Summary Winter CO2 gas exchange of the last three flushes of cembran pine (Pinus cembra L.) was studied under ambient conditions at the alpine timberline, an ecotone with strong seasonal changes in climate. During the coldest months of the year, December to March, gas exchange was almost completely suppressed and even the highest irradiances and temperatures did not cause a significant increase in net photosynthesis compared to spring and fall. In general, daily CO2 balance was negative between December and March except during ex-tended warm periods in late winter. However, because twig respiration was also reduced to a minimum during the Decem-ber--March period, daily carbon losses were minimal. Total measured carbon loss during the winter months was small, equalling the photosynthetic production of one to two warm days in spring or summer when average air temperature was above 6 °C.
Keywords: carbon balance, photosynthesis, respiration.
Introduction
Evergreen conifers are the most common woody plants at the alpine timberline, an ecotone with strong seasonal changes in climate. Short growing seasons alternate with resting periods. In a 10-year set of growth analysis experiments, Kronfuβ
(1994) showed that, at high altitudes, cembran pine (Pinus cembra L.) has an average extension growth period of about 110 days during the warm summer months of May to August. During the winter, low temperature is the main factor deter-mining the duration and intensity of dormancy (Havranek and Tranquillini 1995).
Seasonal variation in net photosynthesis and respiration at the alpine timberline has been attributed to the prevailing temperature. In the fall, frequent frosts cause a decline in net photosynthesis of cembran pine that does not recover until temperatures rise in spring (Cartellieri 1935, Pisek and Winkler 1958, Tranquillini 1959, 1979).
Despite the many studies on gas exchange in conifers, there have been few in situ measurements of gas exchange on mature trees during winter dormancy (Havranek and Tranquillini 1995). Gas exchange at the alpine timberline has been investi-gated using cut twigs under laboratory conditions (Cartellieri
1935, Pisek and Winkler 1958), but there is little information on seasonal variations in gas exchange of conifers at the alpine timberline, especially during the winter months. Therefore, I examined seasonal changes in gas exchange of a subalpine cembran pine in situ under field conditions and estimated its CO2 balance during the winter season.
Materials and methods
Study site and plant material
Measurements were made on a mature, 12-m high cembran pine tree growing in a podsol on a south-west slope at 1950 m a.s.l. near the Klimahaus Research Station on Mt. Patscherk-ofel (47° N, 11° E) near Innsbruck, Austria. Measurements were made from December 9, 1993 to April 21, 1994 and from November 2, 1994 to May 22, 1995, the beginning of bud break. The field site is characterized by a cool subalpine climate with low soil temperatures, a possibility of frost in all months and a continuous snow cover from October until May.
Climate and gas exchange measurements
An aluminum platform provided access to the top of the tree. An LI-190 PAR quantum sensor (Li-Cor, Inc., Lincoln, NE) and a Skye SKH 2013 air temperature/humidity sensor (Skye Instruments, Powys, U.K.) were installed on a horizontal alu-minum rod about 1 m to the side of the top of the tree. Soil temperatures were measured with copper constantan thermo-couples at depths of 2, 15 and 50 cm.
The CO2 gas exchange of the last three flushes was meas-ured continuously by means of a temperature-controlled cham-ber (Walz, Effeltrich, Germany) on sun-exposed branches approximately 2 m from the top of the tree. Temperature in the chamber was controlled and followed ambient air temperature. Atmospheric humidity inside the chamber was monitored with a capacitive humidity sensor (Vaisala, Helsinki, Finland). All of the pneumatic tubing was heated and insulated. The shoot in the chamber was changed every 7 to 8 weeks. Snow was not removed artificially from the chamber after snow fall.
Actual needle temperature on the sun-exposed side was measured with copper constantan thermocouples, one inside the chamber and one outside. Thermocouple junctions, 0.1 mm
Carbon dioxide gas exchange of cembran pine (
Pinus cembra
) at the
alpine timberline during winter
GERHARD WIESER
Forstliche Bundesversuchsanstalt, Rennweg 1, A-6020 Innsbruck, Austria
Received March 7, 1996
in diameter, were formed into soldered loops of approximately 1.5 mm diameter, slipped over the needles and gently crimped in place. Copper constantan thermocouples were also used to measure stem temperatures 5 cm below the stem surface, 1.5 m aboveground.
