www.elsevier.com / locate / bres
Research report
cAMP-induced stellation in primary astrocyte cultures with regional
heterogeneity
*
Chung-Kil Won, Young S. Oh
Departments of Medicine and Neurobiology, Sparks Center 865, University of Alabama at Birmingham, 1530 3rd Ave. South, Birmingham,
AL35294, USA
Received 27 June 2000; accepted 29 August 2000
Abstract
It is well known that increased cAMP levels in cultured astrocytes can convert flat polygonal shaped astrocytes into process-bearing, stellate astrocytes. In this study, we have examined the possible existence of astrocyte regional heterogeneity in morphological changes in response to cAMP stimulation. Primary astrocyte cultures were prepared from six different regions of neonatal rat brains, including cerebral cortex, hippocampus, brain stem, mid brain, cerebellum, and hypothalamus. After about 2 weeks in culture, the astrocyte culture medium was changed to DMEM containing various concentrations of 8-CPT-cAMP, a membrane permeable cAMP analog, for 2 h. We found that 250mM 8-CPT-cAMP produced a maximum effect causing.95% stellation in all regional astrocytes except hypothalamic astrocytes (56% stellation). At lower cAMP concentrations, cell stellation most effectively occurred in cerebellar astrocytes. To examine further the regional heterogeneity of astrocyte morphological changes, glutamate was added together with 8-CPT-cAMP to block cAMP-induced astrocyte stellation. Interestingly, glutamate blockage on cAMP-induced astrocyte stellation was brain region-specific in that cerebral and hippocampal astrocytes were effectively blocked by glutamate when compared to other regional astrocytes. Furthermore, glutamate inhibited isoproterenol-induced astrocyte stellation in a region-specific manner similarly as in cAMP-induced stellation. The present study demonstrates that astrocytes derived from different regions of the neonatal rat brain maintain different levels of morphological plasticity in culture. 2000 Elsevier Science B.V. All rights reserved.
Theme: Development and regeneration
Topic: Glia and other non-neuronal cells
Keywords: Cerebrum; Hippocampus; Cerebellum; Mid brain; Brain stem; Hypothalamus
1. Introduction thicker and longer processes and increased cellular content of glial fibrillary acidic protein (GFAP) has been observed Primary cultures of astrocyte are diverse in their mor- in the CNS after various types of injury caused by phology [8,24], and many factors can influence the mor- physical, chemical, and pathological trauma [15,23]. It is phology of cultured astrocytes, such as the presence or the suggested that astrocyte morphology changes in vivo may absence of neurons in culture [14,22], reagents which can affect neuronal excitability and / or synaptic activity by increase intracellular cAMP levels [13,26,34,40], and the changing the volume of the extracellular space [16]. At presence of b-amyloid peptide [33] or various hormones present, the level of regional heterogeneity in astrocyte [31,43]. Astrocyte morphology in vivo can also change in morphology changes in response to various stimuli is not response to various stimuli. For example, during lactation well understood.
[38] or dehydration state [16], astrocyte morphology can There is increasing evidence suggesting regional hetero-change responding to these normal physiological chal- geneity of astrocyte functions. For example, dopamine lenges. The appearance of reactive astrocytes in vivo with receptors were expressed in astrocytes cultured from the striatum but not from other brain regions [17]. A phos-phoinositide-linked peptide response was different among
*Corresponding author. Tel.: 11-205-934-3806; fax: 1
1-205-934-cortical, cerebellar, and spinal cord astrocytes [10].
An-1147.
E-mail address: [email protected] (Y.S. Oh). giotensin II could activate phospholipase C (PLC) and
C.-K. Won, Y.S. Oh / Brain Research 887 (2000) 250 –258 251
induce prostaglandin release from medullary and cerebellar treated circular glass coverslips. The cells were then astrocytes but not from cortical and hypothalamic as- maintained for an additional 3–5 days before experiments. trocytes [42]. Furthermore, astrocytes cultured from differ- At this stage, we found that GFAP-positive cells were ent regions of the brain display different degrees of gap- greater than 90% in astrocyte cultures derived from junction coupling [20], different levels of muscarinic different regions of the brain. The culture medium was
1
acetylcholine receptors [4], differential modulation of Na changed every 3 days. Immunocytochemistry was per-channel mRNA expression [29], and different rate of formed as previously reported [28] using monoclonal anti-uptake in serotonin and glutamate [3]. These highly GFAP antibody (Roche Mol. Biochem.).
