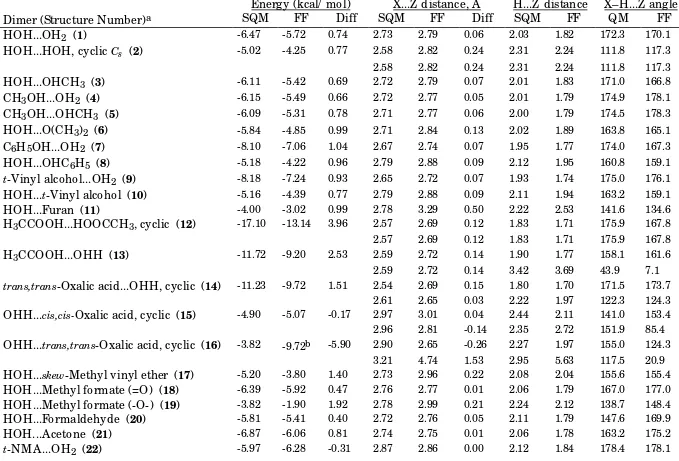
Table B-I. _____________________________________________________________________________
Consensus BCI vs. Scaled HF/-6-31G* Intermolecular-Interaction Energies and Distances.Energy (kcal/ mol)
X...Z distance, Å
H...Z distance
X–H...Z angle
Dimer (Structure Number)
aSQM
FF
Diff
SQM
FF
Diff
SQM
FF
QM
FF
HOH...OH
2(
1)
-6.47 -5.72 0.74 2.73 2.79 0.06 2.03 1.82 172.3 170.1HOH...HOH, cyclic
Cs(
2)
-5.02 -4.25 0.77 2.58 2.82 0.24 2.31 2.24 111.8 117.32.58 2.82 0.24 2.31 2.24 111.8 117.3
HOH...OHCH
3(
3)
-6.11 -5.42 0.69 2.72 2.79 0.07 2.01 1.83 171.0 166.8CH
3OH...OH
2(
4)
-6.15 -5.49 0.66 2.72 2.77 0.05 2.01 1.79 174.9 178.1CH
3OH...OHCH
3(
5)
-6.09 -5.31 0.78 2.71 2.77 0.06 2.00 1.79 174.5 178.3HOH...O(CH
3)
2(
6)
-5.84 -4.85 0.99 2.71 2.84 0.13 2.02 1.89 163.8 165.1C
6H
5OH...OH
2(
7)
-8.10 -7.06 1.04 2.67 2.74 0.07 1.95 1.77 174.0 167.3HOH...OHC
6H
5(
8)
-5.18 -4.22 0.96 2.79 2.88 0.09 2.12 1.95 160.8 159.1t
-Vinyl alcohol...OH
2(
9)
-8.18 -7.24 0.93 2.65 2.72 0.07 1.93 1.74 175.0 176.1HOH...
t-Vinyl alcohol (
10)
-5.16 -4.39 0.77 2.79 2.88 0.09 2.11 1.94 163.2 159.1HOH...Furan (
11)
-4.00 -3.02 0.99 2.78 3.29 0.50 2.22 2.53 141.6 134.6H
3CCOOH...HOOCCH
3, cyclic (
12)
-17.10 -13.14 3.96 2.57 2.69 0.12 1.83 1.71 175.9 167.82.57 2.69 0.12 1.83 1.71 175.9 167.8
H
3CCOOH...OHH (
13)
-11.72 -9.20 2.53 2.59 2.72 0.14 1.90 1.77 158.1 161.62.59 2.72 0.14 3.42 3.69 43.9 7.1
trans,trans
-Oxalic acid...OHH, cyclic (
14)
-11.23 -9.72 1.51 2.54 2.69 0.15 1.80 1.70 171.5 173.72.61 2.65 0.03 2.22 1.97 122.3 124.3
OHH...
cis,cis-Oxalic acid, cyclic (
15)
-4.90 -5.07 -0.17 2.97 3.01 0.04 2.44 2.11 141.0 153.4 2.96 2.81 -0.14 2.35 2.72 151.9 85.4OHH...
trans,trans-Oxalic acid, cyclic (
16)
-3.82 -9.72b -5.90 2.90 2.65 -0.26 2.27 1.97 155.0 124.3 3.21 4.74 1.53 2.95 5.63 117.5 20.9HOH...
skew-Methyl vinyl ether (
17)
-5.20 -3.80 1.40 2.73 2.96 0.22 2.08 2.04 155.6 155.4HOH...Methyl formate (=O) (
18)
-6.39 -5.92 0.47 2.76 2.77 0.01 2.06 1.79 167.0 177.0HOH...Methyl formate (-O-) (
19)
-3.82 -1.90 1.92 2.78 2.99 0.21 2.24 2.12 138.7 148.4HOH...Formaldehyde (
20)
-5.81 -5.41 0.40 2.72 2.76 0.05 2.11 1.79 147.6 169.9HOH...Acetone (
21)
-6.87 -6.06 0.81 2.74 2.75 0.01 2.06 1.78 163.2 175.2HOH...
t-NMA (
23)
-8.03 -7.01 1.01 2.70 2.75 0.05 2.00 1.77 167.7 172.8Formamide dimer, cyclic (
24)
-14.78 -10.69 4.09 2.76 2.84 0.08 2.00 1.83 171.5 170.92.76 2.84 0.08 2.00 1.83 171.6 170.9
Formamide dimer, parallel (
25)
-8.12 -6.49 1.64 2.80 2.80 0.00 2.09 1.78 156.6 175.4t
-NMA dimer, antiparallel (
26)
-7.70 -8.24 -0.54 2.83 2.80 -0.03 2.08 1.79 171.8 166.4t
-NMA antiparallel stacked (
27)
-5.35 -5.58 -0.23 3.58 3.61 0.03 4.04 3.48 74.6 88.93.58 3.61 0.03 4.04 3.48 74.6 88.9
HOH...N-Methylformamide (
28)
-7.90 -6.72 1.18 2.71 2.76 0.05 2.01 1.78 164.8 174.2N-Methylformamide...OH
2(
29)
-6.05 -6.47 -0.42 2.84 2.84 0.00 2.09 1.82 173.7 174.6t
-N-OH,N-Meacetamide...OH
2(
30)
-8.21 -7.28 0.93 2.62 2.73 0.11 1.94 1.75 159.3 176.0HOH...
