www.elsevier.com / locate / bres
Research report
Specific subnuclei of the nucleus tractus solitarius play a role in
determining the duration of inspiration in the rat
a b a a ,
*
Adam M. Wasserman , Niaz Sahibzada , Yvonne M. Hernandez , Richard A. Gillis
a
Department of Pharmacology Georgetown University Medical Center Washington, DC 20007 USA
b
Department of Psychology University of the District of Columbia Washington, DC 20008 USA Accepted 2 July 2000
Abstract
Our previous data obtained in the cat suggest that the neurons of the ventrolateral subnucleus of the tractus solitarius (vlNTS) act as an inspiratory off-switch and terminate the inspiratory phase of the respiratory cycle (Berger et al., Eur. J. Pharmacol. 277 (1995) 195–208; Gillis et al., Neurosci. Abstr. 23 (1997) 725). The purpose of the present study was to determine whether inhibition of the region of the vlNTS of the rat using drugs that hyperpolarize, disfacilitate or block both axonal conduction and action potential generation would alter the inspiratory phase of the respiratory cycle. Experiments were conducted in anesthetized, vagotomized and spontaneously breathing rats while monitoring diaphragmatic electromyogram activity. Vagus nerves were sectioned in order to rule out prolongation of inspiration evoked by microinjection of agents into the vlNTS which block excitatory drive from lung afferent inputs. Bilateral microinjection of the inhibitory amino acidg-aminobutyric acid (GABA) 25 nmol / 45 nl produced an immediate prolongation of inspiratory duration (484618 to 1291684 ms) and an apneustic pattern of breathing. Other effects observed were a significant shortening of expiratory duration (778636 to 432638 ms), rise in blood pressure (8364 to 10866 mmHg) and a small but significant increase in heart rate (439617 to
1 452618 beats / min). Bilateral microinjection of the ionotropic glutamate receptor antagonist kynurenic acid (1 nmol) and the Na channel blocker tetrodotoxin (10 pmol) into the region of the vlNTS consistently produced a similar prolongation of inspiratory duration and an apneustic pattern of breathing. These results support the hypothesis that neurons in the region of the vlNTS promote the transition from inspiration to expiration and function as part of the ‘Inspiratory Off Switch’. 2000 Elsevier Science B.V. All rights reserved.
Theme: Endocrine and autonomic regulation
Topic: Respiratory regulation
Keywords: Apneusis; Respiration; Nucleus tractus solitarius; Inspiratory Off Switch
1. Introduction motoneurons’’ [3], their role in CNS control of breathing is now considered to be relatively minor, especially in Respiratory rhythm and pattern formation are thought to rodents [2,22,35,39]. Indeed, removal of the DRG appears be products of respiratory neurons concentrated into two to have no effect on rhythm generator neurons in a slice or discrete areas of the medulla oblongata [14,23]. One of brainstem–spinal cord preparation [34,39]. These findings these areas of respiratory neurons is located in the dor- raise the question as to what role is played by the DRG in somedial medulla around the obex and has been designated the control of breathing and addressing this question is the the dorsal respiratory group (DRG). In the cat, the DRG focus of the present study.
corresponds most closely to the ventrolateral nucleus of the Currently, the consensus of opinion is that the DRG can solitary tract. While these neurons were at one time convey respiratory rhythmic drive to phrenic and intercost-suggested as the ‘‘principal respiratory drive to phrenic al motoneurons [5]. This opinion is based on studies carried out in cats where neurons of the DRG, specifically those neurons located in the vlNTS monosynaptically excite phrenic and intercostal motoneurons [12,22]. In the *Corresponding author: Tel.: 11-202-687-1607; fax: 1
1-202-687-rat, however, very few neurons of the vlNTS monosynapti-6437.
E-mail address: [email protected] (R.A. Gillis). cally excite motoneurons in the spinal cord that control 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
muscles for breathing [9,31,43]. The vlNTS respiratory incision below the chest cavity and inserting a hooked neurons can also transfer afferent information from the bipolar platinum–iridium electrode to the right half of the lungs to VRG bulbospinal inspiratory neurons [5]. How- costal diaphragm. The electrode was coupled to a Tek-ever, data obtained for both the cat and rat indicate that tronix AM 502 differential AC preamplifier (Tektronix, this is not the role of the vlNTS neurons but is the function Wilsonville, OR). The output signal of the amplifier was of neurons located in the medial subnucleus of the tractus fed into an audiometer and displayed on a storage oscillos-solitarius (mNTS) [6,26]. In a recent study of the role of cope and computer monitor. Data were stored on computer DRG neurons in control of respiration in the rat it was (Apple Macintosh PowerPC connected to MacLab) for concluded based on cross-correlation analysis that DRG later viewing and analysis. The dEMG signal and the neurons did not appear to convey respiratory rhythmic software-generated integration (iEMG) of the raw signal drive to phrenic and intercostal motoneurons [42]. It was using a 100-ms time window were continuously recorded also concluded that DRG neurons had little role in and stored for off-line analysis. Heart rate was obtained transmitting afferent information from the lungs to VRG from the blood pressure signal and was averaged over bulbospinal inspiratory neurons. eight beat bins to minimize beat-to-beat fluctuations.