heterogeneous characteristics of different regional
as-trocytes may suggest a specialized role of asas-trocytes in 2.2. Evaluation of astrocyte stellation different regions of the CNS during information processing
and brain development. Cells were fixed in 4% paraformaldehyde and stained
In the present study, we have examined the regional with coomassie blue (0.25% Coomassie brilliant blue, heterogeneity of astrocyte morphology changes in response R-250, 50% methanol, and 10% acetic acid) to determine to cAMP treatment, which is a well known factor to affect the percentage of stellate cells in culture. In order to astrocyte morphology changes [26,34,40], and blockage of eliminate any serum effect on different regional astrocytes, cAMP- and isoproterenol-induced astrocyte stellation by astrocytes cultured on 24 well plates were maintained in glutamate in six different regional astrocytes cultured from the absence of serum for 24 h, and the astrocyte medium cerebral cortex, hippocampus, brain stem, mid brain, was changed to fresh DMEM containing various con-cerebellum, and hypothalamus. The results suggest that centrations of 8-CPT-cAMP (Biolog Life Science Institute) there is a regional heterogeneity of astrocyte morphology or isoproterenol (Sigma), respectively, in the absence or changes in response to cAMP treatment and glutamate the presence of L-glutamate (Sigma). cAMP- or
iso-effect on astrocyte stellation. proterenol-induced astrocyte stellation was determined after incubating the cells for 2 h in a 378C CO incubator,2 and the levels of stellation among different regional
2. Materials and methods astrocytes were compared. We have chosen 2 h cAMP or isoproterenol stimulation since our preliminary study
2.1. Primary astrocyte cultures showed the maximum astrocyte stellation could be
achieved after 2 h of stimulation in all regional astrocytes. Astrocytes were cultured as previously described [28] As a control, DMEM changes without 8-CPT-cAMP or from postnatal day 0–1 Sprague–Dawley rats with a minor isoproterenol did not induce any astrocyte morphological modification to enrich flat, polygonal-shape astrocytes. changes in all regional astrocytes (data not shown). Briefly, six different regions of the rat brain, including Cells with processes longer than their perinuclear diame-cerebral cortex, hippocampus, cerebellum, mid brain (su- ters were defined as stellate cells according to the criteria perior and inferior colliculi), brain stem (pons and medul- used by Kimelberg et al. [19] and Shao et al. [40]. Stained la), and hypothalamus were isolated, minced, and incu- cells were mounted on slide glass and viewed in a bated in a solution containing 30 units papain / ml (Roche), transmitted light with a 103 objective lens using an Earle’s salts, 0.5 mM EDTA, and 1.65 mM L-cysteine at inverted TMS-F microscope (Nikon) that was connected
378C for 15 min followed by trituration in a complete with a CCD camera (Pixera). For each coverslip, three culture medium (Dulbecco’s Modified Eagle’s Medium, randomly chosen fields were counted (about 40–60 cells in DMEM, containing 10% fetal bovine serum obtained from each field), and the percentage of stellate cells was Hyclone and penicillin / streptomycin, 100 units / ml each). determined (thus, a minimum of 150 cells per each
2
Resuspended cells were plated onto 75 cm culture flasks coverslip was counted). Usually two coverslips, sometimes 7