t-N-OH,N-Methylacetamide (
31)
-4.10 -3.68 0.42 2.82 2.87 0.06 2.12 1.90 168.5 178.0H
2NH...HNH
2, cyclic
C2h(
32)
-3.51 -2.98 0.53 3.01 3.11 0.10 2.61 2.40 123.7 125.43.01 3.11 0.10 2.60 2.40 124.0 125.4
H
2NH...NH
3, linear
Cs(
33)
-3.38 -3.61 -0.23 3.15 3.04 -0.12 2.42 2.01 179.0 178.13.15 3.04 -0.12 3.64 3.37 69.8 62.7
HOH...NH
3(
34)
-7.22 -6.28 0.94 2.80 2.82 0.03 2.09 1.84 176.4 173.8HOH...NH
2CH
3(
35)
-7.18 -5.80 1.38 2.77 2.88 0.11 2.06 1.90 170.6 172.7Imidazole...OH
2(
36)
-7.00 -6.48 0.52 2.79 2.87 0.08 2.03 1.85 177.9 177.1HOH...Imidazole (
37)
-7.77 -5.86 1.91 2.73 2.83 0.09 2.10 1.90 151.1 156.6Indole...OH
2(
38)
-6.33 -5.92 0.41 2.81 2.88 0.07 2.07 1.86 168.3 179.0Pyrrole...OH
2(
39)
-5.89 -5.36 0.54 2.82 2.89 0.07 2.07 1.87 179.5 180.0HOH...Pyridine (
40)
-6.63 -4.99 1.64 2.77 2.88 0.11 2.12 1.96 155.6 157.7Formamidine...H
2O, cyclic (
41)
-11.03 -8.55 2.48 2.82 3.05 0.23 2.20 2.18 143.2 142.22.70 2.81 0.11 2.05 1.86 151.5 161.4
HOH...Formaldehydeimine (
42)
-6.74 -6.50 0.24 2.76 2.78 0.03 2.11 1.81 153.9 168.5Guanidine...OHH (
43)
-7.96 -6.12 1.84 2.88 2.98 0.10 2.25 2.06 145.4 147.82.77 2.97 0.20 2.20 2.14 141.1 141.6
Vinylamine...OH
2(
44) -3.80 -4.34 -0.54 3.04 2.90 -0.14 2.20 1.87 163.8 174.3Aniline...OH
2(
45)
-4.63 -4.46 0.17 2.94 2.91 -0.03 2.20 1.88 173.4 172.7HSH...OH
2(
46)
-2.93 -2.61 0.32 3.33 3.41 0.08 2.29 2.07 175.3 172.6HOH...S(CH
3)
2(
47)
-3.56 -2.81 0.75 3.12 3.45 0.33 3.13 2.48 97.9 172.2OHH...CH
3SSCH
3, cyclic (
48) -3.78 -2.50 1.28 3.38 3.63 0.26 2.84 2.67 147.0 174.5HOH...Thiophene (
49)
-2.66 -2.83 -0.17 3.59 3.74 0.15 3.50 2.92 108.4 142.23.59 3.74 0.15 3.33 3.59 121.0 91.1
HOH...SHC
6H
5(
50)
-2.20 -2.83 -0.63 4.56 4.33 -0.23 4.27 3.51 132.4 143.4HSH...SH
2(
51)
-0.96 -1.17 -0.21 4.15 4.04 -0.11 3.19 2.71 175.6 167.2HOH...SH
2(
52)
-2.27 -1.81 0.46 3.47 3.52 0.04 2.83 2.58 175.5 163.0CH
3COOH...NH
3, bidentate (
53)
-11.94 -9.52 2.42 2.63 2.72 0.09 1.90 1.73 168.3 172.32.89 3.06 0.17 2.53 2.45 119.0 117.1
HOH...PyridineN-oxide (
54)
-10.69 -7.75 2.94 2.63 2.74 0.11 1.94 1.76 161.0 179.2MethylethylamineN-oxide...OHH(
55)
-15.48 -9.21 6.27 2.49 2.69 0.20 1.83 1.74 148.9 162.82.64 3.06 0.42 2.17 2.53 124.2 110.8
Methylethylhydroxylamine...OH
2(
56)
-8.13 -5.08 3.05 2.66 2.77 0.11 2.10 1.79 140.6 179.12.69 3.36 0.67 2.19 3.44 133.1 77.2
HOH...FCH
3(
59)
-4.87 -3.43 1.44 2.70 2.74 0.04 2.06 1.77 153.8 176.9HOH...Chloropropane (
58)
-3.09 -2.76 0.33 3.34 3.36 0.03 2.82 2.42 143.6 162.1H
2NH...O(CH
3)
2(
59)
-2.83 -3.02 -0.20 3.04 3.08 0.05 2.36 2.06 155.9 174.7Methylammonium...OH
2(
60)
-19.30 -18.43 0.86 2.70 2.68 -0.02 1.79 1.64 173.7 173.5Guanidinium..OHH (
61)
-18.48 -18.83 -0.35 2.85 2.77 -0.08 2.08 1.86 147.8 147.32.85 2.77 -0.08 2.08 1.86 147.7 147.3
Imidazolium..OH
2(
62)
-16.95 -17.12 -0.17 2.71 2.64 -0.07 1.81 1.61 174.5 175.9Formamidinium..OH
2(
63)
-16.98 -17.98 -1.01 2.70 2.61 -0.09 1.91 1.58 148.7 176.1Formamidinium...OH
2, cyclic
C2v(
64)
-19.59 -18.95 0.64 2.83 2.79 -0.05 2.09 1.89 142.6 144.52.83 2.79 -0.05 2.09 1.89 142.6 144.5
Formaldehydeiminium...OH
2(
65)
-19.98 -20.19 -0.20 2.66 2.64 -0.02 1.77 1.60 163.4 177.6OHH...(-)O
2CCH
3, bidentate (
66)
-21.85 -19.39 2.46 2.77 2.79 0.02 2.06 1.93 142.8 144.52.78 2.79 0.01 2.08 1.93 141.8 144.5 a
For representational drawings of the dimer structures, see Figure 1 of: T. A. Halgren,
J. Comput. Chem.,
17. 520-552
(1996).
