T:
Toxicol
og
y
&
Chemical
Fo
od
Safet
y
Detection of Warfare Agents in Liquid Foods
Using the Brine Shrimp Lethality Assay
Stephen E. Lumor, Francisco Diez-Gonzalez, and Theodore P. Labuza
Abstract: The brine shrimp lethality assay (BSLA) was used for rapid and non-specific detection of biological and chemical warfare agents at concentrations considerably below that which will cause harm to humans. Warfare agents detected include T-2 toxin, trimethylsilyl cyanide, and commercially available pesticides such as dichlorvos, diazinon, dursban, malathion, and parathion. The assay was performed by introducing 50µL of milk or orange juice contaminated with each analyte into vials containing 10 freshly hatched brine shrimp nauplii in seawater. This was incubated at 28◦C for 24 h, after which mortality was determined. Mortality was converted to probits and the LC50was determined for each analyte by plotting probits of mortality against analyte concentration (log10). Our findings were the following: (1) the lethal effects of toxins dissolved in milk were observed, with T-2 toxin being the most lethal and malathion being the least, (2) except for parathion, the dosage (based on LC50) of analyte in a cup of milk (200 mL) consumed by a 6-y-old (20 kg) was less than the respective published rat LD50values, and (3) the BSLA was only suitable for detecting toxins dissolved in orange juice if incubation time was reduced to 6 h. Our results support the application of the BSLA for routine, rapid, and non-specific prescreening of liquid foods for possible sabotage by an employee or an intentional bioterrorist act.
Keywords: brine shrimp lethality assay, mortality, pesticides, toxins, warfare agents
Practical Application: The findings of this study strongly indicate that the brine shrimp lethality assay can be adapted for nonspecific detection of warfare agents or toxins in food at any point during food production and distribution.
Introduction
Bioterrorism is regarded as a possible threat to the food supply mostly due to the ease with which biological and chemical agents can be acquired, and the vulnerabilities along the food supply chain. Protection of consumers from this threat depends largely on timely detection of these agents in the event of an attack on the food supply. Several analytical methods based on chromatography, mass spectrometry, nuclear magnetic resonance, and immunode-tection are currently in use to detect the presence of specific agents or toxins. However, the wide diversity of toxic compounds poses a challenge to timely detection, and it is infeasible to use several spe-cific methods to adequately protect consumers on a routine basis. Moreover, most of these methods would be extremely expensive if used on a routine basis. The Dept. of Homeland Security has called for the development of novel, rapid, and nonspecific meth-ods for the detection of toxic compounds before the intentionally adulterated food enters the retail chain. These methods would also be suitable for routine prescreening of food products for possible sabotage by a disgruntled employee.
The brine shrimp lethality assay (BSLA) is a rapid nonspecific assay that has found use for preliminary assessment of acute tox-icity in pharmaceutical and toxicology investigations. It uses the
MS 20100662 Submitted 6/14/2010, Accepted 10/28/2010. Authors are with Dept. of Food Science and Nutrition, Univ. of Minnesota, 1334 Eckles Ave., Saint Paul, MN 55108, U.S.A. Direct inquiries to author Labuza (E-mail: tplabuza@ umn.edu).
mortality of brine shrimp nauplii in the presence of toxins or bioactive compounds as a measure of toxicity. It was proposed by Michael and others (1956) and has been utilized for the detec-tion of fungal toxins (Harwig and Scott 1971; Harwig and others 1979; Kumarasamy 2003), plant extract bioactivity (Meyer and others 1982; McLaughlin and others 1991; Pisutthanan and oth-ers 2004), cyanobacterial toxin (Metcalf and othoth-ers 2002), and pesticides (Barahona and Sanchez-Fortun 1999). It has also been used for the assessment of pharmacological activity of marine ex-tracts (Carballo and others 2002) as well as toxicity of heavy metals in the marine environment (Saliba and Krzyz 1976; MacRae and Pandey 1991; Martinez and others 1999). The underlying princi-ple of the BSLA is that the dosage or concentration of a chemical that may be therapeutic in humans would be toxic to the brine shrimp because of its small size. Thus the test would indicate the presence of a chemical that may be harmful to humans in a higher dosage. However, this method has rarely been used for the detection of chemical contaminants in processed foods or food systems.