(Corning) at high density (.2310 cells / flask), and three coverslips, were included for each experimental maintained overnight at 378C in vitro in a 5% CO / 95%2 condition (thus, a minimum of 300 cells was counted per air atmosphere. On the following day, the culture medium experimental condition), and each experimental condition was replaced with a fresh complete culture medium, and was repeated from 3 to 4 independent culture preparations maintained for 7–10 days in vitro. After the cells became (thus, a total of 900–1,200 cells were counted in each data confluent, the culture flask was replaced with 10 ml of points in Figs. 1, 2, 4 and 5). The percentage of stellate fresh complete medium and was shaken overnight on an cells in each experimental condition was expressed as orbital shaker (Lab-line Instruments) at a speed of 250 rpm average6standard deviation. Student’s t-test was used for in a 378C CO incubator. On the next day, the culture flask2 statistical analysis. The experiments were repeated from at was washed five times with phosphate buffered saline least three independent astrocyte culture preparations (PBS), and then the attached astrocytes were dissociated corresponding to each brain region. Sister cultures of
4 2
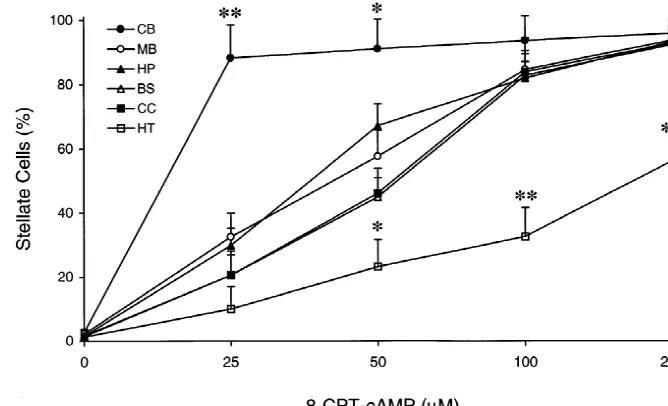
Fig. 1. Dose–response curve of astrocyte morphology changes in response to 8-CPT-cAMP, a membrane permeable cAMP analog. Astrocytes were maintained in the absence of serum for 24 h, and then the cells were treated with various concentrations of 8-CPT-cAMP for 2 h. Note that cerebellar astrocytes were the most sensitive astrocytes to 8-CPT-cAMP treatment while hypothalamic astrocytes were the least sensitive to 8-CPT-cAMP treatment. Each datum point represents the mean6S.D. from 3–4 independent culture preparations. *, P,0.05; **, P,0.01 compared to all other regional astrocytes. CB, cerebellum; MB, mid brain; HP, hippocampus; BS, brain stem; CC, cerebral cortex; HT, hypothalamus.
we have not observed any dramatic changes in the able cAMP analog, induced astrocyte stellation with a percentage of GFAP-positive cells among different as- dose-dependent manner in all regional astrocytes (Fig. 1),
trocyte preparations. and there was a regional heterogeneity in astrocyte
mor-phology changes in response to 8-CPT-cAMP. Cerebellar astrocytes were the most sensitive cells to 8-CPT-cAMP
3. Results treatment, whereas hypothalamic astrocytes were the least sensitive among examined six different regional astrocytes. 3.1. The effect of 8-CPT-cAMP The higher sensitivity of cerebellar astrocytes as compared to other regional astrocytes could be clearly seen at 25mM We found that 8-CPT-cAMP, a highly membrane perme- 8-CPT-cAMP treatment (Fig. 1). At this cAMP
C.-K. Won, Y.S. Oh / Brain Research 887 (2000) 250 –258 253
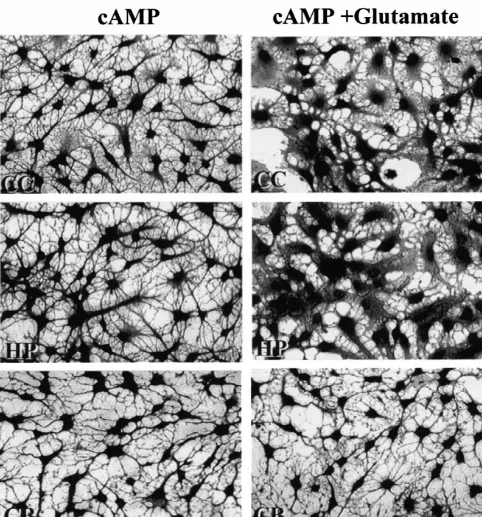
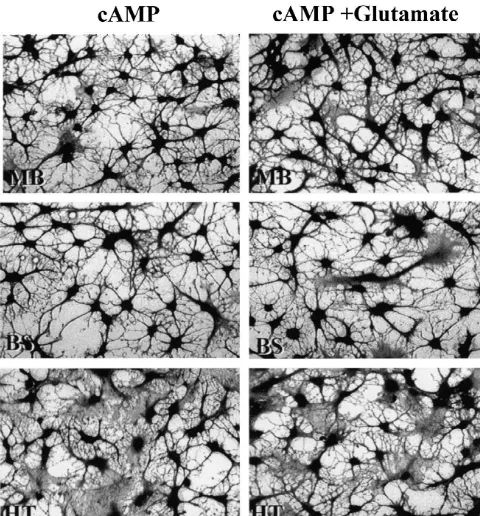
Fig. 3. Photomicrographs showing the effect of glutamate on cAMP-induced astrocyte stellation in different regional astrocytes. Left panel: astrocytes were treated with 250mM 8-CPT-cAMP alone for 2 h. Right panel: astrocytes were treated with 250mM 8-CPT-cAMP plus 500mM glutamate for 2 h. Note that the effective blockage of cAMP-induced astrocyte stellation by glutamate in cerebral cortex (CC) and hippocampal (HP) astrocytes but not in cerebellar (CB), mid brain (MB), brain stem (BS), and hypothalamus (HT) astrocytes.