Our previous data obtained in the cat suggest another possible role for the vlNTS, namely to act as an inspiratory
off switch and terminate the inspiratory phase of the 2.2. Stereotaxic surgery and microinjection procedure respiratory cycle [4,15]. In these studies we found that
microinjection of NMDA receptor antagonists into the Animals were oriented ventral-side down and placed in vlNTS produced an increase in inspiratory duration and an a Kopf small-animal stereotaxic frame (Tujunga, CA). The apneustic breathing pattern. Since numerous investigators dorsal medulla was exposed via a limited occipital have reported the existence of respiratory-related neurons craniotomy with reflection of the dura and retraction of the in the vlNTS and adjacent interstitial subnucleus of the cerebellum in an analogous fashion as described in the cat tractus solitarius (iNTS) of the rat [6,9,26,28,29,38,41,42], [33]. Preliminary studies (not shown) using the calamus we set out to test the hypothesis that these neurons in the scriptorius (i.e., caudal tip of the area postrema) as our rat can function as inspiratory off-switch neurons. reference point were used to determine microinjection coordinates for the specific subnuclei of the NTS. Briefly, Fast Green FCF dye (5%) was microinjected into a series of coordinates and the resulting dye locations compared 2. Methods with known locations of various subnuclei according the studies of Kalia and Sullivan [20] to determine the 2.1. General experimental preparation coordinates used for our experiments. Coordinates used for vlNTS microinjections were the following: 0.5 mm rostral Experiments were performed on 40 adult male Sprague– to calamus scriptorius; 0.9–1.1 mm lateral to midline; Dawley rats (Taconic, Germantown, NY) weighing 260– 0.6–0.8 mm below dorsal surface of the brainstem. 410 g. Anesthesia was instituted with a 3-ml / kg i.p. Coordinates for medial NTS (mNTS) injections were 0.5 / injection of a cocktail containing urethane (800 mg) and 0.4–0.6 / 0.4 mm, for rostrocaudal, mediolateral and
2.3. Assessment of apneusis and analysis of data the placement of microinjection sites in the rostrocaudal axis use as a reference point the opening of the central To the best of our knowledge, no strict criterion has canal into the fourth ventricle (Fig. 1) which we will been employed to distinguish an ‘apneustic’ breath from a abbreviate as CC / IV. The reason for this is that the latter breath demonstrating prolonged inspiratory duration. The reference point can be localized to one 50-mm slice distinction between these two patterns of breathing rests whereas assessment of calamus scriptorius in coronal slices upon the difference in length of time spent in maximal or can be indistinct and may appear to occur on any one of near-maximal inspiratory effort. In the present study, we two or three sections. We also will not use the term ‘obex’ defined an apneustic breath as one that maintained a as a reference point as this term has been variously maximal or near maximal level of inspiratory effort for described as occurring at the most caudal extent of the area
.650 ms and with a total inspiratory duration (T )i .900 postrema [28,29] as well as in coronal sections in which ms. Onset of apneusis was judged to occur at the second area postrema is well developed [11].
apneustic breath. Duration of apneustic breathing was
determined as the time from the second apneustic breath 2.6. Data analysis (i.e., onset) to the occurrence of the second-to-last
apneus-tic breath. All post-drug cardiorespiratory values were Data presented are the means6standard error of the taken at the time of maximal prolongation of T . Thei mean. Statistical analysis was performed using a paired means of five successive respiratory cycles were used to t-test for analysis comparing baseline with post-drug calculate the values reported in these studies. values. An unpaired t-test was used to compare baseline values between vagotomized and non-vagotomized groups. 2.4. Experimental procedure When comparing incidences of apneustic breathing be-tween treatment groups and vehicle controls, a Fisher After bilateral insertion of micropipettes, animals were Exact test was used. In all cases, P,0.05 was the criterion allowed to stabilize for at least 5 min before a baseline used for statistical significance.
recording was obtained. Subsequent to this, drugs of interest or vehicle (saline) were microinjected bilaterally
over a period of approximately 1 min. In two cases, saline 3. Results was loaded into the adjacent barrel and microinjected first
and cardiorespiratory effects recorded for at least 15 min 3.1. Microinjection sites prior to microinjection of drug. In six early experiments,
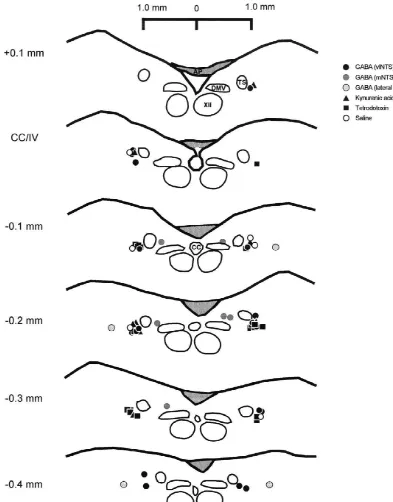
Fast Green FCF dye 5% loaded into the barrel adjacent to All microinjection sites were found to be located within the drug was used to mark drug injection sites to verify a region 0.4 mm caudal to 0.1 mm rostral to the opening of injection site location. This was done only after the the central canal into the fourth ventricle (CC / IV; Fig. 1). experiment had been completed in order to avoid respirato- Microinjections into the region of the vlNTS were found to ry effects of the dye itself. Subsequent studies did not use be distributed from an area lateral to the solitary tract to a Fast Green dye as microinjection sites could be determined region lateral and ventral to the solitary tract. These from microscopic analysis of tissue sections alone. microinjection sites are located within the general borders of the ventrolateral and interstitial subnuclei of the solitary
