www.elsevier.com / locate / bres
Research report
Hypothermia inhibits ischemia-induced efflux of amino acids and
neuronal damage in the hippocampus of aged rats
a ,
*
a a a bHiroaki Ooboshi
, Setsuro Ibayashi , Kentaro Takano , Seizo Sadoshima , Akira Kondo ,
b a
Hideyuki Uchimura , Masatoshi Fujishima
a
Department of Medicine and Clinical Science, Graduate School of Medical Sciences, Kyushu University, Maidashi 3-1-1, Higashi-ku,
Fukuoka812-8582, Japan
b
Center for Emotional and Behavioral Disorders, Hizen National Mental Hospital, Saga, Japan Accepted 15 August 2000
Abstract
Brain hypothermia has been reported to protect against ischemic damages in adult animals. Our goal in this study was to examine whether brain hypothermia attenuates ischemic neuronal damages in the hippocampus of aged animals. We also determined effects of hypothermia on ischemia-induced releases of amino acids in the hippocampus. Temperature in the hippocampus of aged rats (19–23 months) was maintained at 368C (normothermia), 338C (mild hypothermia) or 308C (moderately hypothermia) using a thermoregulator during 20 min of transient forebrain ischemia. Cerebral ischemia increased extracellular concentrations of glutamate and aspartate by 6-and 5-fold, respectively, in the normothermic group. Mild 6-and moderate hypothermia, however, markedly inhibited the rise of these amino acids to less than 2-fold. Elevation of extracellular taurine, a putative inhibitory amino acid, was 16-fold in the normothermic rats. Mild hypothermia attenuated ischemia-induced increase in taurine (10-fold), and moderate hypothermia inhibited the increase. Ischemic damages, evaluated by histopathological grading of hippocampal CA1 area 7 days after ischemia, was significantly ameliorated in the mild (1.360.5, mean6S.E.M.) and moderate hypothermic rats (0.860.3) compared with the normothermic ones (3.460.4). These results suggest that brain hypothermia protects against ischemic neuronal damages even in the aged animals, and the protection is associated with inhibition of excessive effluxes of both excitatory and inhibitory amino acids. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Ischemia
Keywords: Hypothermia; Aged; Cerebral ischemia; Microdialysis; Glutamate; Taurine
1. Introduction susceptible to transient cerebral ischemia than adult SHR
[37].
Stroke is the leading cause of death in the Japanese Brain hypothermia is reported to protect neuronal cells elderly [34], and aging is one of the major risk factors for against ischemic insults in the adult and infant animals cerebrovascular disease. Although the importance of [6,4,23,36]. However, effects of hypothermia on the is-studies that use aged animals to examine brain ischemia chemic damage in the aged animals have not been well has been claimed [25], such experiments are limited clarified. Our first goal in this study was to examine [12,14]. We have developed the experimental model for whether brain hypothermia attenuates the ischemia-induced brain ischemia using aged spontaneous hypertensive rats neuronal damage in the hippocampus of aged SHR. (SHR), more relevant models for human stroke. Recently Increases in extracellular excitatory amino acids have we reported that the hippocampus of aged SHR was more been reported to play a pivotal role in ischemic neuronal damages [10,31,32,34]. Extracellular inhibitory amino acids also increase during cerebral ischemia [16,18,38],
*Corresponding author. Tel.: 181-92-642-5256; fax: 1
81-92-642-and the vulnerability of hippocampus in the aged rats is
5271.
E-mail address: [email protected] (H. Ooboshi). suggested to be related with changes in inhibitory amino
1 2
acids [30]. Therefore, the second goal in this study was to K , 155.5 mM Cl , pH 7.4) was infused in the hippocam-determine effects of hypothermia on the effluxes of pus through the dialysis probe at a rate of 4.0ml / min with excitatory and inhibitory amino acids in the aged rats. We a syringe pump (Eicom Co., Kyoto, Japan). The perfusate monitored the level of extracellular amino acids using a was collected every 10 min into a plastic tube and stored at microdialysis method, and compared with the severity of 2808C for the later measurement of amino acids.
histopathological damages. Regional brain temperature in
the hippocampus was satisfactorily controlled by the 2.4. Brain hypothermia specific thermoregulator [19].