Fig. 3. (continued )
treatment among different regional astrocytes appeared to cAMP-induced (250mM 8-CTP-cAMP) cortical astrocyte be: cerebellum.mid brain, hippocampus$brain stem, and stellation in a dose-dependent manner (Fig. 2). In addition,
cortex.hypothalamus. we found that hippocampal astrocyte stellation induced by
cAMP could also be blocked by L-glutamate. In contrast,
3.2. The effect of glutamate on astrocyte stellation L-glutamate had a minimal effect on cAMP-induced
as-trocyte stellation in mid brain, brain stem, cerebellar, and Previous studies demonstrated that L-glutamate could hypothalamic astrocytes (Figs. 2 and 3). Maximum
C.-K. Won, Y.S. Oh / Brain Research 887 (2000) 250 –258 255
glutamate concentration is consistent with the previous finding by Shao et al. [40] in their cortical astrocyte cultures. We further examined the effect of glutamate blockage on astrocyte stellation at the submaximal dose of 8-CPT-cAMP, i.e., 25mM. The same results were obtained at this concentration; that is,L-glutamate could effectively
block cAMP-induced astrocyte stellation in both cortical and hippocampal astrocytes but not in other regional astrocytes (Fig. 4). Moreover, we found that the effect of
L-glutamate was not mimicked by 500 mM NMDA, but
was mimicked by 500 mM D/ l-aspartate in blocking cAMP-induced cortical or hippocampal astrocyte stella-tion, suggesting that the inhibitory effect ofL-glutamate on
astrocyte stellation was mediated by glutamate transporters not by glutamate receptors (data not shown).
Next, we have tested whether there is a regional heterogeneity of glutamate blockage on astrocyte stellation induced by another reagent. For this purpose, we used
Fig. 4. The effect of glutamate on astrocyte stellation induced by 25mM
8-CPT-cAMP, a submaximal dose to induce astrocyte stellation. As- isoproterenol, a specific agonist against the b-adrenergic trocytes were either treated with 25mM 8-CPT-cAMP alone (open bar) or receptor, because isoproterenol is known to induce as-25mM 8-CPT-cAMP plus 500mM glutamate (closed bar) for 2 h. The
trocyte morphology changes into stellate-shapes by binding
same results obtained at the maximal dose of 8-CPT-cAMP, 250 mM,
tob-adrenergic receptors on cell membranes and
increas-were observed at this cAMP concentration; that is, glutamate effectively
ing intracellular cAMP levels in cultured astrocytes
blocked cAMP-induced astrocyte stellation in cortical and hippocampal
astrocytes but not in other regional astrocytes. Each datum point [27,40,44]. In this regard, we have recently found that 1 represents the mean6S.D. from 3 to 4 independent culture preparations. mM isoproterenol can induce maximal astrocyte stellation CB, cerebellum; MB, mid brain; HP, hippocampus; BS, brain stem; CC,
in cultured astrocytes (Won and Oh, unpublished data), and
cerebral cortex; HT, hypothalamus.
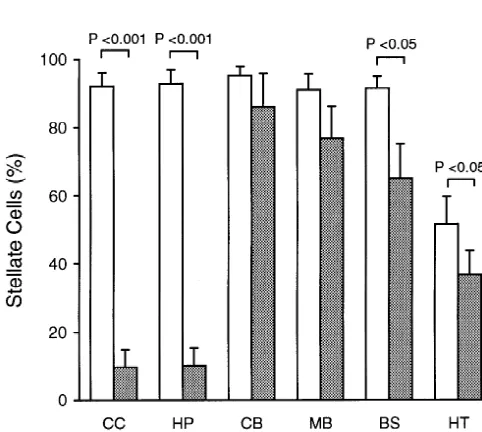
thus we have tested the effect of glutamate on 1 mM isoproterenol-induced astrocyte stellation. As in 8-CPT-cAMP experiments, 500mM glutamate effectively blocked isoproterenol-induced astrocyte stellation in cortical and hippocampal astrocytes (Fig. 5). Glutamate also blocked isoproterenol-induced astrocyte stellation in brain stem and hypothalamic astrocytes at a statistically significant level (P,0.05), but not in other regional astrocytes (Fig. 5), suggesting that the mechanism of glutamate blockage on cAMP-induced or isoproterenol-induced astrocyte stella-tion is similar.