The objective of this study was to determine relative toxicities of toxic chemicals dissolved in whole milk and orange juice to brine shrimp by calculating LC50 (concentration of analyte that kills half of the shrimp population) and comparing results to those of toxins dissolved in dimethyl sulfoxide (DMSO). Compounds screened in this study included T-2 toxin, trimethylsilyl cyanide, and pesticides such as malathion, parathion, dichlorvos, diazinon, and dursban. Milk was chosen as a key food because of the high vulnerability in the production chain (Wein and Liu 2005; Lui and Wein 2008; Poore 2010) while orange juice was chosen for its
C
2010 Institute of Food TechnologistsR
T16 Journal of Food Science rVol. 76, Nr. 1, 2011 doi: 10.1111/j.1750-3841.2010.01966.x
T:
Toxicol
og
y
&
Chemical
Fo
od
Safet
y
Detection of warfare agents in foods . . .
acidic pH. Since many of these compounds are poorly soluble in water, they were dissolved in DMSO to effect their easy dispersion into water, milk, and orange juice.
Materials and Methods
Brine shrimp lethality assay
Brine shrimp eggs were purchased from Fisher Scientific Co. (Pittsburgh, Pa., U.S.A.) while seawater, DMSO chemical toxins were purchased from Sigma-Aldrich Inc. (St. Louis, Mo., U.S.A.). Brine shrimp eggs were hatched in seawater at 28◦C and used after 48 h. The analytes (toxins) were prepared by dissolution in DMSO after which they were introduced into whole milk and orange juice. The assays were performed in triplicate by introducing 50µL of analyte (dissolved in DMSO, milk, or juice) into vials containing 10 freshly hatched (48 h) brine shrimp nauplii. The volumes in the vials were then made up to 5 mL with seawater, and the setups
incubated at 28 ◦C. Mortality (%) was determined after 24 h. Depending on the medium in which the analyte was dissolved in, 50µL of DMSO, milk, or orange juice alone were used as the control.
Processing of results
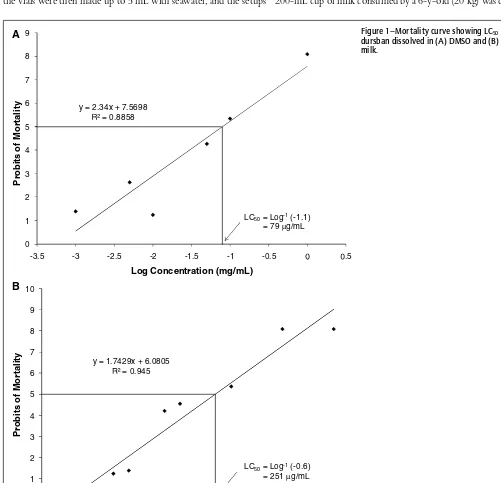
Brine shrimp mortality (%) was converted to probits by using Finney’s probit analysis table (Finney 1952), and a plot of probits (average of 3 determinations) vs log concentration was made. A probit of 8.09 corresponds to 100% mortality, and the concen-tration of analyte that corresponds to a probit of 5 is the LC50 (concentration of analyte in the 50µL sample [µg/mL] that kills half the shrimp population). Probit analysis is a type of regres-sion used to transform a sigmoid binomial dose–response curve into a linear distribution. Using the LC50 value, the hypotheti-cal dosage (mg/kg body weight) of each analyte contained in a 200-mL cup of milk consumed by a 6-y-old (20 kg) was calculated
y = 2.34x + 7.5698 = 0.8858
0 1 2 3 4 5 6 7 8 9
-3.5 -3 -2.5 -2 -1.5 -1 -0.5 .5
P
robit
s
of
M
o
rt
a
lit
y
Log Concentration (mg/mL)
LC50= Log-1(-1.1)
= 79 µg/mL
y = 1.7429x + 6.0805 = 0.945
0 1 2 3 4 5 6 7 8 9 10
-4 -3 -2 -1 2
P
robit
s
of
M
o
rt
a
lit
y
Log Concentration (mg/mL)
LC50= Log-1(-0.6)
= 251 µg/mL
A
B
Figure 1–Mortality curve showing LC50for
dursban dissolved in (A) DMSO and (B) whole milk.