Brain temperature was modulated by a selective brain thermoregulator (metallic plate brain cooling device) which
2. Materials and methods we developed (model BTC-100, Unique Medical Co.,
Tokyo, Japan). Briefly, an aluminum metallic plate (22
2.1. Animals mm316 mm31 mm) consisted of two thermomodules
(one for heating and the other for cooling using a
water-Nineteen aged female SHR, 19–23 months old and circulating system) was placed on the surface of rat’s
weighed 195–215 g, were used in this study. Rats were scalp, and the thermocouple was inserted through an maintained in Animal Center, Kyushu University under elliptocal center hole (8 mm36 mm) in the plate. The specific pathogen-free conditions, and fed stock chow diet device with a continuous-monitoring system can quickly and tap water ad libitum. All experimental procedures were and precisely adjust the cerebral cortex at desired brain performed in accordance with the Physiological Society of temperature [19]. Following a resting period of 90 min, the Japan Guiding Principles for the Care and Use of Animals baseline CBF and arterial blood pressure were determined in the Field of Physiological Sciences. Female SHR were with hippocampal temperature of 368C using the ther-chosen because of their low mortality rate (under 30% at moregulator. Then, the temperature of the hippocampus 20 months), while a half of male SHR expired between 15 was adjusted to 308C (moderate hypothermia, n56) or
and 20 months. 338C (mild hypothermia, n56) or remained 368C
(nor-mothermia, n57) until 80 min after brain ischemia. Rectal
2.2. Surgery temperature was maintained at 378C using a heat pad
throughout the experiment. Both carotid arteries were Cerebral ischemia was produced by bilateral carotid ligated for 20 min, followed by 80 min of recirculation. artery occlusion [13]. Briefly, each rat was anesthetized CBF was determined at every 10 min of ischemia and with amobarbital (100 mg / kg i.p.) and breathed room air recirculation. Arterial blood gases, pH and hematocrit were spontaneously. Both femoral arteries were cannulated, one measured at the resting period, 20 min of ischemia and 80 for sampling blood to measure arterial blood gases and pH min of recirculation.
with an IL meter Model 1304 (Instrumentation Laboratory Inc., Lexington, MA, USA) and the other for continuous
2.5. HPLC analysis recording of blood pressure. Bilateral common carotid
artery was exposed through a ventral incision in the neck,
Concentrations of amino acids were measured using carefully separated from the vagosympathetic trunks, and
HPLC combined with fluorescent detection after pre-loosely encircled with sutures for later retraction.
column derivatization. Each sample was automatically mixed with o-phthalaldehyde and 2-mercaptoethanol for 2 2.3. Microdialysis
min, and then injected into the HPLC system, which consisted of an Eicom pump (Eicom Co., Kyoto, Japan) at Concentrations of extracellular amino acids and cerebral
a flow rate of 1.0 ml / min, a reverse phase column blood flow (CBF) in the hippocampal CA1 subfield were
(Eicompac MA-5 ODS, 4.63250 mm, Eicom Co., Kyoto, simultaneously determined using a microdialysis technique
Japan) and a fluorescent detector (Shimadzu Co., Tokyo, and a hydrogen clearance method, respectively [29]. Each
Japan). The mobile phase was 0.1 M sodium phosphate rat was fixed in a head holder, and a small burr hole was
(pH 6.0) containing 30% (vol / vol) methanol. Concen-made in the parietal region. A dialysis probe with 1-mm
trations of glutamate, aspartate, glycine, taurine, GABA membrane (CMA-10: Carnegie Medicine, Stockholm,
and alanine were determined by comparison of standard Sweden) and a Teflon-coated electrode with
micro-ther-solution. mocouple (300 mm in diameter, 1-mm portion at its tip
uncoated) for measurement of both CBF and brain
tem-perature were placed stereotaxically in the right hippocam- 2.6. Histological examination pal CA1; 4.3 mm posterior and 1.5 mm lateral to the