2.5. Histology tract as defined by Kalia and Sullivan [20].
Non-vagotom-ized animals that received GABA microinjections to the Upon completion of the experiment, animals were region of the vlNTS are not depicted in Fig. 1. These were sacrificed by administration of an overdose of pentobarbi- omitted to reduce clutter but were found to be contained tal sodium through the arterial line. The brain was rapidly within the region of the vlNTS in locations that fall within removed and placed in a solution of 6% buffered parafor- those depicted here. Injection sites medial to the solitary maldehyde and transferred within 24–48 h into 20% tract were contained in an area slightly dorsal to the lateral sucrose in phosphate-buffered saline for at least 24 h half of the DMV. Injection sites directed lateral to the before sectioning at 50 mm for histological analysis. solitary tract were found to be located in the reticular area Sections were stained with neutral red or cresyl violet to 0.4–0.6 mm lateral to the vlNTS region at a dorsoventral facilitate identification of nuclear groups, and the site of level similar to the vlNTS sites (Fig. 1).
microinjection was determined in the following manner: as
the ventral-most extent of identified pipette tracks or the 3.2. GABA microinjection into the vlNTS site of highest density of Fast Green FCF dye (5%).
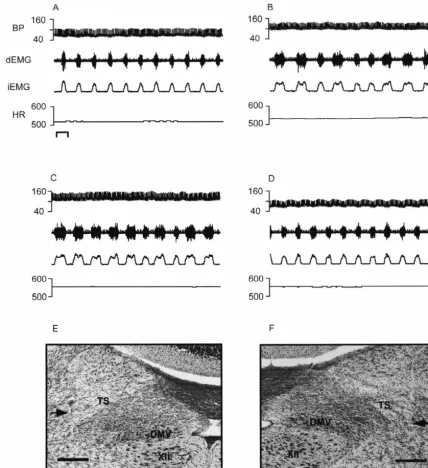
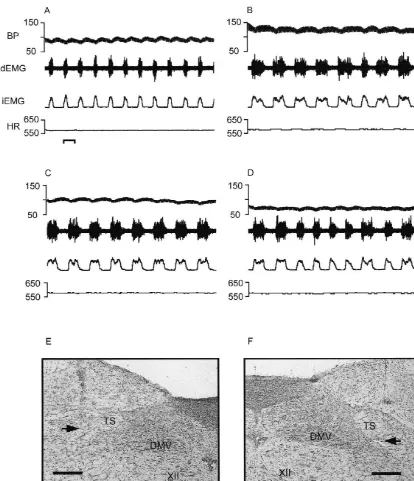
microin-Fig. 1. Summary of microinjection sites. Camera lucida reconstruction of microinjection sites of drugs administered to vagotomized rats. Coordinates listed describe location relative to the opening of the central canal into the fourth ventricle (CC / IV), see Section 2 for description. Abbreviations: AP, area postrema; CC, central canal; XII, hypoglossal nucleus; DMV, dorsal motor nucleus of the vagus, TS, solitary tract.
Table 1
a
Cardiorespiratory response to bilateral microinjection of drugs into the nucleus of the solitary tract
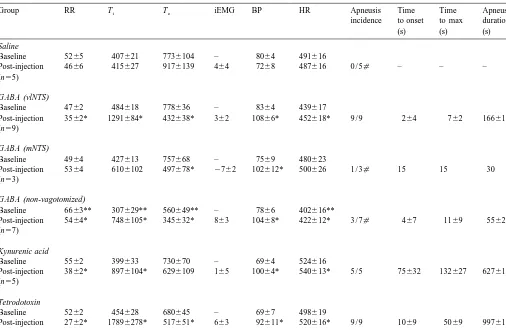
Group RR Ti Te iEMG BP HR Apneusis Time Time Apneusis
incidence to onset to max duration
Abbreviations and units: RR, respiratory rate (breaths / min); T , inspiratory duration (ms); T , expiratory duration (ms); iEMG, integrated diaphragmatici e
EMG signal (% change from baseline); BP, blood pressure (mmHg); HR, heat rate (beats / min). All post-injection values were obtained at the time of maximal effect on Ti. All animals were vagotomized unless otherwise indicated.
*P,0.05 versus baseline values;[P,0.05 versus GABA microinjection into vlNTS of vagotomized animals; **P,0.05 versus baseline of vagotomized
animals.
response although this was not always the case. Pressure pressure and heart rate produced by GABA were quantita-was not increased in one experiment in which apneusis tively similar to those changes observed in vagotomized was produced (data not shown). A small (3%) but signifi- animals (Table 1).
cant increase in heart rate was also observed in this group.