T:
Detection of warfare agents in foods . . .
for each agent. These were compared to published LD50 values (determined in rats by oral administration) of the analytes.
Results and Discussion
Results of this investigation revealed the suitability of the brine shrimp lethality assay for nonspecific detection of analytes at very low concentrations. The pesticides, dichlorvos, dursban, parathion, malathion, and diazinon, as well as T-2 toxin and trimethylsilyl cyanide were screened for relative toxicities in whole milk. Figure 1 shows the mortality curve for dursban in DMSO (Figure 1a) and in whole milk (Figure 1b). LC50 values of the toxins screened are given in Table 1. Of the analytes screened, T-2 toxin was the most lethal whereas the least lethal was malathion (in DMSO). Except for trimethylsilyl cyanide, the LC50values for analytes dissolved in whole milk prior to the assay were slightly higher than values obtained for analytes dissolved in DMSO as might be expected. It is believed that the reduction in lethality of the analytes in milk was partially due to the nutrition provided to the shrimp by the milk components, whereas shrimp that had no access to milk, the control treatment, were more susceptible to the lethal effects of the toxins as indicated by the lower LC50 values of the analytes dissolved in DMSO. It also may be in part by the
Table 1– Concentration of toxic compounds needed to kill 50% of test shrimp (LC50values) in DMSO and whole milk.
LC50(µg/mL)
aLD50 bDosage in
(µg/kg cup of milk
body Whole (µg/kg body
Analyte weight) DMSO milk weight)
T-2 toxin 2700 4 5 50
Dichlorvos 17000 66 790 7900
Diazinon 696000 45 660 6600
Dursban 82000 79 251 2510
Malathion 290000 3019 13804 13804
Parathion 2000 631 724 7240
Trimethylsilyl cyanide Not available 860 670 6700
aLD
50values (rat, oral route) were obtained from the respective MSDS supplied by the
vendor (Sigma-Aldrich).
bHypothetical dosage of toxic compound in a cup (200 mL) of milk that would be
consumed by a 6-y-old (20 kg) based on the calculated LC50values.
better dispersion with DMSO as well as chemical interactions of the agents with milk components.
Based on the LC50values, the hypothetical dosage of analytes in a cup (200 mL) of milk consumed by a 6-y-old child of weight 20 kg was calculated for each toxin (Table 1). These were com-pared to published LD50values (lethal dosage of analyte that kills 50% of the population) of the respective toxins as determined in rats by oral administration. The LC50 for the pesticide durs-ban (in milk) was 251µg/mL. Thus, the dosage of dursban in a cup (200 mL) of milk consumed by a 6-y-old (20 kg) would be 2510µg/kg body weight, which is considerably lower than the LD50 value for dursban in rats (82000 µg/kg body weight) by the oral route (note that the LD50 values [rat, oral route] were obtained from the respective material safety data sheets [MSDS] supplied by the vendor [Sigma-Aldrich]). This difference was also true for the other analytes except for parathion, whose calculated dosage (7200 µg/kg body weight) in a cup of milk consumed by a 6-y-old child was higher than the presumed human LD50 (2000 µg/kg body weight). This is an indication that most of these analytes can be detected in milk at concentrations consider-ably below that which will cause harm in humans.
Results obtained for analytes dissolved in orange juice showed an unusual trend (Table 2). The LC50values were lower than those
Table 2– Concentration of toxic compounds needed to kill 50% of test shrimp (LC50 values) in DMSO and orange juice
(extrap-olated values).
Trimethylsilyl cyanide Not available 860 1.33×10−3 aLD
50values (rat, oral route) were obtained from the respective MSDS supplied by the
vendor (Sigma-Aldrich).