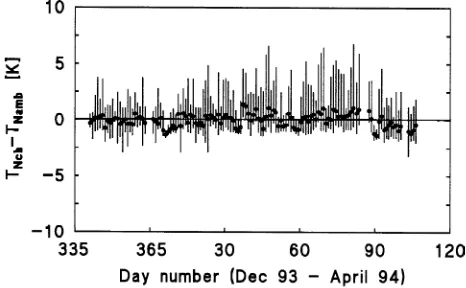
During winter, the chamber tracked ambient conditions fairly well. The mean temperature difference between needles inside and outside the chamber was close to zero (Figure 1). Mean maximum overheating of needles in the chamber was 2.3 °K and mean maximum undercooling was −1.1 °K com-pared to needles outside the chamber; and the absolute maxi-mum difference was +6.8 °K and −3.2 °K, respectively (Figure 1). The observed differences in temperature between needles inside the chamber and needles outside the chamber were within the range found in pine needles within one branch (author’s unpublished observations).
All data were recorded with a Campbell CR10 data logger (Campbell Scientific Ltd., Shepshed, U.K.), programed to re-cord the 10-minute means of measurements taken every min-ute. All CO2 gas exchange parameters were calculated according to von Caemmerer and Farquhar (1981) and related to needle dry weight. Specific leaf area was 61.3 cm2 gdw−1.
Analysis of data was based on half-hour means.
Results
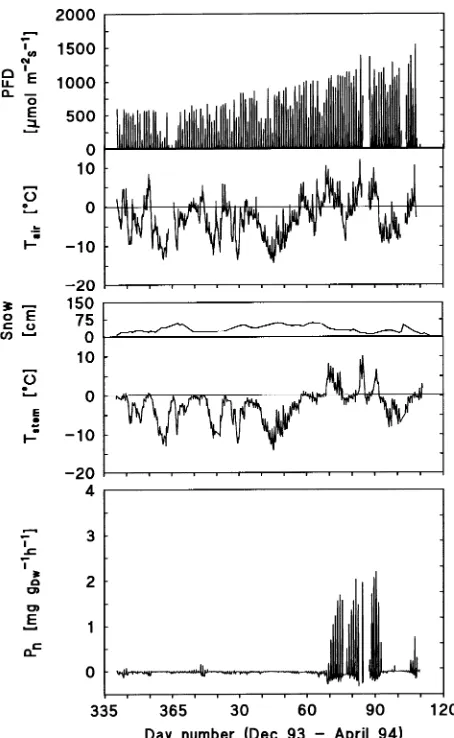
Although the winter climate at the alpine timberline on Mt. Patscherkofel is characterized by low temperatures, air tem-peratures did not remain continuously below 0 °C in any winter month, in either 1993--94 or 1994--95 (Figure 2, and also see Figure 7). The lowest half-hour mean temperature recorded during the study was −19.2 °C on January 5, 1995 and the highest half-hour mean temperature recorded was 14.8 °C on May 6, 1995 (Figure 2). In both winters, snow cover persisted from early December until the end of April. Under the snow, soil temperatures in the top 2-cm layer were always above
−3.8 °C, and at depths below 15 cm soil temperatures never dropped below 0.2 °C.
During the winter, net photosynthesis and nighttime respira-tion were almost completely suppressed (Figure 2). The de-cline in CO2 gas exchange started in November as a consequence of shorter days, lower irradiance and near-freez-ing temperatures. Recovery of photosynthesis began in sprnear-freez-ing in response to the diminishing occurrence of frost and higher air and stem temperatures (Figure 2).
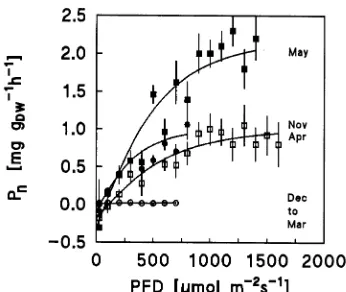
Of all environmental variables examined, daily maximum net photosynthesis was best correlated with minimum stem temperature of the previous night, in both the fall and spring (Figure 3). Seasonal changes in CO2 gas exchange were corre-lated with ambient light and temperature conditions at the timberline site. In general, photosynthetic rates were negli-gible during the winter months (December to March) even at the highest irradiances (Figure 4) and temperatures (Figure 5) when compared with photosynthetic rates during the spring and fall. Furthermore, at a given temperature, respiration dur-ing the night was substantially lower in winter than in sprdur-ing and autumn, indicating a decline in total respiration rate to the maintenance rate (Figure 6).
