9–31.2% compared with those given unenriched
Ž . Ž .
diet 19.3–23.7%, Fig. 2 in contrast to the previous findings Gapasin et al., 1998 . After 60-day extensive culture in earthen nursery ponds, incidence of deformity was
Ž .
significantly lower P-0.05 in milkfish fingerlings fed DHA-enriched live food
Ž6.8–17.8% than those given unenriched diet 19.4–32.4%, Fig. 3 . Total length was. Ž . Ž .
observed to be significantly better P-0.05 in 85-day old milkfish juveniles fed
Ž .
DHA-enriched rotifers and Artemia during the hatchery phase compared with the
Ž .
control Fig. 4 . Although mean percent survival was slightly higher in the DHA-treated
Ž . Ž .
fish range: 93.2–100% than the untreated fish range: 87.3–99.4% , values were not
Ž .
statistically different Fig. 5 .
4. Discussion
Live food enriched with the EFAs DHA and EPA improved larval performance in
Ž . Ž .
striped bass and palmetto bass Tuncer and Harrell, 1992 , cod Takeuchi et al., 1994 ,
Ž . Ž .
red sea bream Furuita et al., 1996a , yellowtail Furuita et al., 1996b , striped jack
ŽTakeuchi et al., 1996 and the summer flounder Baker et al., 1998 . In the present. Ž .
investigation, no significant difference was observed in growth of milkfish larvae fed
Ž .
DHA-enriched live food high DHArEPA ratio compared with those fed unenriched
Žcontrol diet low DHA. Ž rEPA ratio after 25-day larval culture. However, extensive.
rearing of milkfish in nursery ponds showed that DHA-treated fish exhibited
signifi-Ž
cantly better growth than the control confirming our earlier results Gapasin et al.,
. Ž .
1998 . Mourente et al. 1993 reported the best growth rate in gilthead sea bream larvae fed HUFA-enriched rotifers with high DHArEPA ratio. In milkfish, the effects of DHA-enriched live food on growth may not be readily discerned over a short period
Žhatchery phase but rather after extended rearing nursery stage .. Ž . Ž .
Gapasin et al. 1998 observed no significant difference in survival of 25-day old milkfish larvae fed HUFA-enriched live food or those fed unenriched diet. The
Ž
DHArEPA ratios reported in the previous study were less than 1.0 that is 0.71–0.74
.
and 0.33–0.41 for HUFA-enriched rotifers and Artemia, respectively . In the present
Ž .
study, however, survival was significantly better consistent for the two trials in milkfish fed DHA-enriched live food compared with the fish fed unenriched diet
Ž
ratios of more than 1.0 that is 1.49 and 1.26 for the DHA-enriched rotifers and
.
Artemia, respectively; Tables 1 and 2 . Results suggest that increasing the DHArEPA
Ž .
ratio to a value of at least G1.0 in the live food further improved survival of the
Ž .
hatchery-reared milkfish larvae. For marine fish larvae, Tucker 1992 recommended a dietary ny3 HUFA content of 2–4%, including at least 1.0% EPA and 1.0% DHA.
Ž . Ž
Sargent’s 1995 estimate of 2:1 ratio for DHA and EPA based on the values found in
.
most fish eggs , however, is more reasonable. While the optimal DHA and EPA levels required by milkfish larvae have not yet been determined, a dietary DHA level of about
Ž .
0.6–1.0% dry weight basis and DHArEPA ratio of at least G1.0 in the live food would be appropriate for normal growth and good survival in intensive larviculture
Ž .
system. In flatfish, Estevez 1996 reported higher pigmentation success in the Japanese
Ž .
flounder fed diets with a DHArEPA ratio of 1.0. Similarly, Rainuzzo et al. 1994 found a positive correlation between pigmentation success and the DHArEPA ratio in
Ž .
the total and polar lipid fraction of the turbot larvae. Baker et al. 1998 also noted a direct relation between normal pigmentation and levels of DHA in the diet of summer flounder larvae where the DHArEPA ratios in the enriched live food ranged from
Ž
1.11–1.71. These reports underscore the importance of DHA and EPA in appropriate
.
proportions in survival, growth and development of various flatfish species. In milkfish,
Ž .
the need for a high DHArEPA ratio probably at least G1.0 in the larval diet is
Ž .
validated by the fact that the DHArEPA ratios in the newly spawned milkfish eggs 6.8
Ž . Ž .
and whole-body tissue of wild milkfish fry 2.3 were also high Table 3 . Abundance of
Ž
DHA in the tissue of the wild fry further suggests that the zooplankton diet e.g.,
.
copepods of milkfish larvae in the wild is relatively superior in terms of nutritional
Ž
quality and quantity compared with the live food given in the hatchery despite being
.
fortified with HUFA-rich marine fish oils or commercial enrichers . Marine copepods
Žand their naupliircopepodites are considered excellent live food for fish larvae not.
only of their small size but more importantly, because of their high nutritive value.