A summary of microinjection sites is presented in Fig. 1. 3.3. GABA microinjection into the medial NTS (mNTS) GABA was also microinjected bilaterally in seven
subsequent to microinjection. Onset of apneusis was 15 s tetrodotoxin (0.22 mM; 45 nl) into the region of the vlNTS after completion of bilateral microinjection and the effect in nine animals produced a severe apneustic pattern which was over by 30 s after administration (Table 1). Our was quick in onset and long in duration (Table 1; Fig. 5). criterion, as discussed in Section 2, requires two or greater T was more prolonged with this treatment (nearly 4-foldi apneustic breaths to qualify as apneusis. Therefore, this increase in duration) than with GABA (P,0.05) although response was deemed apneusis though all incidences of baseline respiratory values in the two groups were not apneusis observed with GABA microinjected into the significantly different. Decreases in Te and increases in vlNTS involved dozens of apneustic breaths occurring over pressure and heart rate were similar to those observed in a period of 127–195 s duration. The remaining two animals receiving GABA or kynurenic acid microinjec-animals receiving GABA microinjections into the mNTS tions into the vlNTS. Another difference noted in the had no evidence of any apneustic breaths. Expiratory cardiorespiratory response of a subset of animals microin-duration decreased significantly (Table 1) and resembled jected with tetrodotoxin as compared to animals receiving changes observed after vlNTS microinjection. No signifi- GABA and kynurenic acid was a delayed reduction in cant alterations were seen in the amplitude of the dia- iEMG amplitude as well as in mean blood pressure. In two phragmatic electromyogram. Overall, blood pressure was of three animals in which this response occurred, a low observed to increase significantly with mNTS microinjec- amplitude apneustic breathing pattern was observed to tion of GABA (136%, Table 1). This increase was similar develop into an apnea at the same time as this hypotensive in magnitude to pressure alterations observed after GABA response and the animals died. In the other animal, blood microinjection into the vlNTS region (Fig. 3C versus 3E). pressure was low and iEMG amplitude was profoundly In two animals, microinjection of GABA into the sites reduced but a fatal apnea did not develop; in fact, lateral to the vlNTS (Fig. 1) also had no effect on respiratory rate was observed to return towards the cardiorespiratory function (RR, 53 breaths / min pre-in- baseline value.
jection to 56 breaths / min post-injection; iEMG, no
change; T , 379–445 ms; T , 717–613 ms; BP, 78–73i e 3.6. Vehicle microinjection into the ventrolateral nucleus mmHg; HR, 513–516 beats / min). of the solitary tract(vlNTS)
3.4. Blockade of excitatory amino acid To control for possible effects of microinjection of 45 nl neurotransmission in the vlNTS of volume into the vlNTS, the same volume (45 nl of 0.9% saline, pH 7.4) of vehicle was bilaterally microinjected As direct inhibition of neurons in the region of the into the region of the ventrolateral nucleus of the solitary vlNTS was capable of producing a rapid and severe tract while monitoring respiratory parameters in five apneustic pattern of breathing, we sought to determine if animals. Bilateral microinjection of vehicle produced no disfacilitation of neurons in this region could achieve a significant effects on respiration or cardiovascular function similar result. Kynurenic acid is an antagonist at ionotropic when assessed 30 s after microinjection (Table 1). Upon glutamatergic receptors and microinjection of this com- injection there was a transient reduction in respiratory rate pound would block basal glutamatergic input to the region. lasting less than 10 s in all cases and a mild reduction in Kynurenic acid (22 mM; 45 nl) was microinjected into the mean blood pressure which returned to pre-injection levels region of the vlNTS bilaterally in five animals (Fig. 1), all in three of five animals and remained slightly below of which developed apneusis (Fig. 4). In general, the time baseline in two animals.
course of the apneusis produced by kynurenic acid had a longer latency to onset and a longer duration of effect
(Table 1), although, as shown in Fig. 4, onset in one case 4. Discussion was nearly immediate. Interestingly, in contrast to data
obtained with GABA, T was not significantly affected.e Bilateral microinjection of drugs that produce neural This was the only group examined in which apneusis was depression (GABA, kynurenic acid and tetrodotoxin) into not accompanied by a significant reduction in expiratory the region of the ventrolateral NTS in rats consistently duration. Kynurenic acid administration into the vlNTS prolonged T and, in most animals, evoked an apneustici also significantly increased blood pressure. Heart rate also pattern of breathing. Prolongation of T and generation ofi increased, but only by an average of 3%. apneusis resulting from suppression of synaptic activity (GABA, kynurenic acid) and / or action potential gene-3.5. Global inhibition of activity in the vlNTS region ration (tetrodotoxin) supports our hypothesis that neurons in this region have an important role as inspiratory
off-1
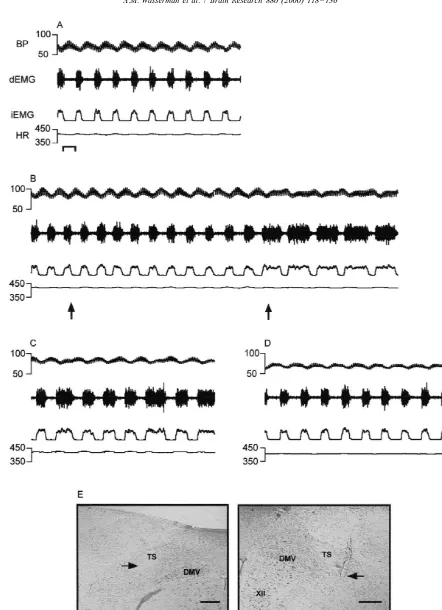
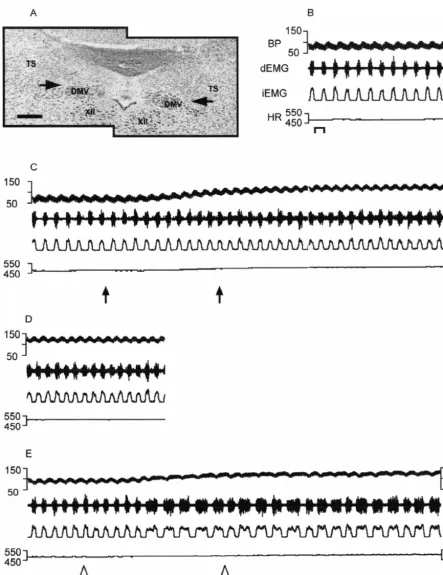
Fig. 4. Bilateral microinjection of kynurenic acid into the ventrolateral NTS produces apneustic breathing in the vagotomized rat. (A) Baseline recording of cardiorespiratory activity; (B) 30 s post bilateral microinjection of kynurenic acid 1 nmol / 45 nl into the vlNTS; (C) 5 min after kynurenic acid microinjection; (D) 20 min after microinjection of kynurenic acid cardiorespiratory activity appears to return to baseline. Bar51 second. Photomicrograph of left and right microinjection sites are depicted in (E) and (F), respectively. Abbreviations as in Fig. 1. Bars50.25 mm.