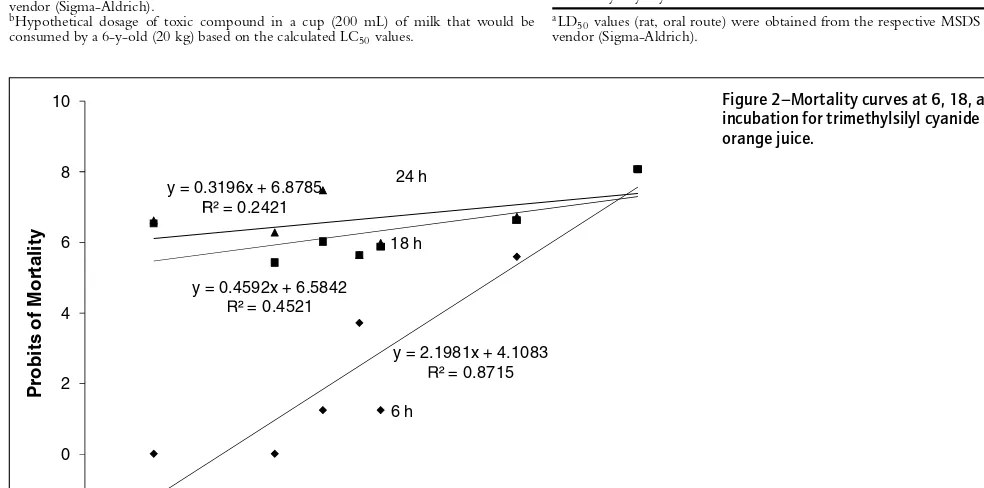
Figure 2–Mortality curves at 6, 18, and 24 h of incubation for trimethylsilyl cyanide dissolved in orange juice.
T:
Detection of warfare agents in foods . . .
obtained for toxins dissolved in DMSO, implying that toxins dis-solved in orange juice were more lethal than when disdis-solved in DMSO only. However, this observation is attributable to the com-bined lethal effects of the toxins and orange juice on the shrimp since unusually high levels of mortality was also observed in the control treatments. It would be tempting to attribute this obser-vation to a change in pH, but this is probably unlikely because the change in pH of seawater upon the introduction of analytes (in orange juice) was 1.04—from 7.92 to pH 6.88—which was probably not enough to cause or enhance lethality. However, this effect may be attributed to the minor components of orange juice since the brine shrimp has been reported to be sensitive to plant extracts containing flavonoids and other polyphenols (Nino and others 2006; Omale and Okafor 2008), which happen to be sig-nificantly present in orange juice (Rapisarda 1999).
To eliminate the enhanced lethal effect of orange juice, mortal-ity of brine shrimp to trimethylsilyl cyanide was observed after 6, 18, and 24 h of incubation (Figure 2). The slope of the mortal-ity curve at 6 h (R2=0.87) incubation was significantly steeper than those obtained at 18 and 24 h of incubation, which indicates that the effect of the analyte was more obvious at shorter incuba-tion times. Moreover, no mortalities were observed in the control treatments at 6 h. Therefore, reliable results can be obtained for toxins dissolved in orange juice if incubation time is significantly reduced to, for example, 6 h. This time-frame is sufficient for the detection of deliberate contaminations at any steps of orange juice processing since commercially packaged orange juice does not typically reach consumers within 6 h of the final processing steps.
This study also examined the effect of ricin and staphylococcal enterotoxin A (SEA) on brine shrimp, but results showed that the shrimp rather used ricin and SEA, both proteins, as sources of energy. The shrimp in the treatment group generally outlived those in the control group (no ricin or SEA) by 4 to 5 d. This is an indication that the BSLA may not be suitable for detecting protein-based biological toxins in food.
Conclusions
Our results support the potential of using the BSLA for rapid and nonspecific detection of warfare agents in whole milk and other liquid foods including bottled water as well as soft drinks at concentrations considerably lower than that which will cause harm to humans. The BSLA results based on 24 h of incubation were not reliable for toxins dissolved in orange juice. Reliable results were obtained when the time of incubation was reduced to 6 h. The sensitivity of this assay can be enhanced by reducing the total volume of the assay from 5 to 2.5 mL or less.
Acknowledgment
This study was funded by the Natl. Center for Food Protec-tion and Defense under DHS Science and Technology Assistance Agreement nr DHS-2007-ST-061-000003 awarded by the U.S. Dept. of Homeland Security. It has not been formally reviewed by DHS. The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the U.S. Dept. of Homeland Security. The Dept. of Homeland Security does not endorse any products or commercial services mentioned in this publication.