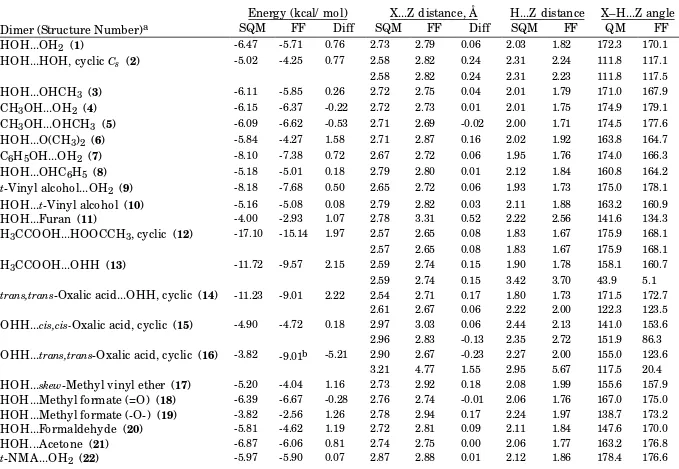
Table B-II.
_________________________________________________________________________________________________ Single-molecule BCI vs. Scaled HF/-6-31G* Intermolecular-Interaction Energies and Distances.Energy (kcal/ mol)
X...Z distance, Å
H...Z distance
X–H...Z angle
Dimer (Structure Number)
aSQM
FF
Diff
SQM
FF
Diff
SQM
FF
QM
FF
HOH...OH
2(
1)
-6.47 -5.72 0.74 2.73 2.79 0.06 2.03 1.82 172.3 170.1HOH...HOH, cyclic
Cs(
2)
-5.02 -4.25 0.77 2.58 2.82 0.24 2.31 2.24 111.8 117.32.58 2.82 0.24 2.31 2.24 111.8 117.3
HOH...OHCH
3(
3)
-6.11 -5.36 0.75 2.72 2.78 0.06 2.01 1.81 171.0 168.1CH
3OH...OH
2(
4)
-6.15 -5.58 0.57 2.72 2.77 0.05 2.01 1.79 174.9 179.8CH
3OH...OHCH
3(
5)
-6.09 -5.37 0.72 2.71 2.76 0.05 2.00 1.78 174.5 177.2HOH...O(CH
3)
2(
6)
-5.84 -3.98 1.87 2.71 2.90 0.19 2.02 1.95 163.8 165.5C
6H
5OH...OH
2(
7)
-8.10 -6.33 1.77 2.67 2.77 0.10 1.95 1.80 174.0 167.4HOH...OHC
6H
5(
8)
-5.18 -3.87 1.31 2.79 2.91 0.12 2.12 1.98 160.8 157.9t
-Vinyl alcohol...OH
2(
9)
-8.18 -8.12 0.06 2.65 2.69 0.04 1.93 1.70 175.0 176.0HOH...
t-Vinyl alcohol (
10)
-5.16 -4.90 0.26 2.79 2.84 0.05 2.11 1.90 163.2 160.7HOH...Furan (
11)
-4.00 -2.93 1.07 2.78 3.31 0.53 2.22 2.56 141.6 134.0H
3CCOOH...HOOCCH
3, cyclic (
12)
-17.10 -15.19 1.91 2.57 2.65 0.08 1.83 1.67 175.9 168.12.57 2.65 0.08 1.83 1.67 175.9 168.1
H
3CCOOH...OHH (
13)
-11.72 -9.54 2.18 2.59 2.74 0.15 1.90 1.79 158.1 160.42.59 2.74 0.15 3.42 3.71 43.9 4.8
trans,trans
-Oxalic acid...OHH, cyclic (
14)
-11.23 -9.02 2.20 2.54 2.71 0.17 1.80 1.73 171.5 172.82.61 2.67 0.06 2.22 2.00 122.3 123.5
OHH...
cis,cis-Oxalic acid, cyclic (
15)
-4.90 -4.73 0.17 2.97 3.03 0.06 2.44 2.13 141.0 153.4 2.96 2.82 -0.13 2.35 2.72 151.9 86.1OHH...
trans,trans-Oxalic acid, cyclic (
16)
-3.82 -9.02b -5.21 2.90 2.67 -0.23 2.27 2.00 155.0 123.5 3.21 4.77 1.56 2.95 5.67 117.5 20.4HOH...
skew-Methyl vinyl ether (
17)
-5.20 -4.02 1.18 2.73 2.93 0.20 2.08 2.01 155.6 157.9HOH...Methyl formate (=O) (
18)
-6.39 -6.65 -0.26 2.76 2.74 -0.01 2.06 1.76 167.0 175.0HOH...Methyl formate (-O-) (
19)
-3.82 -2.56 1.26 2.78 2.94 0.17 2.24 1.97 138.7 173.2HOH...Formaldehyde (
20)
-5.81 -4.61 1.20 2.72 2.81 0.09 2.11 1.84 147.6 169.9HOH...Acetone (
21)
-6.87 -5.94 0.93 2.74 2.75 0.01 2.06 1.77 163.2 176.0HOH...
t-NMA (
23)
-8.03 -7.37 0.65 2.70 2.72 0.02 2.00 1.75 167.7 173.6Formamide dimer, cyclic (
24)
-14.78 -13.04 1.74 2.76 2.78 0.02 2.00 1.76 171.5 171.72.76 2.78 0.02 2.00 1.76 171.6 171.7
Formamide dimer, parallel (
25)
-8.12 -8.26 -0.14 2.80 2.77 -0.03 2.09 1.80 156.6 155.9t
-NMA dimer, antiparallel (
26)
-7.70 -7.77 -0.07 2.83 2.80 -0.03 2.08 1.79 171.8 167.1t
-NMA antiparallel stacked (
27)
-5.35 -5.98 -0.63 3.58 3.52 -0.06 4.04 3.47 74.6 84.83.58 3.52 -0.06 4.04 3.47 74.6 84.8
HOH...N-Methylformamide (
28)
-7.90 -6.88 1.02 2.71 2.75 0.04 2.01 1.77 164.8 175.7N-Methylformamide...OH
2(
29)
-6.05 -5.45 0.60 2.84 2.90 0.06 2.09 1.88 173.7 173.8t
-N-OH,N-Meacetamide...OH
2(
30)
-8.21 -7.22 0.99 2.62 2.73 0.11 1.94 1.75 159.3 175.6HOH...