4. Discussion
In the present study, we have demonstrated that (i) neonatal astrocytes cultured from six different regions of the rat brain display regional heterogeneity in terms of their sensitivity to cAMP-induced morphological changes, namely stellation, and that (ii) glutamate blocks cAMP-induced astrocyte stellation with regional heterogeneity. Astrocytes are the most numerous cells in the brain playing diverse functional roles, including extracellular ion and volume regulation, uptake and release of neurotransmitters,
Fig. 5. The effect of glutamate on isoproterenol-induced astrocyte
stellation. Astrocytes were treated with 1mM isoproterenol alone (open metabolic support for neurons, and formation of blood–
bar) or 1mM isoproterenol plus 500mM glutamate (closed bar) for 2 h. brain barrier [6,35]. Since anatomically distinct brain Glutamate effectively blocked isoproterenol-induced astrocyte stellation regions are involved in different neurological functions, in both cortical (CC) and hippocampal (HP) astrocytes but not in
astrocytes in different brain regions are likely to be
cerebellar (CB), mid brain (MB), brain stem (BS), and hypothalamic
heterogeneous in their major physiological roles and
(HT) astrocytes. Each datum point represents the mean6S.D. from 3 to 4
this speculation, regional heterogeneity of astrocytes has strated by a gene knockout experiment [37]. In rats that been noted by many laboratories. For example, Lee et al. chronically received antisense oligonucleotides against the [20] have found that the strength of gap-junction coupling astrocyte glutamate transporters, the animals showed an among different regional astrocytes is different, and can be elevated level of extracellular glutamate, neurodegenera-ranked as: cerebellum, hippocampus.hypothalamus. tion in striatum and hippocampus, and a progressive cortex.spinal cord. In addition, various levels of GFAP paralysis. Glutamate has been shown to affect astrocyte content [7], phosphoinositide-linked peptide response [10], morphology changes in vitro [1,40]. Shao et al. [40] and dopamine receptor expression [17] among different demonstrated that glutamate could block astrocyte stella-regional astrocytes have been observed. Therefore, the tion induced byb-adrenergic receptor stimulation, such as present study extends another aspect of astrocyte regional isoproterenol treatment, in primary cortical astrocyte cul-heterogeneity in regard to different levels of morphological tures. They showed that the effect of glutamate on plasticity in response to cAMP treatment and glutamate astrocyte stellation was not due to inhibition of cAMP effect on cAMP-induced astrocyte stellation. formation or glutamate receptor stimulation, but by gluta-It is well known that increased intracellular cAMP levels mate uptake into the cells via glutamate transporters, in astrocytes by treatment with a membrane permeable which would cause cell swelling and inhibit the process of cAMP analog [13,26,34,40] or by b-adrenergic stimula- cell stellation. They have proposed a hypothesis that tion, e.g., isoproterenol treatment [40], leads to astrocyte astrocyte morphology in vivo may be dynamically reg-morphology change from flat polygonal shaped astrocytes ulated by different neurotransmitters, such as norepineph-into process-bearing, stellate astrocytes. The process of rine and glutamate, during neuronal activity in the brain. astrocyte stellation has been found to be a tubulin-[48], For example, during glutamatergic neuronal activity, gluta-microtubule-[13], and actin filaments-dependent [5]. Thus, mate released from neuronal terminals can be taken up by during the conversion of flat astrocytes into stellate astrocytes to cause astrocyte swelling and result in de-morphology, a series of coordinated alteration and reor- creased volume of the extracellular space. Consistent with ganization of cytoskeletal proteins occurs in astrocytic this hypothesis, it is demonstrated that cortical extracellu-cytoplasm. Interestingly, GFAP, a major component of lar space shrinks more than 30% during extensive neuronal astrocyte intermediate filament, seems not to be critically activity [21].