Metabolic activity increased during long-lasting warm periods in late winter. For example, in March 1994, which was unusu-ally mild, respiration increased and net photosynthetic rates reached up to 30% of summer values (Figure 7) during a period Figure 1. Observed daily mean and range in the difference between the
temperature of cembran pine needles inside (TNch) and outside (TNamb) the gas exchange chamber from December 9, 1993 to April 21, 1994.
when maximum daily air temperatures were high and mini-mum night temperatures were above zero.
Monthly photosynthetic production was negative from De-cember through February in both years. Although the period of negative photosynthetic production normally extended to April (e.g., for the winter 1994--1995 (Figure 2)), photosyn-thetic production was positive for the unusually mild month of March 1994 (Table 1). In general, total carbon loss of the foliage during the winter was less than 32 mg gdw−1.
Discussion
Variation in net photosynthesis of cembran pine tracked sea-sonal changes in climate at the alpine timberline. In late autumn and early winter, CO2 exchange permanently
de-creased. This decline coincided with shorter days and tempera-tures below 0 °C. These same factors also cause the develop-ment of frost resistance and cellular alterations during pre-dor-mancy (Holzer 1958, Senser and Beck 1979, Havranek and Tranquillini 1995). Near-freezing temperatures during frost hardening of pines alter chlorophyll organization (Öquist and Strand 1986) as well as pigment composition (Ottander et al. 1995) and chlorophyll fluorescence (Nagele 1989, Ottander et al. 1995, Westin et al. 1995).
Several studies have shown that short periods of apparently favorable conditions following a severe frost do not result in CO2 uptake in trees at the alpine timberline (Tranquillini 1957, Pisek and Winkler 1958, Tranquillini 1979), leading Tran-quillini (1979) to conclude that winter dormancy in trees at the alpine timberline is particularly rigid and long lasting. Al-Figure 4. Means of net photosynthesis (Pn) at different ranges of
irradiance (PFD) for current-year to two-year-old needles of cembran pine. Data were selected according to the following climatic condi-tions: vapor pressure deficit ≤ 8 Pa kPa−1; air temperature ≥ 5 °C in November, April, May, but ≥ 2 °C during December to March. Each data point is the mean of 10 to 50 half-hour mean values based on diurnal courses during the investigation period 1994--1995. Vertical bars represent the standard error of the mean.
Figure 5. Means of net photosynthesis (Pn) at different ranges of air temperatures (T) for current-year to two-year-old needles of cembran pine. Data were selected according to the following climatic condi-tions: vapor pressure deficit ≤ 8 Pa kPa−1, photon flux density ≥ 500
µmol m−2 s−1. Each data point is the mean of 10 to 50 half-hour mean values based on diurnal courses during the investigation period 1994--1995. Vertical bars represent the standard error of the mean.
Figure 6. Means rates of nighttime respiration (Rnight) over different ranges of air temperatures (T) for current-year to two-year-old needles of cembran pine. Each data point is the mean of 20 to 80 half-hour mean values based on diurnal courses during the investigation period 1994--1995. Vertical bars represent the standard error of the mean. Figure 3. Maximum daily net photosynthesis (Pn,max) in relation to the
though daily CO2 balance was negative between December and March, I observed an increase in gas exchange, with photosynthetic rates reaching up to 30% of summer values
during an extended warm period in March 1994 when mini-mum air temperatures were above zero, and soil, stem and branches were not frozen. Similarly, in February 1993 at the same study site, I measured photosynthetic rates in Norway spruce needles that increased as much as 15% of summer values following several days of thaw with nighttime tempera-tures just below freezing (author’s unpublished observations). In central alpine valleys, where the duration of the winter suppression in gas exchange is shorter than at high altitudes, winter photosynthetic rates of up to 30 and 50% of summer values have been recorded in Norway spruce (Zöschg 1988) and Scots pine (Meusburger 1988), respectively. Positive net photosynthesis during favorable days in midwinter has also been shown for Scots pine in central Sweden at 150 m a.s.l (Troeng and Linder 1982) as well as in young red spruce grown in Vermont at 104 m a.s.l. (Schaberg et al. 1995). However, when branches were transferred from the field to optimum conditions in the laboratory during the winter, photosynthetic capacity did not recover to summer values (Pisek and Winkler 1958, Schwarz 1971), suggesting that photosynthetic capacity during winter is suppressed not only by low temperatures but also by an endogenous factor that is not lost when the branches are excised. This suggestion is consistent with chlorophyll fluorescence measurements (Nagele 1989).