t-N-OH,N-Methylacetamide (
31)
-4.10 -3.85 0.25 2.82 2.87 0.05 2.12 1.90 168.5 175.0H
2NH...HNH
2, cyclic
C2h(
32)
-3.51 -4.02 -0.51 3.01 3.08 0.07 2.61 2.37 123.7 125.13.01 3.08 0.07 2.60 2.37 124.0 125.1
H
2NH...NH
3, linear
Cs(
33)
-3.38 -4.02 -0.64 3.15 3.01 -0.15 2.42 1.97 179.0 178.23.15 3.01 -0.15 3.64 3.34 69.8 62.4
HOH...NH
3(
34)
-7.22 -6.63 0.59 2.80 2.81 0.01 2.09 1.83 176.4 173.9HOH...NH
2CH
3(
35)
-7.18 -5.61 1.57 2.77 2.87 0.10 2.06 1.90 170.6 173.0Imidazole...OH
2(
36)
-7.00 -6.42 0.58 2.79 2.87 0.08 2.03 1.85 177.9 176.5HOH...Imidazole (
37)
-7.77 -6.15 1.62 2.73 2.81 0.07 2.10 1.87 151.1 158.6Indole...OH
2(
38)
-6.33 -6.15 0.18 2.81 2.86 0.06 2.07 1.84 168.3 179.1Pyrrole...OH
2(
39)
-5.89 -5.69 0.20 2.82 2.88 0.06 2.07 1.86 179.5 180.0HOH...Pyridine (
40)
-6.63 -5.03 1.60 2.77 2.88 0.11 2.12 1.95 155.6 158.5Formamidine...H
2O, cyclic (
41)
-11.03 -8.59 2.44 2.82 3.02 0.20 2.20 2.14 143.2 143.62.70 2.81 0.12 2.05 1.87 151.5 160.1
HOH...Formaldehydeimine (
42)
-6.74 -5.58 1.16 2.76 2.84 0.08 2.11 1.87 153.9 166.7Guanidine...OHH (
43)
-7.96 -7.26 0.69 2.88 2.96 0.08 2.25 2.04 145.4 149.52.77 2.86 0.10 2.20 2.01 141.1 144.6
Vinylamine...OH
2(
44) -3.80 -4.14 -0.34 3.04 2.91 -0.13 2.20 1.89 163.8 174.3Aniline...OH
2(
45)
-4.63 -4.40 0.22 2.94 2.91 -0.03 2.20 1.89 173.4 172.6HSH...OH
2(
46)
-2.93 -2.67 0.26 3.33 3.40 0.07 2.29 2.06 175.3 172.7HOH...S(CH
3)
2(
47)
-3.56 -3.06 0.50 3.12 3.47 0.35 3.13 2.50 97.9 173.7OHH...CH
3SSCH
3, cyclic (
48) -3.78 -2.67 1.12 3.38 3.63 0.25 2.84 2.66 147.0 172.7HOH...Thiophene (
49)
-2.66 -2.95 -0.29 3.59 3.72 0.13 3.50 2.84 108.4 151.03.59 3.72 0.13 3.33 3.69 121.0 84.4
HOH...SHC
6H
5(
50)
-2.20 -2.84 -0.65 4.56 4.34 -0.22 4.27 3.53 132.4 143.3HSH...SH
2(
51)
-0.96 -1.21 -0.24 4.15 4.03 -0.12 3.19 2.71 175.6 167.2HOH...SH
2(
52)
-2.27 -1.84 0.42 3.47 3.51 0.04 2.83 2.57 175.5 163.1CH
3COOH...NH
3, bidentate (
53)
-11.94 -10.18 1.76 2.63 2.71 0.08 1.90 1.72 168.3 170.52.89 2.97 0.08 2.53 2.31 119.0 120.9
HOH...PyridineN-oxide (
54)
-10.69 -8.34 2.35 2.63 2.71 0.08 1.94 1.73 161.0 177.4MethylethylamineN-oxide...OHH(
55)
-15.48 -9.22 6.26 2.49 2.69 0.20 1.83 1.74 148.9 162.82.64 3.06 0.42 2.17 2.53 124.2 110.8
Methylethylhydroxylamine...OH
2(
56)
-8.13 -5.47 2.65 2.66 2.75 0.09 2.10 1.77 140.6 178.52.69 3.37 0.68 2.19 3.53 133.1 72.8
HOH...FCH
3(
59)
-4.87 -3.49 1.38 2.70 2.73 0.03 2.06 1.76 153.8 175.5HOH...Chloropropane (
58)
-3.09 -2.72 0.37 3.34 3.36 0.03 2.82 2.44 143.6 158.7H
2NH...O(CH
3)
2(
59)
-2.83 -2.65 0.18 3.04 3.14 0.10 2.36 2.11 155.9 175.4Methylammonium...OH
2(
60)
-19.30 -18.33 0.97 2.70 2.68 -0.02 1.79 1.64 173.7 170.8Guanidinium..OHH (
61)
-18.48 -18.83 -0.35 2.85 2.77 -0.08 2.08 1.86 147.8 147.32.85 2.77 -0.08 2.08 1.86 147.7 147.3
Imidazolium..OH
2(
62)
-16.95 -17.12 -0.17 2.71 2.64 -0.07 1.81 1.61 174.5 175.9Formamidinium..OH
2(
63)
-16.98 -17.98 -1.01 2.70 2.61 -0.09 1.91 1.58 148.7 176.1Formamidinium...OH
2, cyclic
C2v(
64)
-19.59 -18.95 0.64 2.83 2.79 -0.05 2.09 1.89 142.6 144.52.83 2.79 -0.05 2.09 1.89 142.6 144.5
Formaldehydeiminium...OH
2(
65)
-19.98 -18.16 1.83 2.66 2.70 0.04 1.77 1.66 163.4 179.2OHH...(-)O
2CCH
3, bidentate (
66)
-21.85 -19.49 2.35 2.77 2.79 0.02 2.06 1.92 142.8 144.62.78 2.79 0.01 2.08 1.92 141.8 144.6 a
For representational drawings of the dimer structures, see Figure 1 of: T. A. Halgren,
J. Comput. Chem.,
17. 520-552
(1996).