involved in the astrocyte stellation process because stella- In this study, we demonstrated that cAMP- and iso-tion can occur in LRM55 glioma cells, which express proterenol-induced astrocyte stellation were the most effec-negligible amount of GFAP [39] and in astrocytes derived tively blocked by glutamate in cortical and hippocampal from GFAP-knock out mice [32]. Recently, Padmanabhan primary astrocyte cultures. This finding is consistent with et al. [30] have demonstrated that changes in astrocyte the report by Amundson et al. [3] who demonstrated that adhesion to the substratum are crucial in cAMP-induced there was a regional heterogeneity in the rate of glutamate process formation. They found that cAMP-induced as- uptake among different regional astrocytes; the relative trocyte stellation was closely related with a decrease in order of glutamate uptake was hippocampus.cortex.
tyrosine phosphorylation of focal adhesion kinase (FAK or hypothalamus.mid brain, brain stem.cerebellum. So far, pp125FAK) and its associated protein, paxillin. It was five glutamate transporters with distinct structures have proposed that cAMP might directly activate tyrosine been cloned, and two types (GLAST and GLT-1) are phosphatase, which would decrease tyrosine phosphoryla- primarily expressed in astrocytes [11]. Using immuno-tion of FAX and paxillin resulting in the redistribuimmuno-tion of histochemistry, Furuta et al. [12] have found that the these proteins in the cell to induce astrocyte stellation. expression of these two glutamate transporters is differen-Assuming that the process of cAMP-induced astrocyte tially regulated during CNS development. For example, stellation among different regional astrocytes is similar, it they found that at neonatal stage cortical and hippocampal can be speculated that regional heterogeneity of astrocyte astrocytes expressed low-to-moderate levels of GLAST stellation may be related to an expression of different and GLT-1, while cerebellar astrocytes expressed only isoform and / or differential expression level of a certain faint or undetectable levels of these transporters. Interest-cytoskeletal, cytoskeletal-associated, or cytoskeletal ingly, cultured neonatal astrocytes predominantly express protein-modifying protein in a brain region-specific man- GLAST [41]. Using anti-GLAST antibody, we have
ner. recently found that the expression level of GLAST in
Glutamate is the major excitatory neurotransmitter in the cortical and hippocampal astrocytes is much higher than mammalian central nervous system, and it is essential to the levels in other regional astrocytes in culture (Won and maintain a low extracellular glutamate concentration be- Oh, unpublished data). Therefore, the effective blockage of cause excessive activation of glutamate receptors can glutamate on cortical and hippocampal astrocyte stellation damage neurons [9,36]. Clearance of glutamate released by is most likely related to the higher expression level of neurons is primarily carried out by astrocytes through their glutamate transporters in these two regional astrocytes as
1
C.-K. Won, Y.S. Oh / Brain Research 887 (2000) 250 –258 257
of mouse brain astrocytes in culture vary with their tissue of origin
cortex and hippocampus is functionally well correlated
but differ from those of neurons, Eur. J. Neurosci. 6 (1994)
with the major localization of glutamatergic neuronal
1702–1709.
pathways in these two brain regions. It is well known that [5] D.M. Barodo, W. Mellado, M.L. Shelanski, Astrocyte process glutamate receptors, such as NMDA and AMPA receptors, growth induction by actin breakdown, J. Cell Biol. 117 (1992)
are highly expressed in cortex and hippocampus [25]. 357–367.
[6] B.A. Barres, New roles for glia, J. Neurosci. 11 (1991) 3685–3694.
In the developing brain, one of the major roles of
[7] C. Beyer, B. Epp, J. Fassberg, I. Reisert, C. Pilgrim, Region- and
astrocytes include guidance for neuronal migration and
sex-related differences in maturation of astrocytes in dissociated cell
production of neurotrophic factors for neuronal survival cultures of embryonic rat brain, Glia 3 (1990) 55–64.
and maturation. In the vertebrate brain, neurogenesis [8] J.A. Black, H. Sontheimer, S.G. Waxman, Spinal cord astrocytes in mostly occurs before birth. In the postnatal period, a vitro: Phenotypic diversity and sodium channel immunoreactivity,
Glia 7 (1993) 272–285.
second wave of neurogenesis can occur in cerebellum,
[9] D. Choi, M. Maulucci-Gedde, A. Kriegstein, Glutamate
neuro-hippocampus, and olfactory bulb. Interestingly, unlike
toxicity in cortical cell culture, J. Neurosci. 7 (1987) 357–368.
hippocampus or olfactory bulb, where neurogenesis occurs
[10] A.J. Cholewinski, M.R. Hanley, G.P. Wilkin, A
phosphoinositide-only in a restricted area, in cerebellum huge numbers of linked peptide response in astrocytes: evidence for regional hetero-interneurons and granule cells are generated during the first geneity, Neurochem. Res. 13 (1988) 389–394.