bregma and 3.0 mm in depth from the surface of the brain. After 80 min of recirculation, the dialysis probe and
1 21
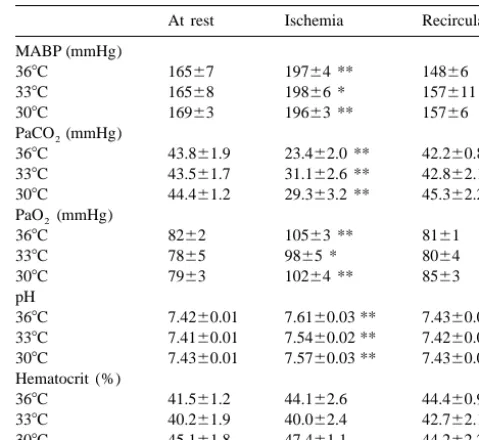
Table 1
the hole in the skull. The femoral arteries were ligated, and
Mean arterial blood pressure (MABP), arterial gases, pH and hematocrit
incisions in the head, neck and leg were sutured. Each rat
at resting period, 10 min of ischemia and 80 min of recirculation
was brought back to the cage and freely fed water and
At rest Ischemia Recirculaton
food. Seven days after forebrain ischemia, five rats in the
368C groups and four rats in each hypothermic group were MABP (mmHg)
368C 16567 19764 ** 14866
anesthetized with amobarbital (100 mg / kg i.p.). Brains
338C 16568 19866 * 157611
were transcardially perfused with 4% paraformaldehyde in
308C 16963 19663 ** 15766
1 / 15 M phosphate buffer (pH 7.3) after a brief wash-out PaCO (mmHg)
2
period with heparinized saline. Each brain was removed 368C 43.861.9 23.462.0 ** 42.260.8
and fixed in 4% neutral formaldehyde for 7 days. Paraffin 338C 43.561.7 31.162.6 ** 42.862.1
308C 44.461.2 29.363.2 ** 45.362.2
sections were taken at the level of the hippocampus in each
PaO (mmHg)2
rat and were stained with hematoxylin and eosin. Ischemic
368C 8262 10563 ** 8161
neuronal damage of the hippocampal CA1 subfield in each 338C 7865 9865 * 8064
hemisphere was graded from 0 to 3 (3, majority of neurons 308C 7963 10264 ** 8563
damaged; 2, many neurons damaged; 1, a few neurons pH
368C 7.4260.01 7.6160.03 ** 7.4360.01
damaged; or 0, normal) by a neuropathologist (A.K.)
338C 7.4160.01 7.5460.02 ** 7.4260.02
without knowledge of the experimental conditions, and the
308C 7.4360.01 7.5760.03 ** 7.4360.01
summed value of both hemispheres was regarded as Hematocrit (%)
ischemic score for each animal. 368C 41.561.2 44.162.6 44.460.9
338C 40.261.9 40.062.4 42.762.1 308C 45.161.8 47.461.1 44.262.2
2.7. Statistical analysis Values are means6S.E.M. (n56–7). Paired data were compared with
control values by analysis of variance and Dunnett’s t-test (* P,0.05, ** P,0.01). No significant difference was found among the groups.
All values were presented as mean6S.E.M. The statisti-cal differences among and within groups for physiologistatisti-cal
parameter, CBF and concentrations of amino acids were 3.2. Basal amino acid level analyzed by two-way repeated analysis of variance
fol-lowed by Dunnett’s t-test. Ischemic scores for neuronal Concentrations of the last three dialysates at the resting damages were compared among groups by nonparametric period were averaged and regarded as basal values, which, Kruskal–Wallis’ h-test followed by Dunnett’s t-test. in the 36, 33, or 308C group, were 5668 nM, 56613 or 4868 for glutamate, 2466 nM, 2464 or 2666 for aspartate, 307646 nM, 395634 or 304628 for glycine, 243642 nM, 214636 or 219652 for taurine and 234627
3. Results nM, 301634 or 256645 for alanine, respectively.
Al-3.1. Physiological parameters
Table 1 depicts physiological parameters at the resting period, 10 min of ischemia, and 80 min of recirculation. Mean arterial blood pressure increased by approximately 30 mmHg during carotid artery occlusion, and recovered to the resting values during recirculation. Each rat developed respiratory alkalosis during ischemia. There was no signifi-cant difference in any physiological parameters among the groups. Changes in CBF to the hippocampus are present in Fig. 1. CBF before ischemia was 46.362.2 ml / 100 g / min, 44.465.0 and 47.764.7 in the 36, 33 and 308C group, respectively. Brain hypothermia for 20 min did not alter the blood flow. Bilateral carotid artery occlusion for 20 min reduced CBF to less than 12 ml / 100 g / min in all groups. CBF increased from 85 to 160% of the resting value immediately after recirculation, followed by mild
Fig. 1. Changes in hippocampal blood flow in the 368C (n57), 338C
hypoperfusion (60–80% of the resting value) in all groups.
(n56) and 308C (n56) groups. Data are expressed as mean6S.E.M.