Table B-III.
_________________________________________________________________________________________________ Unique-bond BCI vs. Scaled HF/-6-31G* Intermolecular-Interaction Energies and Distances.Energy (kcal/ mol)
X...Z distance, Å
H...Z distance
X–H...Z angle
Dimer (Structure Number)
aSQM
FF
Diff
SQM
FF
Diff
SQM
FF
QM
FF
HOH...OH
2(
1)
-6.47 -5.71 0.76 2.73 2.79 0.06 2.03 1.82 172.3 170.1HOH...HOH, cyclic
Cs(
2)
-5.02 -4.25 0.77 2.58 2.82 0.24 2.31 2.24 111.8 117.12.58 2.82 0.24 2.31 2.23 111.8 117.5
HOH...OHCH
3(
3)
-6.11 -5.85 0.26 2.72 2.75 0.04 2.01 1.79 171.0 167.9CH
3OH...OH
2(
4)
-6.15 -6.37 -0.22 2.72 2.73 0.01 2.01 1.75 174.9 179.1CH
3OH...OHCH
3(
5)
-6.09 -6.62 -0.53 2.71 2.69 -0.02 2.00 1.71 174.5 177.6HOH...O(CH
3)
2(
6)
-5.84 -4.27 1.58 2.71 2.87 0.16 2.02 1.92 163.8 164.7C
6H
5OH...OH
2(
7)
-8.10 -7.38 0.72 2.67 2.72 0.06 1.95 1.76 174.0 166.3HOH...OHC
6H
5(
8)
-5.18 -5.01 0.18 2.79 2.80 0.01 2.12 1.84 160.8 164.2t
-Vinyl alcohol...OH
2(
9)
-8.18 -7.68 0.50 2.65 2.72 0.06 1.93 1.73 175.0 178.1HOH...
t-Vinyl alcohol (
10)
-5.16 -5.08 0.08 2.79 2.82 0.03 2.11 1.88 163.2 160.9HOH...Furan (
11)
-4.00 -2.93 1.07 2.78 3.31 0.52 2.22 2.56 141.6 134.3H
3CCOOH...HOOCCH
3, cyclic (
12)
-17.10 -15.14 1.97 2.57 2.65 0.08 1.83 1.67 175.9 168.12.57 2.65 0.08 1.83 1.67 175.9 168.1
H
3CCOOH...OHH (
13)
-11.72 -9.57 2.15 2.59 2.74 0.15 1.90 1.78 158.1 160.72.59 2.74 0.15 3.42 3.70 43.9 5.1
trans,trans
-Oxalic acid...OHH, cyclic (
14)
-11.23 -9.01 2.22 2.54 2.71 0.17 1.80 1.73 171.5 172.72.61 2.67 0.06 2.22 2.00 122.3 123.5
OHH...
cis,cis-Oxalic acid, cyclic (
15)
-4.90 -4.72 0.18 2.97 3.03 0.06 2.44 2.13 141.0 153.6 2.96 2.83 -0.13 2.35 2.72 151.9 86.3OHH...
trans,trans-Oxalic acid, cyclic (
16)
-3.82 -9.01b -5.21 2.90 2.67 -0.23 2.27 2.00 155.0 123.6 3.21 4.77 1.55 2.95 5.67 117.5 20.4HOH...
skew-Methyl vinyl ether (
17)
-5.20 -4.04 1.16 2.73 2.92 0.18 2.08 1.99 155.6 157.9HOH...Methyl formate (=O) (
18)
-6.39 -6.67 -0.28 2.76 2.74 -0.01 2.06 1.76 167.0 175.0HOH...Methyl formate (-O-) (
19)
-3.82 -2.56 1.26 2.78 2.94 0.17 2.24 1.97 138.7 173.2HOH...Formaldehyde (
20)
-5.81 -4.62 1.19 2.72 2.81 0.09 2.11 1.84 147.6 170.0HOH...Acetone (
21)
-6.87 -6.06 0.81 2.74 2.75 0.00 2.06 1.77 163.2 176.8HOH...
t-NMA (
23)
-8.03 -7.50 0.53 2.70 2.71 0.01 2.00 1.74 167.7 173.8Formamide dimer, cyclic (
24)
-14.78 -13.98 0.80 2.76 2.76 0.00 2.00 1.74 171.5 171.32.76 2.76 0.00 2.00 1.74 171.6 171.3
Formamide dimer, parallel (
25)
-8.12 -8.00 0.12 2.80 2.79 -0.01 2.09 1.83 156.6 154.4t
-NMA dimer, antiparallel (
26)
-7.70 -8.03 -0.33 2.83 2.77 -0.05 2.08 1.76 171.8 169.1t
-NMA antiparallel stacked (
27)
-5.35 -5.88 -0.53 3.58 3.40 -0.19 4.04 3.26 74.6 88.83.58 3.68 0.10 4.04 3.65 74.6 83.7
HOH...N-Methylformamide (
28)
-7.90 -7.06 0.84 2.71 2.73 0.03 2.01 1.75 164.8 176.3N-Methylformamide...OH
2(
29)
-6.05 -5.74 0.31 2.84 2.88 0.04 2.09 1.87 173.7 173.9t
-N-OH,N-Meacetamide...OH
2(
30)
-8.21 -7.92 0.29 2.62 2.70 0.08 1.94 1.72 159.3 176.2HOH...