[11] N.C. Danbolt, F.A. Chaudhry, Y. Dehnes, K.P. Lehre, L.M. Levy,
few weeks of postnatal age [2]. In fact, cerebellum
Properties and localization of glutamate transporters, Prog. Brain
contains more than half of all the neurons found in the
Res. 116 (1998) 21–41.
brain. In this regard, the most dynamic morphological
[12] A. Furuta, J.D. Rothstein, L.J. Martin, Glutamate transporter protein
plasticity of cerebellar astrocytes among six different subtypes are expressed differentially during rat CNS development, J. regional astrocytes in response to cAMP treatment (this Neurosci. 17 (1997) 8363–8375.
study) as well as isoproterenol treatment and serum [13] J.E. Goldman, B. Abramson, Cyclic AMP-induced shape changes of astrocytes are accompanied by rapid depolymerization of actin,
deprivation [45] may relate to the dynamic developmental
Brain Res. 528 (1990) 189–196.
stage of cerebellum in neonates. This speculation is also
[14] M. Hatten, Neuronal regulation of astroglial morphology and
consistent with the observation that hypothalamic as- proliferation in vitro, J. Cell Biol. 100 (1985) 384–396.
trocytes are the least sensitive astrocytes to cAMP treat- [15] M.E. Hatten, R.K.H. Liem, M.L. Shelanski, C.A. Mason, Astroglia
ment, as hypothalamus is a well-matured brain region in CNS injury, Glia 4 (1991) 233–243.
[16] N. Hawrylak, J.C. Fleming, A.K. Salm, Dehydration and rehydration
before birth [18]. The permissive role of astrocytic
mor-selectively and reversibly alter glial fibrillary acidic protein
im-phological plasticity to cerebellar development is a new
munoreactivity in the rat supraoptic nucleus and subjacent glial
concept and needs to be tested further. Finally, as the limitans, Glia 22 (1998) 260–271.
conclusion of the present study is based on primary [17] E. Hosli, L. Hosli, Autoradiographic studies on the uptake of
astrocyte cultures in vitro, it will be necessary in the future 3H-dopamine by neurons and astrocytes in explant and primary cultures of rat CNS: effects of uptake inhibitors, Int. J. Dev.
to examine regional heterogeneity of neonatal astrocyte
Neurosci. 15 (1997) 45–53.
morphology changes in vivo in response to cAMP
stimula-[18] J.D. Ifft, An autoradiographic study of the time of final division of
tion. neurons in rat hypothalamic nuclei, J. Comp. Neur. 144 (1972)
193–204.
[19] H.K. Kimelberg, S. Narumi, R.S. Bourke, Enzymatic and mor-phological properties of primary astrocyte cultures, and enzyme
Acknowledgements
development in vivo, Brain Res. 153 (1978) 55–77.
[20] S.H. Lee, W.T. Kim, A.H. Cornell-Bell, H. Sontheimer, Astrocytes
We would like to thank Ms. Martha Yeager for the exhibit regional specificity in gap-junction coupling, Glia 11 (1994) 315–325.
secretarial assistance and Dr. David G. Warnock for his
[21] H. Lux, U. Heinemann, I. Dietzel, Ionic changes and alterations in
continuous support and encouragement. This work was
the size of the extracellular space during epileptic activity, Adv.
supported by grants from the NIH (NS-34877) and
Epi-Neurol. 44 (1986) 619–639.
lepsy Foundation of America. [22] S. Matsutani, N. Yamamoto, Neuronal regulation of astrocyte
morphology in vitro is mediated by GABAergic signaling, Glia 20 (1997) 1–9.
[23] M.K. McMillian, L. Thai, J.-S. Hong, J.P. O’Callaghan, K.R.
References Pennypacker, Brain injury in a dish: a model for reactive gliosis,
TINS 17 (1994) 138–142.
[1] K. Abe, H. Saito, L-Glutamate suppresses amyloid b-protein-in- [24] R.H. Miller, V. Szigeti, Clonal analysis of astrocyte diversity in duced stellation of cultured rat cortical astrocytes, J. Neurochem. 74 neonatal rat spinal cord cultures, Development 113 (1991) 353–362. (2000) 280–286. [25] D. Monaghan, R. Bridges, C. Cotman, The excitatory amino acid [2] J. Altman, Postnatal development of the cerebellar cortex in the rat, receptors: Their classes, pharmacology, and distinct properties in the J. Comp. Neur. 145 (1972) 353–398. function of the central nervous system, Annu. Rev. Pharmacol. [3] R.H. Amundson, S.K. Goderie, H.K. Kimelberg, Uptake of Toxicol. 29 (1989) 365–402.