The transition to winter dormancy was accompanied by a change in the temperature response of respiration. At a given ambient temperature, twig respiration was substantially lower during winter than in spring and autumn, and thus reduced carbon loss during the long subalpine winter. Similarly, stem respiration of cembran pine was also found to be low during winter compared with summer values (Havranek 1981). How-ever, in May, when buds began to expand and were about 1.5 cm in length, there was an increase in twig respiration probably because of a strong demand for carbohydrates in the developing buds (cf. Sprugel et al. 1995).
At the alpine timberline, winter dormancy begins to break in spring. Full recovery of photosynthesis occurred after the air and stem temperatures had increased and the aboveground tissue had thawed. Furthermore, in spring, a reorganization of cell structures (Holzer 1958, Senser and Beck 1979) and pig-ment composition (Ottander et al. 1995) has been observed.
At the alpine timberline, monthly carbon balance of cem-bran pine twigs was negative for three to four months. The total measured carbon loss of the twigs during the winter months was only 22--31 mg CO2 per gram needle dry weight, which is equal to the photosynthetic production of one to two warm days in summer or spring, when average air temperature is above 6 °C. This estimate of total carbon loss was more than 10 times lower than the value determined for cembran pine by Tranquillini (1959). However, Tranquillini’s estimate was based on the temperature respiration relationship measured during the summer, which would be equivalent to the tempera-ture respiration relationship estimated for May in this study, which was significantly displaced on the y-axis above values measured during the winter. The measured CO2 balance of cembran pine was also less than the value of 140 mg CO2 per gram of photosynthetically active tissue calculated for bristle-Table 1. Monthly carbon balance (mg gdw−1) of the last three flushes
of cembran pine during the winter of 1993--1994 and 1994--1995.
Month 1993--1994 1994--1995
November -- 43.80
December −11.90 −5.88
January −10.42 −6.62
February −8.74 −1.75
March 93.00 −8.18
April 41.76 72.72
May -- 351.17
cone pine on the basis of gas exchange rates measured under controlled temperatures in the field and temperature data (Schulze et al. 1967).
During the leafless period from October to April, whole-tree respiration of a European larch at the timberline was calculated to be only 2.3% of its annual photosynthetic carbon gain (Havranek and Tranquillini 1995). Similarly, continuous gas exchange measurements during winter in Switzerland demon-strated that the monthly CO2 balance of mature Norway spruce shoots was slightly negative between December and March in trees growing at 1600 m a.s.l., whereas it was positive in trees growing in the valley in all months (Häsler 1991). Even in the boreal zone, CO2 balance of Scots pine was negative only from December to February (Troeng and Linder 1982).
In conclusion, in response to the long and severe winters at the alpine timberline, CO2 gas exchange and twig respiration of forest trees were severely reduced, presumably reflecting an acclimation to the low temperatures that are characteristic of the region. Although winter dormancy is reported to be par-ticularly rigid and long lasting (Tranquillini 1979), I observed that net photosynthesis can become significant during ex-tended warm periods in late winter. Total carbon loss by the foliage throughout the winter was minimal, equalling the pho-tosynthetic production of one to two warm days in spring or summer. I conclude that these adaptations of gas exchange contribute to the survival of conifers at the alpine timberline.
Acknowledgment
I thank Th. Gigele for excellent technical assistance during both winters of investigation.
References
Cartellieri, E. 1935. Jahresgang von osmotischem Wert, Transpiration und Assimilation einiger Ericaceen der alpinen Zwergstrauchheide und von Pinus cembra. Jahrb. f. wiss. Bot. 82:460--506.
Häsler, R. 1991. Vergleich der Gaswechselmessungen der drei Jahre (Juli 1986--Juni 1989). In Luftschadstoffe und Wald. Ed. M. Stark. Verlag der Fachvereine, Zürich, pp 177--184.