t-N-OH,N-Methylacetamide (
31)
-4.10 -4.27 -0.18 2.82 2.83 0.02 2.12 1.86 168.5 175.3H
2NH...HNH
2, cyclic
C2h(
32)
-3.51 -3.29 0.22 3.01 3.08 0.07 2.61 2.37 123.7 125.23.01 3.08 0.07 2.60 2.37 124.0 125.2
H
2NH...NH
3, linear
Cs(
33)
-3.38 -4.04 -0.66 3.15 3.00 -0.15 2.42 1.97 179.0 178.23.15 3.00 -0.15 3.64 3.35 69.8 61.6
HOH...NH
3(
34)
-7.22 -6.61 0.61 2.80 2.81 0.01 2.09 1.83 176.4 173.8HOH...NH
2CH
3(
35)
-7.18 -6.28 0.90 2.77 2.84 0.07 2.06 1.86 170.6 173.1Imidazole...OH
2(
36)
-7.00 -6.46 0.55 2.79 2.86 0.08 2.03 1.85 177.9 174.9HOH...Imidazole (
37)
-7.77 -6.31 1.46 2.73 2.80 0.06 2.10 1.86 151.1 159.4Indole...OH
2(
38)
-6.33 -6.34 -0.01 2.81 2.85 0.04 2.07 1.83 168.3 178.9Pyrrole...OH
2(
39)
-5.89 -5.70 0.20 2.82 2.88 0.06 2.07 1.86 179.5 180.0HOH...Pyridine (
40)
-6.63 -5.63 1.00 2.77 2.84 0.07 2.12 1.90 155.6 162.1Formamidine...H
2O, cyclic (
41)
-11.03 -9.02 2.01 2.82 2.98 0.17 2.20 2.09 143.2 144.52.70 2.80 0.11 2.05 1.86 151.5 158.9
HOH...Formaldehydeimine (
42)
-6.74 -5.91 0.84 2.76 2.82 0.06 2.11 1.85 153.9 167.1Guanidine...OHH (
43)
-7.96 -7.39 0.56 2.88 2.95 0.07 2.25 2.01 145.4 150.32.77 2.86 0.09 2.20 2.01 141.1 143.5
Vinylamine...OH
2(
44) -3.80 -4.34 -0.54 3.04 2.91 -0.13 2.20 1.89 163.8 177.7Aniline...OH
2(
45)
-4.63 -4.46 0.16 2.94 2.92 -0.02 2.20 1.90 173.4 174.9HSH...OH
2(
46)
-2.93 -2.68 0.25 3.33 3.40 0.07 2.29 2.06 175.3 172.7HOH...S(CH
3)
2(
47)
-3.56 -3.04 0.52 3.12 3.45 0.34 3.13 2.48 97.9 177.0OHH...CH
3SSCH
3, cyclic (
48) -3.78 -2.67 1.11 3.38 3.63 0.26 2.84 2.67 147.0 172.9HOH...Thiophene (
49)
-2.66 -2.95 -0.29 3.59 3.72 0.13 3.50 2.83 108.4 151.93.59 3.72 0.13 3.33 3.70 121.0 83.7
HOH...SHC
6H
5(
50)
-2.20 -2.79 -0.59 4.56 4.51 -0.05 4.27 3.70 132.4 142.5HSH...SH
2(
51)
-0.96 -1.21 -0.25 4.15 4.03 -0.12 3.19 2.71 175.6 167.3HOH...SH
2(
52)
-2.27 -1.84 0.43 3.47 3.51 0.04 2.83 2.57 175.5 163.0CH
3COOH...NH
3, bidentate (
53)
-11.94 -10.25 1.68 2.63 2.71 0.08 1.90 1.72 168.3 170.92.89 2.98 0.08 2.53 2.32 119.0 120.3
HOH...PyridineN-oxide (
54)
-10.69 -8.23 2.46 2.63 2.71 0.08 1.94 1.73 161.0 177.7MethylethylamineN-oxide...OHH(
55)
-15.48 -10.53 4.96 2.49 2.67 0.18 1.83 1.73 148.9 156.92.64 2.90 0.26 2.17 2.29 124.2 115.3
Methylethylhydroxylamine...OH
2(
56)
-8.13 -5.55 2.57 2.66 2.75 0.09 2.10 1.77 140.6 177.82.69 3.36 0.67 2.19 3.55 133.1 71.1
HOH...FCH
3(
59)
-4.87 -3.48 1.39 2.70 2.73 0.03 2.06 1.76 153.8 175.5HOH...Chloropropane (
58)
-3.09 -2.91 0.17 3.34 3.40 0.06 2.82 2.45 143.6 163.2H
2NH...O(CH
3)
2(
59)
-2.83 -2.76 0.07 3.04 3.09 0.06 2.36 2.07 155.9 173.5Methylammonium...OH
2(
60)
-19.30 -18.33 0.97 2.70 2.68 -0.02 1.79 1.64 173.7 170.8Guanidinium..OHH (
61)
-18.48 -18.82 -0.34 2.85 2.78 -0.08 2.08 1.86 147.8 147.32.85 2.77 -0.08 2.08 1.86 147.7 147.4
Imidazolium..OH
2(
62)
-16.95 -17.14 -0.19 2.71 2.64 -0.07 1.81 1.61 174.5 175.9Formamidinium..OH
2(
63)
-16.98 -17.44 -0.46 2.70 2.63 -0.08 1.91 1.59 148.7 175.4Formamidinium...OH
2, cyclic
C2v(
64)
-19.59 -19.55 0.04 2.83 2.78 -0.05 2.09 1.88 142.6 144.42.83 2.77 -0.06 2.09 1.87 142.6 144.7
Formaldehydeiminium...OH
2(
65)
-19.98 -18.16 1.82 2.66 2.70 0.04 1.77 1.66 163.4 179.2OHH...(-)O
2CCH
3, bidentate (
66)
-21.85 -19.50 2.34 2.77 2.78 0.01 2.06 1.92 142.8 144.62.78 2.79 0.02 2.08 1.93 141.8 144.6 a
For representational drawings of the dimer structures, see Figure 1 of: T. A. Halgren,
J. Comput. Chem.,
17. 520-552
(1996).