Miyazaki et al. defined these neurons as pump cells due to lateral to the solitary tract, at a site where they would be their activation by lung inflation, despite the fact that pump inhibited by drugs that were used in our study. Indeed, in a cells have previously been defined as lacking centrally second study of these neurons, Miyazaki et al. [29] generated respiratory rhythm [1]. Most importantly, 38% reported that the accentuated firing at the time of phase-(i.e., 20 out of 52) of these pump cells were most active switching was due to glutamatergic input and that blockade during the period of transition from inspiration to expira- of GABAA receptors could amplify this glutamatergic tion. The inspiratory off switch (IOS) is operative during input. Presumably, blockade of the glutamatergic input this phase transition and Miyazaki et al. propose that this with kynurenic acid or turning down the ‘amplifier’ by
1
Fig. 5. Effects of bilateral microinjection of tetrodotoxin into the ventrolateral NTS of the rat. (A) Baseline recording of cardiorespiratory activity. Bar51 s. (B) Recording obtained 1.5 min post-bilateral microinjection of tetrodotoxin 10 pmol / 45 nl into the vlNTS. Onset of apneusis was 34 s after administration of drug. (C) Four minutes after bilateral microinjection of tetrodotoxin; (D) 5 min post-tetrodotoxin microinjection. Photomicrographs of left and right microinjection sites are depicted in (E) and (F), respectively. Abbreviations as in Fig. 1. Bars50.25 mm.
We stress that the pump neurons exhibiting enhanced ed drugs. Presumably, activity of these neurons is involved discharge around the transition period from late inspiration in termination of inspiration; therefore, inhibition of these to early expiration were located primarily immediately neurons with drugs such as GABA, kynurenic acid, and lateral as well as ventrolateral to the solitary tract, with a tetrodotoxin, as was done in our study, would be expected few neurons scattered within the solitary tract [28,29]. to prolong T and produce apneusis. Conversely, excessivei These appear to be within the borders defined by Kalia and stimulation of these specialized pump cells by microinjec-Sullivan [20] as comprising the ventrolateral as well as tion of glutamate into the ventrolateral region of the NTS interstitial subnuclei (niTS) of the solitary tract in the rat. would be expected to inhibit inspiration and lead to apnea. Although the target site for our drug microinjections was This effect has indeed been found to occur [24].
kynurenic acid and tetrodotoxin results in prolongation of the cat reported that apneustic breathing could be produced T and apneusis were obtained in rats with vagus nervesi from the Pre-Boetzinger Complex by partial blockade of sectioned. A similar qualitative result was also obtained synaptic inhibition [36].