Ž .
Evjemo and Olsen 1997 reported the predominant fatty acids in the marine copepods
Ž .
Temora longicornis and Eurytemora sp. were DHA, EPA and palmitic acid 16:0 with DHArEPA ratios ranging from 1.83–1.97. High amounts of DHA were detected in the fatty acid composition of the copepods Oithona sp., Pseudodiaptomus spp. and Acartia
Ž
tsuensis with DHArEPA values of 1.28, 1.37 and 2.64, respectively Toledo et al.,
. Ž .
1999 . With the exception of palmitic acid 16:0 , the predominance of DHA and EPA
Ž . Ž
in the tissues of the wild mikfish fry this study and the copepods Evjemo and Olsen,
.
1997; Toledo et al., 1999 indicate the preference and importance of these EFAs in the
Ž .
diet of milkfish larvae and perhaps in other marine fish species as well . The fatty acid pattern of the predator larvae generally reflects that of its prey. Thus, it is important in enrichment that the fatty acid profile of live food produced in the hatchery emulates or approximates the fatty acid patterns of the natural food organisms in the wild through the use of appropriate commercial enrichers and proper enrichment procedures.
Incidence of opercular deformity was significantly lower in the DHA-treated fish compared with the untreated fish despite the eggs coming from different broodstock
Ž .
confirmed our previous results Gapasin et al., 1998 . Similar abnormalities have also
Ž . Ž .
been reported in salmonids Bruno, 1990 , European sea bass Daoulas et al., 1991 ,
Ž . Ž .
and seemed to be associated with intensive culture. While it was observed that high dietary DHArEPA ratio decreased incidence of opercular abnormalities in milkfish
Ž .
larvae, it is not clear why or how these EFAs in appropriate proportions function to alleviate such deformities. Further study is therefore required to understand such syndrome.
Ž .
ARA is an important constituent of the food chain Nichols et al., 1982 . The presence of ARA in moderate amounts, as well as other ny6 PUFAs which are
Ž .
generally of plant origin, in the tissue of wild milkfish fry Table 3 suggests that the fish at such developmental stage also ingest microalgae, apart from zooplankton, as
Ž . Ž .
food. The present data confirmed Banno’s 1980 and Kinoshita’s 1981 observations that wild milkfish fry in shore waters feed mainly on copepods and diatoms. As fish grows from one developmental stage to the other, there is a corresponding ontogenetic
Ž .
shift in its nutrient requirements Dabrowski, 1986 . With the yolk fully resorbed and lacking a functional stomach, first feeding milkfish larvae must rely on a steady exogenous supply of HUFA-rich diet of zooplankton from the natural environment. As
Ž . Ž
milkfish larvae metamorphoses into a fry postlarvae , it becomes an omnivore Banno,
. Ž
1980; Kinoshita, 1981 . As the fry subsequently becomes a young juvenile or
finger-.
ling , the cardiac stomach develops acid- and pepsin-secreting glands while mucosal
Ž .
folds acquire secondary branching Ferraris et al., 1987 . At this stage, the morphology
Ž .
of the digestive system of juvenile and also of the adult milkfish indicates that it has
Ž . Ž
become an herbivore with generalist tendencies Kinoshita, 1981 . Algal mat local term
.
lablab , natural food base of milkfish in ponds, is a complex community of unicellular
Ž .
algae, diatoms and other organisms Rabanal, 1966 and reportedly constitutes in part 2.0% 18:2 ny6, 4.0% 18:3ny3 but no detectable amounts of 20:4 ny6, 20:5ny3 or
Ž .
22:5ny3 Gorriceta, 1982 . The presence of ARA, EPA and DHA in the liver, despite their absence in lablab, suggests that young milkfish juveniles have the capacity to
Ž
convert dietary ny3 and ny6 PUFAs into long-chain HUFAs Benitez and Gorriceta,
. Ž . Ž .