There was no significant difference in hippocampal blood Bilateral carotid artery occlusion reduced the blood flow to less than 12 flow during and after brain ischemia among the groups ml / 100 g / min in all groups. (b) P,0.05, (a) P,0.01 compared with the
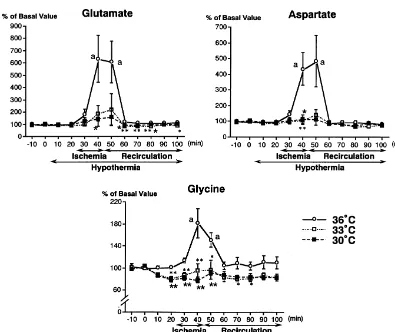
though concentrations of GABA were not detectable at the 3.4. Inhibitory amino acids resting periods, the basal values of the other amino acids
did not differ among the three groups, and changes in Twenty min after induction of hypothermia, concen-concentrations of amino acids were shown as percentages trations of taurine were significantly reduced to 80% of the
of basal values. basal value. In the 368C group, taurine increased to a
greater extent (16-fold) than glutamate and aspartate during and soon after cerebral ischemia (Fig. 3). On the
3.3. Excitatory amino acids other hand, changes in taurine levels in the 308C group
were only 3-fold and not significant compared with basal Concentrations of glutamate before ischemia were not levels. The elevation of taurine in the 338C group, how-altered by brain hypothermia. The extracellular glutamate ever, was still marked (10-fold, P,0.05). Concentrations significantly increased approximately 6-fold (P,0.01) of GABA increased to 171.0632.3 nM during ischemia in during bilateral carotid artery occlusion in the 368C group five of seven rats in the 368C group. In contrast, only one (Fig. 2). Concentrations of glutamate returned to the basal of six rats in each hypothermic group showed detectable level after 20 min of recirculation. Ischemia-induced GABA concentrations (338C group, 88.5 nM; 308C, 58.8 elevation of glutamate was markedly attenuated (F 2,165 nM).
6.17; P,0.02) to less than 2-fold the basal values in both
hypothermia groups. The time course of extracellular 3.5. Other amino acids aspartate was similar to glutamate (Fig. 2), showing 5-fold
increases by ischemia in the normothermic group, but no The ischemia-induced increase of glycine was relatively
significant elevation in hypothermic groups. small (2-fold) but significant in the normothermic group
Fig. 2. Changes in concentrations of glutamate, aspartate and glycine in the dialysate in the 368C (n57), 338C (n56) and 308C (n56) groups. Data are expressed as mean6S.E.M. of percentages of the resting value. Differences among the groups were significant in the concentrations of glutamate (F 2,1656.17; 0.02), aspartate (F 2,1654.55; P,0.05) and glycine (F 2,16513.0; P,0.001) by two-way repeated measured analysis of variance. * P,0.05,
w w w
Fig. 3. Changes in concentrations of taurine and alanine in dialysate in the 368C (n57), 338C (n56) and 308C (n56) groups. Data are expressed as mean6S.E.M. of percentages of the resting value. Differences among the groups were significant in the concentrations of taurine (F 2,1655.59; P,0.02)
w
and alanine (F 2,16512.2; P,0.001) by two-way repeated measured analysis of variance. * P,0.05, ** P,0.01, difference of 338C from 368C, P,0.05,
w w
P,0.01, difference of 308C from 368C by one-way analysis of variance and Dunnett’s t-test. The ischemia-induced increases in taurine and alanine were significant in the 368C and 338C groups. (b) P,0.05, (a) P,0.01 compared with the basal value by one-way analysis of variance and Dunnett’s t-test.
(Fig. 2). The extracellular glycine decreased by 20% at 20 tamate, aspartate) and inhibitory (taurine, GABA) amino min after induction of hypothermia, which lasted during acids in the hippocampus, which was for the first time ischemia and recirculation. Concentrations of alanine in demonstrated in the aged ischemic model.