with microinjection of GABA in rats with vagus nerves Although the drugs that we studied, namely, GABA, left intact. This result is consistent with our earlier findings kynurenic acid and tetrodotoxin all prolonged Ti and in cats showing that prolongation of T and apneusis cani produced apneusis without significantly affecting the am-occur in both vagotomized and non-vagotomized animals plitude of the iEMG, there were some differences among when an antagonist of NMDA receptors is microinjected the effects of these drugs. One difference was seen with into the vlNTS [4]. It should be noted that while mi- kynurenic acid which caused apneusis but did not sig-croinjection of GABA into the vlNTS / niTS region of rats nificantly affect T . In contrast, GABA and tetrodotoxine with vagus nerves intact always prolonged Ti in our shortened T significantly. Another difference in responsee studies, apneusis was observed in less than one-half of was seen with tetrodotoxin which, in a subpopulation of these animals. In comparison, GABA microinjection pro- animals, produced a secondary reduction in iEMG and duced apneusis in all rats with cervical vagotomy. The mean arterial blood pressure minutes after the induction of apparent resistance to apneusis development in rats with apneusis. GABA and kynurenic acid microinjections never vagus nerves intact might be due to the fact that two produced these delayed reductions in iEMG amplitude. We sources of excitatory drive are influencing the pump cells have no explanation for the divergent effect of kynurenic described by Miyazaki et al. [29]. That is, excitatory inputs acid on Te compared with other drugs studied, but the from the periphery, via the afferent vagal fibers from the delayed effect of tetrodotoxin on iEMG might be related to lung, and from within the brain are driving these neurons its ability to inhibit sodium-dependent axonal conduction. at or near the time of phase-switching (see Fig. 12 of That is, anterograde labeling methods in the rat have Miyazaki et al. [29]). In vagus nerve intact animals, revealed that a subset of axonal projections of pre-microinjected GABA would need to inhibit both these motoneurons of the rostral VRG (rVRG) course dorsally in excitatory inputs onto the pump neurons whereas, in the medulla through the region of the NTS prior to vagotomized rats, GABA would only have to inhibit the descending through the contralateral medulla to innervate central excitatory input to these pump cells. the phrenic motor nucleus in the spinal cord [9,13,45]. Resistance to apneusis in animals with intact vagus Axonal recordings demonstrating an inspiratory pattern nerves may also be explained by the findings of Bonham have been described in this NTS region as well [45]. It and McCrimmon [6] that report the presence of pump cells therefore seems possible that the delayed effect of TTX to that are not restricted to the vlNTS. These investigators reduce iEMG amplitude is due to the slow development of observed pump cells dorsal and medial to other respiratory a use-dependent block of axonal conduction in bulbospinal neurons in what they consider constitutes the dorsal fibers of passage from the VRG to the phrenic motor respiratory group in the rat. With intact vagus nerves these nucleus.
pump cells, excited by slowly adapting pulmonary stretch The drugs that we used as tools to examine the role of receptors (SAR), could function to terminate inspiration the vlNTS region in the control of respiration in the rat not but might escape the influence of microinjections of only affected respiration but also affected blood pressure. GABA that was targeted to suppress neuronal activity in Specifically, microinjection of GABA, kynurenic acid and the immediate vicinity of the vlNTS. In animals with tetrodotoxin into the vlNTS region resulted in significant vagus nerves sectioned these pump cells ‘outside’ the increases in mean blood pressure. This response has been vlNTS would not be excited by SAR leaving the respirato- reported by other investigators who have used GABAA ry cycle more sensitive to alterations in the function of receptor agonists and kynurenic acid in the medial NTS
vlNTS neurons. [10,16]. The typical effect of microinjection of either
GABAer-gic input; thus, the GABA receptors present on these has been observed in the in vitro neonatal rodent brain-neurons are available to respond to exogenously microin- stem–spinal cord preparations after elimination of pontine jected GABA. The result is an inhibition of these neurons and vagal afferents [17]. The lack of a profound influence and a rise in arterial blood pressure. Tetrodotoxin, pre- of the vlNTS, the pons and vagal afferents on inspiratory sumably, silences these same second order NTS neurons duration of the in vitro neonatal rodent brainstem–spinal that exert tonic inhibition of central sympathetic outflow. cord preparations is difficult to resolve at the present time. The anatomical location of the majority of second-order In summary, our data provide evidence that the neurons neurons involved in the baroreceptor reflex pathway is the within the ventrolateral region of the nucleus of the mNTS [27,32,44]; however, the vlNTS as well as the niTS solitary tract play an important role in determining the and dorsolateral NTS have been found to be targets for duration of inspiration in the rat. Microinjection of drugs baroreceptor afferents from the carotid sinus in the rat [18]. which inhibit neuronal activation and / or action potential Hence, microinjection of drugs into the vlNTS region in conduction in this area of the dorsomedial medulla reliably our study appears to be influencing a significant number of produce apneustic breathing. Additionally, these studies the second-order inhibitory NTS neurons that are com- were largely conducted in vagotomized rats, which were ponents of the baroreceptor reflex pathway. Furthermore, significantly more susceptible to induction of apneusis by section of cervical vagus nerves does not appear to affect vlNTS inhibition than those with vagus nerves intact, this the pressor response to GABA microinjected into the suggests vagal afferents, most likely from pulmonary vlNTS, and this agrees with the findings of Ito and Sved stretch receptors mediating the Hering–Breuer reflex path-[19] who observed no difference in the depressor response way, supply excitatory inputs to neurons in this region. to the GABA receptor antagonist bicuculline microinject-A Thus, the region of the vlNTS appears to function as an ed into the mNTS of control versus acutely sinoaortic integral part of the ‘Inspiratory Off Switch’ in the rat. denervated rats.
Although we were frequently not able to avoid blood
pressure effects when we targeted our drugs for neurons in Acknowledgements the vlNTS region, the effects on inspiratory duration were
site specific and limited to the vlNTS region. GABA The authors would like to thank Dr. Manual Ferreira Jr. targeted to neurons of the mNTS region did not induce for expert assistance and Roxanne Brown and Bernice significant prolongation of T overall, though in one animali Williams for technical help. This work was supported by an exceptionally mild and short-lived apneusis occurred. National Institutes of Health Grants NS 28130 (R.A.G.), Additionally, the respiratory changes noted with GABA NS 36035 (N.S.) and a National Institutes of Health microinjection into the vlNTS region occurred independent Predoctoral Fellowship DA005889 (A.M.W.).
of blood pressure changes in a few animals (data not shown).