Havranek, W.M. 1981. Stammatmung, Dickenwachstum und Photo-synthese einer Zirbe (Pinus cembra L.) an der Waldgrenze. Mitt. Forstl. Bundesversuchsanst. Wien 142:443--467.
Havranek, W.M. and W. Tranquillini. 1995. Physiological processes during winter dormancy and their ecological significance. In Eco-physiology of Coniferous Forests. Eds. W.K. Smith and T.M. Hinckley. Academic Press, San Diego, pp 95--124.
Holzer, H. 1958. Die winterlichen Veränderungen der Assimilation-szellen von Zirbe (Pinus cembra L.) und Fichte (Picea excelsa Link) an der alpinen Waldgrenze. Österr. Bot. Z. 105:323--346. Kronfuβ, H. 1994. Der Einfluβ der Lufttemperatur auf das
Höhen-wachstum der Zirbe. Centralbl. Gesamte Forstwes. 111:165--181.
Meusburger, A. 1988. Der winterliche Gaswechsel der Kiefer in inner-alpinen Tallagen. Diplomarbeit Botanik, Univ. Innsbruck, Innsbruck, Austria, 129 p.
Nagele, M. 1989. Winterliche Veränderungen der Photosyntheseak-tivität ausgewählter Holzpflanzen. Ph.D. Thesis, Univ. Innsbruck, Innsbruck, Austria, 148 p.
Öquist, G. and M. Strand. 1986. Effects of frost hardening on quantum yield, chlorophyll organisation and energy distribution between two photosystems in Scots pine. Can. J. Bot. 64:748--753.
Ottander, C., D. Campbell and G. Öquist. 1995. Seasonal changes in photosystem II organisation and pigment composition in Pinus sylvestris. Planta 197:176--183.
Pisek, A. and E. Winkler. 1958. Assimilationsvermögen und Respira-tion der Fichte (Picea excelsa Link) in verschiedener Höhenlage und der Zirbe (Pinus cembra L.) an der Waldgrenze. Planta 51:518--543.
Schaberg, P.G., R.C. Wilkinson, J.B. Shane, J.R. Donelly and P.F. Cali. 1995. Winter photosynthesis of red spruce from three Vermont seed sources. Tree Physiol. 15:345--350.
Schulze, E.-D., H.A. Mooney and E.L. Dunn. 1967. Wintertime pho-tosynthesis of bristlecone pine (Pinus aristata) in the White Moun-tains of California. Ecology 48:1044--1047.
Schwarz, W. 1971. Das Photosynthesevermögen einiger immergrüner während des Winters und seine Reaktivierungsgeschwindigkeit nach schärferen Frösten. Ber. Dtsch. Bot. Ges. 84:585--594. Senser, M. and E. Beck. 1979. Kälteresistenz der Fichte. II. Einfluβ
von Photoperiode und Temperatur auf die Struktur und photo-chemischen Reaktionen von Chloroplasten. Ber. Dtsch. Bot. Ges. 92:243--259.
Sprugel, D.G., M.G. Ryan, J.R. Brooks, K.A. Vogt and T.A. Martin. 1995. Respiration from the organ level to the stand. In Resource Physiology of Conifers. Eds. W.K. Smith and T.M. Hinckley. Aca-demic Press, San Diego, pp 255--299.
Tranquillini, W. 1957. Standortsklima, Wasserbilanz und CO2 -Gaswechsel junger Zirben (Pinus cembra L.) an der alpinen Waldgrenze. Planta 49:612--661.
Tranquillini, W. 1959. Die Stoffproduktion der Zirbe (Pinus cem-bra L.) an der Waldgrenze während eines Jahres. Planta 54:107--151.
Tranquillini, W. 1979. Physiological ecology of the alpine timberline. Ecological Studies, Vol 31. Springer-Verlag, Berlin, Heidelberg, New York, Tokyo, 137 p.
Troeng, E. and S. Linder. 1982. Gas exchange in a 20-year-old stand of Scots pine. I. Net photosynthesis of current and one-year-old shoots within and between seasons. Physiol. Plant. 54:7--14. von Caemmerer, S. and G.D. Farquhar. 1981. Some relationships
between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376--387.
Westin, J., L.G. Sunblad and J.E. Hellgren. 1995. Seasonal variation in photochemical activity and hardiness in clones of Norway spruce (Picea abies). Tree Physiol. 15:685--693.