the normothermic rats significantly elevated 3-fold during Because most of ischemic stroke occur in the elderly ischemia, and remained at high values during recirculation populations [35] and the age-related vulnerability to is-(Fig. 3). The alanine level was reduced in rats with chemia are reported [12,37], it is important to examine the hypothermia during and after cerebral ischemia (F 2,165 pathophysiology of brain ischemia and to explore effective
12.2; P,0.001). treatment with aged models [23]. Those studies are,
however, limited [11,12,37]. Therefore, our study using the
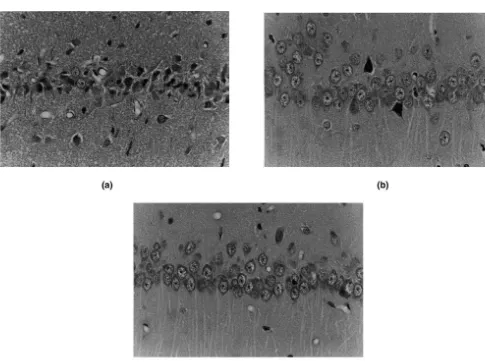
3.6. Histological examination aged hypertensive animals would provide useful
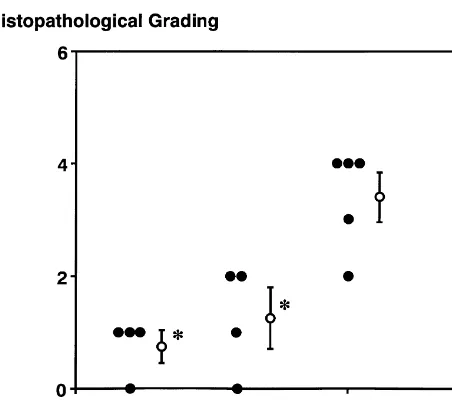
infor-mation for treatment of brain ischemia in aged populations. Photomicrographs of the CA1 subfield of the hippocam- Because CBF and other physiological parameters were pus 7 days after cerebral ischemia are shown in Fig. 4. In similar among normothermic and hypothermic animals in rats of the normothermic group, most pyramidal cells our study, factors other than circulation are suggested to revealed shrunken cytoplasm with acidophilic changes and contribute to the protection of hippocampal neurons. One pyknotic nuclei associated with perinuclear vacuolation. possible mechanism is the altered effluxes of excitatory Glial cells also increased in number. In contrast, degenera- amino acids, because ischemia-induced effluxes of gluta-tive pyramidal cells in the 338C group were scattered, and mate and aspartate are regarded to play crucial roles in the ischemic damages were markedly attenuated in the 308C development of ischemic neuronal damages in the adult group. Scores for ischemic damages (Fig. 5) were sig- animals [10,31,32,34]. Interestingly, our results revealed nificantly attenuated in both mild (1.360.5) and moderate- complete inhibition of effluxes of glutamate and aspartate ly (0.860.3) hypothermic groups as compared with the in the hippocampus by mild decreases of brain temperature
normothermic group (3.460.4). (by 38C). In the previous reports with adult animals
[22,24], mild brain hypothermia (approximately 38C reduc-tion) provided moderate reductions of the
ischemia-in-4. Discussion duced effluxes in the hippocampus. Therefore, mild brain
hypothermia may be more effective in the aged animals to In this study, we demonstrated that ischemic neuronal inhibit effluxes of excitatory amino acids, thereby leading damages in the hippocampus of aged SHR were markedly to effective protection of hippocampal neurons.
protected by mild and moderate reduction of brain tem- On the other hand, inhibitory amino acids, i.e., GABA perature. Furthermore, the protection was associated with and taurine, increase substantially in the extracellular space inhibition of ischemia-induced effluxes of excitatory (glu- during ischemia [14,16], and are suggested to protect neuronal cells against ischemic damages [1,20,33]. The protective effects of inhibitory amino acids are reported to be mediated by hyperpolarization via chloride channels [5,17,28]. Although recent studies suggest that the balance of excitatory and inhibitory amino acids is related to the selective vulnerability of the hippocampus in the adult models [15,21], we have shown that the imbalance is involved in the age-related vulnerability of hippocampus to ischemia [30]. Therefore, the significant elevation (10-fold) of taurine in the mild hypothermia (338C) may have contributed to neuronal protection.