Consistent with our findings that specific subnuclei of
References the NTS play a role in determining the duration of
inspiration in the rat are findings of other investigators who
[1] A.J. Berger, Dorsal respiratory group neurons in the medulla of the have reported that inhibition of vlNTS neurons either by
cat: spinal projections, responses to lung inflation and superior focal cold block or lidocaine microinjection will cause
laryngeal nerve stimulation, Brain Res. 135 (1977) 231–254. apneustic breathing. Koepchen et al. reported that, in the [2] A.J. Berger, K.A. Cooney, Ventilatory effects of kainic acid injection cat, cooling neurons in the region of the vlNTS produces of the ventrolateral solitary nucleus, J. Appl. Physiol. 52 (1982)
131–140. prolongation of inspiratory duration and induction of
[3] A.J. Berger, R.A. Mitchell, J.W. Severinghaus, Regulation of apneusis in the absence of lung inflation [21]. Budzinska et
respiration, New Engl. J. Med. 297 (1977) 138–143. al. also reported that focal cold block of dorsal respiratory
[4] I. Berger, R.A. Gillis, S. Vitagliano, W.H. Panico, S. Magee, M. group neurons causes apneustic-type breathing in cats [8]. Kelly, W.P. Norman, J.E. McManigle, A.M. Taveira Da Silva, Prolongation of inspiratory duration has also been reported NMDA receptors are involved at the ventrolateral nucleus tractus to occur after lidocaine blockade of the NTS region in solitarii for termination of inspiration, Eur. J. Pharmacol. 277 (1995)
195–208. rabbits [7]. In addition, prolongation of inspiratory
dura-[5] A.L. Bianchi, M. Denavit-Saubie, J. Champagnat, Central control of tion and / or apneustic-type breathing have been reported to
breathing in mammals: neuronal circuitry, membrane properties, and occur in some [2,21,37], but not all studies [25,40] in the neurotransmitters, Physiol. Rev. 75 (1995) 1–45.
cat, following lesions in the vlNTS. It should be noted that [6] A.C. Bonham, D.R. McCrimmon, Neurons in a discrete region of data from studies of the in vitro neonatal rodent brain the nucleus tractus solitarius are required for the Breuer-Hering
reflex in rat, J. Physiol (London) 427 (1990) 261–280. stem–spinal cord preparation indicate no apneustic-type
[7] K. Budzinska, J.R. Romaniuk, The role of raphe and tractus respiratory rhythmicity after horizontal transections which
region of the medulla in the cat, Acta Physiol. Scand. 124 (1985) tion and retention in vitro of fluorescently labeled aortic baro-317–328. receptor terminals on neurons from the nucleus tractus solitarius, [9] D. de Castro, J. Lipski, R. Kanjhan, Electrophysiological study of Brain Res. 581 (1992) 339–343.
dorsal respiratory neurons in the medulla oblongata of the rat, Brain [28] M. Miyazaki, A. Arata, I. Tanaka, K. Ezure, Activity of rat pump Res. 639 (1994) 49–56. neurons is modulated with central respiratory rhythm, Neurosci. [10] J.M. Catelli, W.J. Giakas, A.F. Sved, GABAergic mechanisms in Lett. 249 (1998) 61–64.
nucleus tractus solitarius alter blood pressure and vasopressin [29] M. Miyazaki, I. Tanaka, K. Ezure, Excitatory and inhibitory release, Brain Res 403 (1987) 279–289. synaptic inputs shape the discharge pattern of pump neurons of the [11] E.G. Dobbins, J.L. Feldman, Brainstem network controlling de- nucleus of the tractus solitarii in the rat, Exp. Brain Res. 129 (1999)
scending motor drive to phrenic motoneurons in rat, J. Comp. 191–200.
Neurol. 347 (1994) 64–86. [30] P.A. Nunez-Abades, A.M. Morillo, R. Pasaro, Brainstem connect-[12] J. Duffin, J. Lipski, Monosynaptic excitation of the thoracic ions of the rat ventral respiratory subgroups: afferent projections, J.
motoneurons by inspiratory neurons in the nucleus tractus solitarius Autonom. Nerv. Syst. 42 (1993) 99–118.
in the cat, J. Physiol. 390 (1987) 445–451. [31] T. Onai, M. Saji, M. Miura, Projections of supraspinal structures to [13] H.H. Ellenberger, J.L. Feldman, Monosynaptic transmission of the phrenic motor nucleus in rats studied by a horseradish per-respiratory drive to phrenic motoneurons from brainstem bulbospi- oxidase microinjection method, J. Auton. Nerv. Syst. 21 (1987) nal neurons in rats, J. Comp. Neurol. 269 (1988) 47–57. 233–239.
[14] C. von Euler, Brainstem mechanisms for generation and control of [32] T. Onai, M. Saji, M. Miura, Functional subdivisions of the nucleus breathing pattern, in: N.S. Cherniack, J.G. Widdicombe (Eds.), tractus solitarii of the rat as determined by circulatory and respirato-Control of Breathing, Handbook of Physiology, Vol. 2, Am. Physiol. ry responses to electrical stimulation of the nucleus, J. Autonom.