1985 and later confirmed by Bautista and de la Cruz 1988 and Borlongan 1992 . This
Ž
probably explains the high percent survival of 85-day old milkfish juveniles DHA-treated
. Ž .
or not as the fish at this stage and perhaps earlier at metamorphosis are already able to utilize the available natural food. This information has important implications on the feeding management in the culture of milkfish for optimum growth and survival.
Acknowledgements
References
Andrades, J.A., Becerra, J., 1996. Skeletal deformities in larval, juvenile and adult stages of cultured sea
Ž .
bream Sparus aurata L. . Aquaculture 141, 1–11.
Baker, E.P., Alves, D., Bengtson, D.A., 1998. Effects of rotifer and Artemia fatty acid enrichment on survival, growth and pigmentation of summer flounder Paralichthys dentatus larvae. J. World Aquacult. Soc. 29, 494–498.
Ž .
Banno, J.E., 1980. The food and feeding habits of milkfish fry, Chanos chanos Forsskal collected from two habitats along the coast of Hamtik, Antique. MSc Thesis, University of the Philippines-Visayas, Iloilo, Philippines, 77 pp.
Ž . Ž .
Bautista, M.N., de la Cruz, M.C., 1988. Linoleic vy6 and linolenic vy3 acids in the diet of fingerling
Ž .
milkfish Chanos chanos Forsskal . Aquaculture 71, 347–358.
Benitez, L.V., Gorriceta, I.R., 1985. Lipid composition of milkfish grown in ponds by traditional aquaculture.
Ž .
In: Cho, C.Y., Cowey, C.B., Watanabe, T. Eds. , Finfish Nutrition in Asia: Methodological Approaches to
Ž .
Research and Development. International Development Centre IDRC , Ottawa, Canada, pp. 145–152.
Ž .
Borlongan, I.G., 1992. The essential fatty acid requirement of milkfish Chanos chanos Forsskal . Fish Physiol. Biochem. 9, 401–408.
Bruno, D.W., 1990. Miscellaneous external abnormalities of farmed salmonids. Aquacult. Inform. Serv. 11, 1–6.
Castell, J.D., Bell, J.G., Tocher, D.R., Sargent, J.R., 1994. Effects of purified diets containing different combinations of arachidonic and docosahexaenoic acid on the survival, growth and fatty acid composition
Ž .
of juvenile turbot Scophthalmus maximus . Aquaculture 128, 315–333.
Dabrowski, K., 1986. Ontogenetic aspects of nutritional requirements in fish. Comp. Biochem. Physiol. 85A, 639–655.
Daoulas, C., Economou, A.N., Bantavas, I., 1991. Osteological abnormalities in laboratory reared sea-bass
ŽDicentrarchus labrax fingerlings. Aquaculture 97, 169–180..
Estevez, A., 1996. Effects of lipids and vitamin A on pigmentation success of flatfish. PhD Thesis, University of Kagoshima, Japan, 149 pp.
Evjemo, J.O., Olsen, Y., 1997. Lipid and fatty acid content in cultivated live feed organisms compared to marine copepods. Hydrobiologia 358, 159–162.
Ferraris, R.P., Tan, J.D., de la Cruz, M.C., 1987. Development of the digestive tract of milkfish Chanos
Ž .
chanos Forsskal : histology and histochemistry. Aquaculture 61, 241–257.
Folch, J., Lees, M., Stanley, G.H., 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509.
Furuita, H., Takeuchi, T., Toyota, M., Watanabe, T., 1996a. EPA and DHA requirements in early juvenile red sea bream using HUFA enriched Artemia nauplii. Fish. Sci. 62, 246–251.
Furuita, H., Takeuchi, T., Watanabe, T., Fujimoto, H., Sekiya, S., Imaizumi, K., 1996b. Requirements of larval yellowtail for eicosapentaenoic acid, docosahexaenoic acid and highly unsaturated fatty acid. Fish. Sci. 62, 372–379.
Gapasin, R.S.J., Marte, C.L., 1990. Milkfish hatchery operations, Aquaculture Extension Manual No. 17. SEAFDECrAQD, Tigbauan, Iloilo, Philippines, 24 pp.
Gapasin, R.S.J., Bombeo, R., Lavens, P., Sorgeloos, P., Nelis, H., 1998. Enrichment of live food with essential
Ž .
fatty acids and vitamin C: effects on milkfish Chanos chanos larval performance. Aquaculture 162, 269–286.
Gorriceta, I.R., 1982. Studies on the digestive lipases and lipid composition of milkfish, Chanos chanos
ŽForsskal . MSc Thesis, University of the Philippines-Diliman, Quezon City, Philippines, 56 pp..