Few reports are available regarding mechanisms for attenuation of ischemia-induced effluxes of neurotrans-mitters by brain hypothermia. It is reported that hypo-thermia prevents translocation of protein kinase C in cerebral ischemia [8]. Activation of synaptic enzymes such as protein kinase C or calcium-calmodulin kinase II facilitates the release of transmitters [10,27], and protein kinase C is involved in ischemia-induced transmitter release [26]. Therefore, preventing overactivation of
pre-Fig. 5. Histopathological grading of ischemic damages in the hippocam- synaptic enzymes may lead to attenuation of massive pal CA1. Ischemic damages were significantly reduced in both 30 and
releases of neurotransmitters. Another possibility is
pre-338C groups as compared with 368C group.* P,0.05 compared with the
servation of uptake mechanisms of amino acids. The
368C group by nonparametric Kruskal–Wallis’ h-test followed by
[14] N. Futrell, J.H. Garcia, E. Peterson, C. Millikan, Embolic stroke in
glia is suggested to be the major factor for effluxes of
aged rats, Stroke 22 (1991) 1582–1591.
amino acids during brain ischemia, and the uptake carrier
[15] M.Y.-T. Globus, R. Busto, E. Martinez, I. Valdes, W.D. Dietrich,
1 1
is driven by the transmembrane gradients for Na –K [2]. M.D. Ginsberg, Comparative effect of transient global ischemia on Because hypothermia attenuates ischemic depolarization extracellular levels of glutamate, glycine, and g-aminobutyric acid
[9], preservation of ionic gradients by hypothermia may in vulnerable and nonvulnerable brain regions in the rat, J. Neuro-chem. 57 (1991) 470–478.
attenuate the reversed operation of the uptake mechanism.
¨
[16] H. Hagberg, A. Lehmann, M. Sandberg, B. Nystrom, I. Jacobson, A.
In conclusion, mild brain hypothermia markedly
in-Hamberger, Ischemia-induced shift of inhibitory and excitatory
hibited the ischemia-induced effluxes of both excitatory amino acids from intra- to extracellular compartments, J. Cereb. and inhibitory amino acids, and ameliorated ischemic Blood Flow Metab. 5 (1985) 413–419.
neuronal damages in the hippocampus of aged SHR. Mild [17] M.A. Hausser, W.H. Yung, M.G. Lacey, Taurine and glycine activate¨
2
the same Cl conductance in substantia nigra dopamine neurons,
brain hypothermia may lead to one of the useful treatments
Brain Res. 571 (1992) 103–108.
against ischemic damages in the aged model.
¨ ¨
[18] L. Hillard, A. Hallstrom, S. Segersvard, L. Persson, U. Ungerstedt, Dynamics of extracellular metabolites in the striatum after middle cerebral artery occlusion in the rat monitored by intracerebral microdialysis, J. Cereb. Blood Flow Metab. 9 (1989) 607–616. Acknowledgements
[19] S. Ibayashi, K. Takano, H. Ooboshi, T. Kitazono, S. Sadoshima, M. Fujishima, Effect of selective brain hypothermia on regional cerebral
This work was supported by Grants-in-Aid from the blood flow and tissue metabolism using brain thermo-regulator in Ministry of Education, Science and Culture of Japan spontaneously hypertensive rats, Neurochem. Res. 25 (2000) 369–
375.
(04670390, 05305006), the Ministry of Health and Welfare
[20] A. Lehmann, H. Hagberg, A. Hamberger, A role for taurine in the
of Japan (11-008) and the Social Insurance Agency
maintenance of homeostasis in the central nervous system during
Contract Fund commissioned to the Japanese Health
hyperexcitation?, Neurosci. Lett. 52 (1984) 341–346.
Sciences Foundation. [21] B. Lin, M.Y.-T. Globus, W.D. Dietrich, R. Busto, E. Martinez, M.D.
Ginsberg, Differing neurochemical and morphological sequalae of global ischemia: comparison of single- and multiple-insult paradigms, J. Neurochem. 59 (1992) 2213–2223.
References [22] M. Matsumoto, M.S. Scheller, M.H. Zornow, M.A.P. Strant, Effects of S-emopamil, nimodipine, and mild hypothermia on hippocampal [1] M.S. Abel, D.W. McCandless, Elevatedg-aminobutyric acid levels glutamate concentrations after repeated cerebral ischemia in rabbits,
attenuate the metabolic response to bilateral ischemia, J. Neuro- Stroke 24 (1993) 1228–1234.
chem. 58 (1992) 740–744. [23] H. Minamisawa, M-.L. Smith and B.K. Siesjo, The effect of mild¨ [2] D. Attwell, B. Barbour, M. Szatkowski, Nonvesicular release of hypothermia and hypothermia on brain damage following 5, 10, and
neurotransmitter, Neuron 11 (1993) 401–407. 15 min of forebrain ischemia, Ann. Neurol. 28 (1990) 26–33. [4] A. Buchan, W.A. Pulsinelli, Hypothermia but not the N-methyl-D- [24] A. Mitani, K. Kataoka, Critical levels of extracellular glutamate
aspartate antagonist, MK-801, attenuates neuronal damage in gerbils mediating gerbil hippocampal delayed neuronal death during hypo-subjected to transient global ischemia, J. Neurosci. 10 (1990) 311– thermia: bran microdialysis study, Neuroscience 42 (1991) 661–
316. 670.