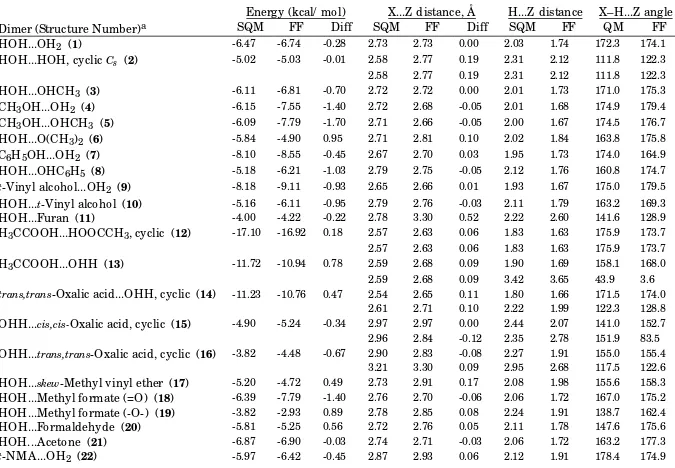
Table B-IV.
__________________________________________________________________________________________________ Unique-bond-LJ12-6 BCI vs. Scaled HF/-6-31G* Intermolecular-Interaction Energies and Distances.Energy (kcal/ mol)
X...Z distance, Å
H...Z distance
X–H...Z angle
Dimer (Structure Number)
aSQM
FF
Diff
SQM
FF
Diff
SQM
FF
QM
FF
HOH...OH
2(
1)
-6.47 -6.74 -0.28 2.73 2.73 0.00 2.03 1.74 172.3 174.1HOH...HOH, cyclic
Cs(
2)
-5.02 -5.03 -0.01 2.58 2.77 0.19 2.31 2.12 111.8 122.32.58 2.77 0.19 2.31 2.12 111.8 122.3
HOH...OHCH
3(
3)
-6.11 -6.81 -0.70 2.72 2.72 0.00 2.01 1.73 171.0 175.3CH
3OH...OH
2(
4)
-6.15 -7.55 -1.40 2.72 2.68 -0.05 2.01 1.68 174.9 179.4CH
3OH...OHCH
3(
5)
-6.09 -7.79 -1.70 2.71 2.66 -0.05 2.00 1.67 174.5 176.7HOH...O(CH
3)
2(
6)
-5.84 -4.90 0.95 2.71 2.81 0.10 2.02 1.84 163.8 175.8C
6H
5OH...OH
2(
7)
-8.10 -8.55 -0.45 2.67 2.70 0.03 1.95 1.73 174.0 164.9HOH...OHC
6H
5(
8)
-5.18 -6.21 -1.03 2.79 2.75 -0.05 2.12 1.76 160.8 174.7t
-Vinyl alcohol...OH
2(
9)
-8.18 -9.11 -0.93 2.65 2.66 0.01 1.93 1.67 175.0 179.5HOH...
t-Vinyl alcohol (
10)
-5.16 -6.11 -0.95 2.79 2.76 -0.03 2.11 1.79 163.2 169.3HOH...Furan (
11)
-4.00 -4.22 -0.22 2.78 3.30 0.52 2.22 2.60 141.6 128.9H
3CCOOH...HOOCCH
3, cyclic (
12)
-17.10 -16.92 0.18 2.57 2.63 0.06 1.83 1.63 175.9 173.72.57 2.63 0.06 1.83 1.63 175.9 173.7
H
3CCOOH...OHH (
13)
-11.72 -10.94 0.78 2.59 2.68 0.09 1.90 1.69 158.1 168.02.59 2.68 0.09 3.42 3.65 43.9 3.6
trans,trans
-Oxalic acid...OHH, cyclic (
14)
-11.23 -10.76 0.47 2.54 2.65 0.11 1.80 1.66 171.5 174.02.61 2.71 0.10 2.22 1.99 122.3 128.8
OHH...
cis,cis-Oxalic acid, cyclic (
15)
-4.90 -5.24 -0.34 2.97 2.97 0.00 2.44 2.07 141.0 152.7 2.96 2.84 -0.12 2.35 2.78 151.9 83.5OHH...
trans,trans-Oxalic acid, cyclic (
16)
-3.82 -4.48 -0.67 2.90 2.83 -0.08 2.27 1.91 155.0 155.4 3.21 3.30 0.09 2.95 2.68 117.5 122.6HOH...
skew-Methyl vinyl ether (
17)
-5.20 -4.72 0.49 2.73 2.91 0.17 2.08 1.98 155.6 158.3HOH...Methyl formate (=O) (
18)
-6.39 -7.79 -1.40 2.76 2.70 -0.06 2.06 1.72 167.0 175.2HOH...Methyl formate (-O-) (
19)
-3.82 -2.93 0.89 2.78 2.85 0.08 2.24 1.91 138.7 162.4HOH...Formaldehyde (
20)
-5.81 -5.25 0.56 2.72 2.76 0.05 2.11 1.78 147.6 175.6HOH...Acetone (
21)
-6.87 -6.90 -0.03 2.74 2.71 -0.03 2.06 1.72 163.2 177.3HOH...
t-NMA (
23)
-8.03 -8.49 -0.46 2.70 2.68 -0.02 2.00 1.69 167.7 177.6Formamide dimer, cyclic (
24)
-14.78 -15.07 -0.29 2.76 2.82 0.07 2.00 1.80 171.5 174.12.76 2.82 0.07 2.00 1.80 171.6 174.1
Formamide dimer, parallel (
25)
-8.12 -9.54 -1.42 2.80 2.83 0.03 2.09 1.87 156.6 155.3t
-NMA dimer, antiparallel (
26)
-7.70 -8.93 -1.23 2.83 2.85 0.02 2.08 1.84 171.8 167.8t
-NMA antiparallel stacked (
27)
-5.35 -7.71 -2.36 3.58 3.27 -0.32 4.04 3.09 74.6 90.93.58 3.42 -0.17 4.04 3.29 74.6 88.6
HOH...N-Methylformamide (
28)
-7.90 -8.15 -0.25 2.71 2.69 -0.01 2.01 1.71 164.8 178.9N-Methylformamide...OH
2(
29)
-6.05 -6.25 -0.19 2.84 2.93 0.09 2.09 1.91 173.7 172.1t
-N-OH,N-Meacetamide...OH
2(
30)
-8.21 -9.25 -1.04 2.62 2.65 0.03 1.94 1.66 159.3 177.5HOH...