3 3
on the morphology and some enzyme activities of primary mono- neurotoxic potency of glutamate agonists in cerebral cortex in layer cultures from rat brain, J. Neurochem. 31 (1978) 1479–1490. dissociated cell culture, J. Neurosci. 12 (1992) 56–61.
1
[28] Y. Oh, S.G. Waxman, Differential Na channelb1 subunit mRNA [37] J. Rothstein, M. Dykes-Hoberg, C. Pardo, L. Bristol, R. Kunci, Y. expression in stellate and flat astrocytes cultured from rat cortex and Kanai, M. Hediger, Knockout of glutamate transporters reveals a cerebellum: a combined in situ hybridization and immunocytoch- major role for astroglial transport in excitotoxicity and clearance of emistry study, Glia 13 (1995) 166–173. glutamate, Neuron 16 (1996) 675–686.
[29] Y. Oh, S.G. Waxman, Novel splice variants of the voltage-sensitive [38] A.K. Salm, K.G. Smithson, G.I. Hatton, Lactation-associated redis-sodium channel alpha subunit, NeuroReport 9 (1998) 1267–1272. tribution of the glial fibrillary acidic protein within the supraoptic [30] J. Padmanabhan, D. Clayton, M.L. Shelanski, Dibutyryl cyclic nucleus, Cell Tiss. Res. 242 (1985) 9–15.
AMP-induced process formation in astrocytes is associated with a [39] W. Shain, D.S. Forman, V. Madelian, J. Turner, Morphology of decrease in tyrosine phosphorylation of focal adhesion kinase and astroglial cells is controlled by beta-adrenergic receptors, J. Cell paxillin, J. Neurobiol. 39 (1999) 407–422. Biol. 105 (1987) 2307–2314.
[31] S. Paul, S. Das, R. Poddar, P.K. Sarkar, Role of thyroid hormone in [40] Y. Shao, M.O.K. Enkvist, K.D. McCarthy, Glutamate blocks as-the morphological differentiation and maturation of astrocytes: troglial stellation: effect of glutamate uptake and volume changes, temporal correlation with synthesis and organization of actin, Euro. Glia 11 (1994) 1–10.
J. Neurosci. 8 (1996) 2361–2370. [41] R. Swanson, J. Liu, J. Miller, J. Rothstein, J. Farrell, B. Stein, M. [32] M. Perkny, C. Eliasson, C.-L. Chien, L.G. Kindblom, L. R, A. Longuemare, Neuronal regulation of glutamate transporter subtype
Hamberger, C. Betsholtz, GFAP-deficient astrocytes are capable of expression in astrocyts, J. Neurosci. 17 (1997) 932–940.
stellation in vitro when cocultured with neurons and exhibit a [42] E.A. Tallant, J.T. Higson, Angiotensin II activates distinct signal reduced amount of intermediate filaments and an increased cell transduction pathways in astrocytes isolated from neonatal rat brain, saturation density, Exp. Cell. Res. 239 (1998) 332–343. Glia 19 (1997) 333–342.
[33] C.J. Pike, B.J. Cummings, R. Monzavi, C.W. Cotman,b-Amyloid- [43] C.D. Toran-Allerand, W. Bentham, R.C. Miranda, J.P. Anderson, induced changes in cultured astrocytes parallel reactive astrocytosis Insulin influences astroglial morphology and glial fibrillary acidic associated with senile plaques in Alzheimer’s disease, Neurosci. 63 protein (GFAP) expression in organotypic cultures, Brain Res. 558
(1994) 517–531. (1991) 296–304.
[34] K. Ramsell, B.-G. Zhao, D. Baker, P. Cobbett, Serum modulates [44] P.J. Voisin, J.M. Girault, J. Labouesse, O.M. Viratelle,b-Adrenergic cyclic AMP-dependent morphological changes in cultured neuro- receptors of cerebellar astrocytes in culture: intact cells versus hypophysial astrocytes, Brain. Res. Bull. 39 (1996) 109–114. membrane preparation, Brain Res. 404 (1987) 65–79.