[5] M.H. Bureau, R.W. Olsen, Taurine acts on a subclass of GABAA [25] C. Millikan, Animal stroke models, Stroke 23 (1992) 795–797. receptors in mammalian brain in vitro, Eur. J. Pharmacol. 207 [26] H. Nakane, H. Yao, S. Ibayashi, T. Kitazono, H. Ooboshi, H. (1991) 9–16. Uchimura, M. Fujishima, Protein kinase C modulates
ischemia-´
[6] R. Busto, W.D. Dietrich, M.Y.-T. Globus, I. Valdes, P. Sheinberg, induced amino acids release in the striatum of hypertensive rats, M.D. Ginsberg, Small differences in intra-ischemic brain tempera- Brain Res. 782 (1998) 290–296.
ture critically determine the extent of ischemic neuronal injury, J. [27] Y. Nishizuka, Studies and perspectives of protein kinase C, Science Cereb. Blood Flow Metab. 7 (1987) 729–738. 233 (1986) 305–312.
¨
[8] M. Cardel, F. Boris-Moller, T. Wieloch, Hypothermia prevents the [28] R.W. Olsen, E.H.F. Wong, G.B. Stauber, D. Murakami, R.G. King, ischemia-induced translocation and inhibition of protein kinase C in J.B. Fischer, Biochemical properties of the GABA / barbiturate / the rat striatum, J. Neurochem. 57 (1991) 1814–1817. benzodiazepine receptor chloride ion channel complex, Adv. Exp. [9] Q. Chen, M. Chopp, G. Bodzin, H. Chen, Temperature modulation Med. Biol. 175 (1984) 205–219.
of cerebral depolarization during focal cerebral ischemia in rats: [29] H. Ooboshi, S. Sadoshima, H. Yao, T. Nakahara, H. Uchimura, M. correlation with ischemic injury, J. Cereb. Blood Flow Metab. 13 Fujishima, Inhibition of ischemia-induced dopamine release by v -(1993) 389–394. conotoxin, a calcium channel blocker, in the striatum of sponta-[10] D.W. Choi, Cerebral hypoxia: some new approaches and unanswered neously hypertensive rats – in vivo brain dialysis study, J.
Neuro-questions, J. Neurosci. 10 (1990) 2493–2501. chem. 58 (1992) 298–303.
[11] D. Corbett, S. Nurse, F. Colbourne, Hypothermic neuroprotection. A [30] H. Ooboshi, H. Yao, S. Ibayashi, S. Sadoshima, M. Fujishima, H. global ischemia study using 18- to 20-month-old gerbils, Stroke 28 Nakane, T. Matsumoto, M. Hirano, H. Uchimura, Ischemia-induced (1997) 2238–2242. release of amino acids in the hippocampus of aged hypertensive rats, [12] M. Davis, A.D. Mendelow, R.H. Perry, I.R. Chambers, O.F. James, J. Cereb. Blood Flow Metab. 15 (1995) 227–234.
recovery of neuronal function following cerebral hypoxia: an in [36] F.A. Welsh, R.E. Sims, V.A. Harris, Mild hypothermia prevents vitro study, Life Sci. 40 (1987) 2059–2066. ischemic injury in gerbil hippocampus, J. Cereb. Blood Flow Metab. [34] R.P. Simon, J.H. Swan, T. Griffiths, B.S. Meldrum, Blockade of 10 (1990) 557–563.
N-methyl-D-aspartate receptors may protect against ischaemic dam- [37] H. Yao, S. Sadoshima, H. Ooboshi, Y. Sato, H. Uchimura, M. age in the brain, Science 226 (1984) 850–852. Fujishima, Age-related vulnerability to cerebral ischemia in sponta-[35] K. Ueda, Y. Hasuo, T. Ohmura, Y. Kiyohara, H. Kawano, I. Kato, A. neously hypertensive rats, Stroke 22 (1991) 1414–1418.