Halver, J.E., Smith, R.R., Tolbert, B.M., Baker, E.M., 1975. Utilization of ascorbic acid in fish. Ann. N.Y. Acad. Sci. 258, 81–102.
Ž .
Hilomen-Garcia, G.V., 1997. Morphological abnormalities in hatchery-bred milkfish Chanos chanos Forsskal fry and juveniles. Aquaculture 152, 155–166.
Ž .
Hilomen-Garcia, G.V., 1998. Sensitivity of fertilized milkfish Chanos chanos Forsskal eggs to mechanical shock and simulated transport. Aquaculture 159, 239–247.
Kanazawa, A., 1985. Essential fatty acid and lipid requirements of fish. In: Cowey, C.B., Mackie, A.M., Bell,
Ž .
Kinoshita, I., 1981. Feeding habit and development of the digestive system in juvenile milkfish, Chanos
Ž .
chanos Forsskal . MSc Thesis, Nagasaki University, Japan, 42 pp.
Koven, W.M., Tandler, A., Sklan, D., Kissil, G.W., 1993. The association of eicosapentaenoic and docosahex-aenoic acids in the main phospholipids of different-age Sparus aurata larvae with growth. Aquaculture 116, 71–82.
Koumoundouros, G., Oran, G., Divanach, P., Stefanakis, S., Kentouri, M., 1997. The opercular complex
Ž .
deformity in intensive gilthead sea bream Sparus aurata L. larviculture. Moment of apparition and
description. Aquaculture 149, 215–226.
Lavens, P., Dhert, Ph., Merchie, G., Stael, M., Sorgeloos, P., 1994. A standard procedure for the mass production on an artificial diet of rotifers with a high nutritional quality for marine fish larvae. In: Chou, L.M., Munro, A.D., Lam, T.J., Chen, T.W., Cheong, L.K.K., Ding, J.K., Hooi, K.K., Khoo, H.W., Phang,
Ž .
V.P.E., Shim, K.F., Tan, C.H. Eds. , Proceedings of the Third Asian Fisheries Forum — Nutrition, 26–30 October 1992, Singapore. pp. 745–748.
Leger, Ph., Naessens-Foucquaert, P., Sorgeloos, E., 1987. International study on Artemia XXXV. Techniques to manipulate the fatty acid profile in Artemia nauplii and the effects on its nutritional effectiveness for the
Ž .
marine crustacean Mysidopsis bahia M. . In: Sorgeloos, P., Bengtson, D.A., Decleir, W., Jaspers, E.
ŽEds. , Artemia Research and Its Applications, vol. 3. Universal Press, Wetteren, Belgium, pp. 411–424..
Lijauco, M.M., Juario, J.V., Baliao, D.D., Grinom, Quinitio, G., 1979. Milkfish culture and management,˜
Extension Manual No. 4. Southeast Asian Fisheries Development Center — Aquaculture Department, Tigbauan, Iloilo, Philippines.
Metcalfe, L.D., Schmitz, A.A., Pelka, J.R., 1966. The rapid preparation of fatty acid methyl esters from lipids for gas chromatographic analysis. Anal. Chem. 38, 514–515.
Mourente, G., Rodriguez, A., Tocher, D.R., Sargent, J.R., 1993. Effects of dietary docosahexaenoic acid
ŽDHA; 22:6 ny3 on lipid and fatty acid compositions and growth in gilthead sea bream Sparus aurata. Ž .
L. larvae during first feeding. Aquaculture 112, 79–98.
Ž .
Mustafa, T., Srivastava, K.C., 1989. Prostaglandins eicosanoids and their role in ectothermic organisms. Adv. Comp. Environ. Physiol. 5, 157–207.
Nichols, P.D., Klumpp, D.W., Johns, R.B., 1982. Lipid components of the seagrasses Posidonia australis and
Heterozostera tasmanica as indicators of carbon source. Phytochem. 21, 1613–1621.
Ž .
Rabanal, H.R., 1966. The culture of lablab, the natural food of milkfish or bangos, Chanos chanos Forsskal fry and fingerling under cultivation. Phil. Fish. J. 35, 22–26.
Rainuzzo, J.R., Reitan, K.I., Jorgensen, L., Olsen, Y., 1994. Lipid composition in turbot larvae fed live feed cultured by emulsions of different lipid classes. Comp. Biochem. Physiol. 107A, 699–710.
Reitan, K.I., Rainuzzo, J.R., Olsen, Y., 1994. Influence of lipid composition of live feed on growth, survival and pigmentation of turbot larvae. Aquacult. Int. 2, 33–48.