References
Barahona MV, Sanchez-Fortun S. 1999. Toxicity of carbamates to the brine shrimpArtemia salinaand the effect of atropine, BW284c51, iso-OMPA and 2-PAM on carbaryl toxicity. Env Pollut 104:469–76.
Carballo JS, Hernandez-Inda ZL, Perez P, Garcia-Gravalos MD. 2002. A comparison between two brine shrimp assays to detectin vitrocytotoxicity in marine natural products. BCM Biotechnol 2:17–21.
Finney, D. J. 1952. Probit analysis: a statistical treatment of the sigmoid response curve. 2nd ed. Cambridge, U.K.: Cambridge Univ. Press. 318 p.
Harwig J, Scott, PM. 1971. Brine shrimp (Artemia salinaL.) larvae as a screening system for fungal toxins. Appl Microbiol 21:1011–6.
Harwig J, Scott PM, Stoltz DR, Blanchfield BJ. 1979. Toxins of mold from decaying tomato fruit. Appl Environ Microbiol 38:267–74
Kumarasamy Y, Nahar L, Cox PJ, Jaspars M, Sarker SD. 2003. Bioactivity of secoiridoid glyco-sides fromCentaurium erythraea. Phytomed 10:344–47.
Lui Y, Wein L. 2008. Mathematically assessing the consequences of food terrorism scenarios. J Food Science 73; M346–53.
MacRae TH, Pandey AS. 1991. Effects of metals on early stages of the brine shrimp,Artemia: a developmental toxicity assay. Arch Environ Contam Toxicol 20:247–52.
Martinez M, Ramo JD, Torreblanca A, Diaz-Mayans J. 1999. Effect of cadmium exposure on zinc levels in the brine shrimpArtemia partenogenetica. Aquaculture 172:315–25.
McLaughlin JL, Chang CJ, Smith DL. 1991. Bench top bioassay for the discovery of bioactive natural products: an update. In: Rahman AU, editor. Studies in natural product chemistry. Amsterdam: Elsevier. p 383–409.
Metcalf JS, Lindsay J, Beattie KA, Birmingham S, Saker ML, Torokne AK, Codd GA. 2002. Toxicity of cylindrospermopsin to brine shrimpArtemia salina: comparison with protein synthesis inhibitors and microcystins. Toxicon 40:1115–20.
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. 1982. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 45: 31–4.
Michael AS, Thompson CG, Abramovitz M. 1956.Artemia salinaas a test organism for bioassay. Science 123:464.
Nino J, Narraez DM, Mosquera OS, Correa YM. 2006. Antibacterial, antifungal and cytotoxic activities of eightAsteraceaeand twoRubiacaeaplants from Colombian biodiversity. Braz J Microbiol 37:566–70.
Omale J, Okafor PN. 2008. Comparative antioxidant capacity, membrane stabilization, polyphe-nol composition and cytotoxicity of the leaf and stem ofCissus multistriata. African J Biotechnol 7:3129–33.
Pisutthanan S, Plianbangchan P, Pisutthanan N, Ruanruay S, Muanrit O. 2004. Brine shrimp lethality activity of Thai medicinal plants in the family Meliaceae. Naresuam Univ J 12:13–8. Poore D. 2010. Protecting your food supply: a practical approach [Internet]. Glendale, Calif.: Food Safety Magazine. Available from: http://www.foodsafetymagazine.com/article. asp?id=3536&s. Accessed 2010 June 01.
Rapisarda P, Tomaino A, Cascio RL, Bonina F, Pasquale AD, Saija A. 1999. Antioxidant effectiveness as influenced by phenolic content of orange juice. J Agric Food Chem 47:4718– 23.
Saliba LJ, Krzyz RM. 1976. Effect of heavy metals on the hatching of brine-shrimp eggs. Marine Poll Bull 7:181–2.
Wein LM, Liu Y. 2005. Analyzing a bioterror attack on the food supply: the case of botulnium toxin in milk. PNAS 102:9984–9.