t-N-OH,N-Methylacetamide (
31)
-4.10 -5.43 -1.33 2.82 2.77 -0.04 2.12 1.79 168.5 175.6H
2NH...HNH
2, cyclic
C2h(
32)
-3.51 -4.26 -0.75 3.01 3.00 0.00 2.61 2.24 123.7 130.33.01 3.00 0.00 2.60 2.24 124.0 130.2
H
2NH...NH
3, linear
Cs(
33)
-3.38 -4.50 -1.12 3.15 3.06 -0.10 2.42 2.02 179.0 176.73.15 3.06 -0.10 3.64 3.10 69.8 77.9
HOH...NH
3(
34)
-7.22 -7.71 -0.49 2.80 2.78 -0.02 2.09 1.79 176.4 176.7HOH...NH
2CH
3(
35)
-7.18 -7.54 -0.36 2.77 2.80 0.02 2.06 1.81 170.6 175.9Imidazole...OH
2(
36)
-7.00 -7.11 -0.10 2.79 2.90 0.11 2.03 1.88 177.9 174.1HOH...Imidazole (
37)
-7.77 -6.64 1.13 2.73 2.84 0.10 2.10 1.85 151.1 178.9Indole...OH
2(
38)
-6.33 -6.98 -0.65 2.81 2.89 0.08 2.07 1.87 168.3 178.0Pyrrole...OH
2(
39)
-5.89 -6.31 -0.42 2.82 2.91 0.09 2.07 1.89 179.5 180.0HOH...Pyridine (
40)
-6.63 -6.20 0.44 2.77 2.82 0.04 2.12 1.84 155.6 170.6Formamidine...H
2O, cyclic (
41)
-11.03 -10.56 0.47 2.82 2.92 0.10 2.20 1.99 143.2 149.92.70 2.76 0.06 2.05 1.78 151.5 167.6
HOH...Formaldehydeimine (
42)
-6.74 -6.72 0.02 2.76 2.81 0.05 2.11 1.82 153.9 179.2Guanidine...OHH (
43)
-7.96 -8.80 -0.85 2.88 2.96 0.08 2.25 2.01 145.4 153.72.77 2.76 0.00 2.20 1.84 141.1 154.3
Vinylamine...OH
2(
44) -3.80 -4.78 -0.98 3.04 2.95 -0.10 2.20 1.92 163.8 179.0Aniline...OH
2(
45)
-4.63 -5.10 -0.48 2.94 2.95 0.01 2.20 1.93 173.4 178.0HSH...OH
2(
46)
-2.93 -3.47 -0.54 3.33 3.26 -0.07 2.29 1.92 175.3 171.4HOH...S(CH
3)
2(
47)
-3.56 -4.10 -0.54 3.12 3.18 0.06 3.13 2.20 97.9 179.8OHH...CH
3SSCH
3, cyclic (
48) -3.78 -3.45 0.33 3.38 3.43 0.06 2.84 2.69 147.0 133.5HOH...Thiophene (
49)
-2.66 -4.48 -1.82 3.59 3.46 -0.13 3.50 3.22 108.4 96.03.59 3.46 -0.13 3.33 2.72 121.0 133.7
HOH...SHC
6H
5(
50)
-2.20 -3.87 -1.67 4.56 4.68 0.12 4.27 4.07 132.4 123.6HSH...SH
2(
51)
-0.96 -1.59 -0.62 4.15 3.67 -0.48 3.19 2.35 175.6 167.0HOH...SH
2(
52)
-2.27 -2.45 -0.18 3.47 3.23 -0.24 2.83 2.28 175.5 168.4CH
3COOH...NH
3, bidentate (
53)
-11.94 -11.82 0.12 2.63 2.70 0.07 1.90 1.70 168.3 175.02.89 2.94 0.04 2.53 2.21 119.0 125.4
HOH...PyridineN-oxide (
54)
-10.69 -9.95 0.74 2.63 2.65 0.02 1.94 1.66 161.0 175.9MethylethylamineN-oxide...OHH(
55)
-15.48 -12.03 3.45 2.49 2.63 0.14 1.83 1.65 148.9 167.72.64 2.95 0.31 2.17 2.42 124.2 110.4
Methylethylhydroxylamine...OH
2(
56)
-8.13 -6.76 1.37 2.66 2.69 0.03 2.10 1.70 140.6 179.02.69 3.28 0.59 2.19 3.42 133.1 73.7
HOH...FCH
3(
59)
-4.87 -3.40 1.47 2.70 2.81 0.11 2.06 1.84 153.8 173.5HOH...Chloropropane (
58)
-3.09 -3.43 -0.34 3.34 3.28 -0.05 2.82 2.33 143.6 165.9H
2NH...O(CH
3)
2(
59)
-2.83 -3.40 -0.57 3.04 3.06 0.02 2.36 2.03 155.9 179.4Methylammonium...OH
2(
60)
-19.30 -18.42 0.88 2.70 2.81 0.11 1.79 1.77 173.7 172.0Guanidinium..OHH (
61)
-18.48 -20.20 -1.72 2.85 2.86 0.01 2.08 1.92 147.8 154.52.85 2.86 0.01 2.08 1.92 147.7 154.5
Imidazolium..OH
2(
62)
-16.95 -16.56 0.39 2.71 2.81 0.10 1.81 1.78 174.5 175.9Formamidinium..OH
2(
63)
-16.98 -16.71 0.27 2.70 2.80 0.10 1.91 1.78 148.7 171.4Formamidinium...OH
2, cyclic
C2v(
64)
-19.59 -21.61 -2.02 2.83 2.86 0.02 2.09 1.90 142.6 154.42.83 2.86 0.02 2.09 1.90 142.6 154.4
Formaldehydeiminium...OH
2(
65)
-19.98 -19.23 0.76 2.66 2.79 0.13 1.77 1.75 163.4 177.6OHH...(-)O
2CCH
3, bidentate (
66)
-21.85 -22.61 -0.76 2.77 2.69 -0.08 2.06 1.80 142.8 147.02.78 2.70 -0.08 2.08 1.81 141.8 146.9 a
For representational drawings of the dimer structures, see Figure 1 of: T. A. Halgren,
J. Comput. Chem.,
17. 520-552