Rodriguez, C., Perez, J.A., Diaz, M., Izquierdo, M.S., Fernandez-Palacios, H., Lorenzo, H., 1997. Influence of
Ž .
the EPArDHA ratio in rotifers on gilthead seabream Sparus aurata larval development. Aquaculture 150, 77–89.
Rodriguez, C., Perez, J.A., Badia, P., Izquierdo, M.S., Fernandez-Palacios, H., Lorenzo-Hernandez, A., 1998.
Ž .
The ny3 highly unsaturated fatty acids requirements of gilthead seabream Sparus aurata L. larvae when using an appropriate DHArEPA ratio in the diet. Aquaculture 169, 9–23.
Sargent, J.R., 1995. Origins and functions of egg lipids: nutritional implications. In: Bromage, N.R., Roberts,
Ž .
R.J. Eds. , Broodstock Management and Egg and Larval Quality. Blackwell, Oxford, pp. 353–372. Sargent, J.R., McEnvoy, L.A., Bell, J.G., 1997. Requirements, presentation and sources of polyunsaturated
fatty acids in marine fish larval feeds. Aquaculture 155, 117–127.
Sargent, J., Bell, J.G., McEnvoy, L.A., Tocher, D.R., Estevez, A., 1999. Recent developments in the essential fatty acid nutrition of fish. Aquaculture 177, 191–199.
SAS Institute, 1988. SASrSTATTM Guide for Personal Computers. 6th edn. SAS Institute, Cary, NC, 1028 pp.
Sorgeloos, P., Lavens, P., Leger, Ph., Tackaert, W., Versichele, D., 1986. Manual for the Culture and Use of Brine Shrimp Artemia in Aquaculture. Faculty of Agriculture, University of Gent, Belgium, 319 pp. Sumagaysay, N.S., Hilomen-Garcia, G.V., Garcia, L.M.B., 1999. Growth and production of deformed and
Ž .
Takeuchi, T., Zheng, K., Yoseda, K., Hirokawa, J., Watanabe, T., 1994. Nutritive value of DHA-enriched rotifer for larval cod. Nippon Suisan Gakkaishi 60, 641–652.
Takeuchi, T., Masuda, R., Ishizaki, Y., Watanabe, T., Kanematsu, M., Imaizumi, K., Tsukamoto, K., 1996. Determination of the requirement of larval striped jack for eicosapentaenoic acid and docosahexaenoic acid using enriched Artemia nauplii. Fish. Sci. 62, 760–765.
Tave, D., Handwerker, T.S., 1994. Semi-operculum: a non-heritable birth defect in Tilapia nilotica. J. World Aquacult. Soc. 25, 333–336.
Tocher, D.R., Mourente, G., Sargent, J.R., 1997. The use of silages prepared from fish neural tissues as
Ž .
enrichers for rotifers Brachionus plicatilis and Artemia in the nutrition of larval marine fish. Aquacul-ture 148, 213–231.
Toledo, J.D., Doi, J.D., Duray, M., 1996. Viability of milkfish eggs and larvae after simulated and actual
Ž .
transport. In: MacKinlay, D., Eldridge, M. Eds. , The Fish Egg: Its Biology and Culture Symposium Proceedings, 14–18 July. San Francisco State University, San Francisco, USA, pp. 51–57.
Toledo, J.D., Golez, M.S., Doi, M., Ohno, A., 1999. Use of copepod nauplii during early feeding stage of grouper Epinephelus coioides. Fish. Sci. 65, 390–397.
Ž .
Tucker, J.W., 1992. Feeding intensively-cultured marine fish larvae. In: Allan, G.L., Dall, W. Eds. , Proceedings of the Aquaculture Nutrition Workshop, Salamander Bay, 15–17 April 1991. NSW Fisheries, Brackish Water Fish Culture Research Station, Salamander Bay, Australia, pp. 129–146.
Ž .
Tuncer, H., Harrell, R.M., 1992. Essential fatty acid nutrition of larval striped bass Morone saxatilis and
Ž .
palmetto bass M. saxatilis=M. chrysops . Aquaculture 101, 105–121.
Watanabe, T., Izquierdo, M.S., Takeuchi, T., Satoh, S., Kitajima, C., 1989. Comparison between eicosapenta-enoic acid and docosahexaeicosapenta-enoic acid in terms of essential fatty acid deficiency in larval red seabream. Nippon Suisan Gakkaishi 55, 1635–1640.