Soc, 1986, pp. 1–67. Nerv. Syst. 21 (1987) 195–202.
[15] R.A. Gillis, Y.M. Hernandez, M. Bingaman, W.H. Panico, A.M. [33] F.D. Pagani, W.P. Norman, D.K. Kasbekar, R.A. Gillis, Effects of Taveira Da Silva, N-Methyl-D-aspartate (NMDA) receptors at the stimulation of the nucleus ambiguuous complex on gastroduodenal ventrolateral nucleus tractus solitarius (NTS) play a role in the motility and secretion, Am. J. Physiol. 246 (1984) G253. termination of inspiration, Neurosci. Abstr. 23 (1997) 725. [34] J.F.R. Paton, J.M. Ramirez, D.W. Richter, Functionally intact in vitro [16] P.G. Guyenet, T.M. Flitz, S.R. Donaldson, Role of excitatory amino preparation generating respiratory activity in neonatal and mature
acids in rat vagal and sympathetic baroreflexes, Brain Res. 407 mammals, Pflugers Arch. 428 (1994) 250–260.
(1987) 272–284. [35] J.F.R. Paton, The ventral respiratory network of the mature mouse [17] D. Hilaire, B. Duron, Maturation of the mammalian respiratory studied in a working heart-brainstem preparation, J. Physiol.
(Lon-system, Physiol. Rev. 79 (1999) 325–360. don) 493 (1996) 819–831.
[18] G.D. Housley, R.L. Martin-Body, N.J. Dawson, J.D. Sinclair, Brain [36] O. Pierrefiche, S.W. Schwarzacher, A.M. Bischoff, D.W. Richter, stem projections of the glossopharyngeal nerve and its carotid sinus Blockade of synaptic inhibition within the pre-Botzinger complex in branch in the rat, Neuroscience 22 (1987) 237–250. the cat suppresses respiratory rhythm generation in vivo, J. Physiol. [19] S. Ito, A.F. Sved, Influence of GABA in the nucleus of the solitary 509 (1998) 245–254.
tract on blood pressure in baroreceptor-denervated rats, Am. J. [37] C.A. Richardson, R.A. Mitchell, Power spectral analysis of inspirat-Physiol. 273 (1997) R1657–R1662. ory nerve activity in the decerebrate cat, Brain Res. 233 (1982) [20] M. Kalia, J.M. Sullivan, Brainstem projections of sensory and motor 317–336.
components of the vagus nerve in the rat, J. Comp. Neurol. 211 [38] K. Saether, G. Hilaire, R. Monteau, Dorsal and ventral respiratory (1982) 248–264. groups of neurons in the medulla of the rat, Brain Res. 419 (1987) [21] H.P. Koepchen, D. Klubendorf, H. Lazar, T. Hukuhara, H. Abel, 87–96.
Conclusions on respiratory rhythmogenesis drawn from lesion and [39] J.C. Smith, H.H. Ellenberger, K. Ballanyi, D.W. Richter, J.L. ¨
cooling experiments predominantly in the region of ventrolateral Feldman, Pre-Botzinger complex: a brainstem region that may nucleus of solitary tract (vlNTS), in: A.L. Bianchi, M. Denavit- generate respiratory rhythm in mammals, Science 254 (1991) 726–
´
Saubie (Eds.), Neurogenesis of Central Respiratory Rhythm: Elec- 729.
trophysiological, Pharmacological and Clinical Aspects, MTP Press, [40] D.F. Speck, J.L. Feldman, The effects of microstimulation and Lancaster, UK, 1985, pp. 77–80. microlesions in the ventral and dorsal respiratory groups in medulla [22] J. Lipski, L. Kubin, J. Jodkowski, Synaptic action of Rbneurons on of cat, J. Neurosci. 2 (1982) 744–757.
phrenic motoneurons studied with spike-triggered averaging, Brain [41] M. Takeda, S. Matsumoto, Discharge patterns of dorsal and ventral Res. 288 (1983) 105–118. respiratory group neurons during spontaneous augmented breaths [23] S. Long, J. Duffin, The neuronal determinants of respiratory rhythm, observed in pentobarbital anesthetized rats, Brain Res. 749 (1997)
Prog. Neurobiol. 27 (1986) 101–182. 95–100.
[24] V. Marchenko, H.N. Sapru, Different patterns of respiratory and [42] G.F. Tian, J. Duffin, The role of dorsal respiratory group neurons cardiovascular responses elicited by chemical stimulation of dorsal studied with cross-correlation in the decerebrate rat, Exp. Brain Res. medulla in the rat, Brain Res. 857 (2000) 99–109. 121 (1998) 29–34.
[25] D.R. McCrimmon, D.F. Speck, J.L. Feldman, Role of the ventrola- [43] H. Yamada, K. Ezure, M. Manabe, Efferent projections of inspirat-teral region of the nucleus of the tractus solitarius in processing ory neurons of the ventral respiratory group. A dual labeling study respiratory afferent input from vagus and superior laryngeal nerves, in the rat, Brain Res. 455 (1988) 283–294.
Exp. Brain Res. 67 (1987) 449–459. [44] J. Zhang, S.W. Mifflin, Receptor subtype specific effects of GABA [26] D.R. McCrimmon, A.C. Bonham, S.K. Coles, The Breuer-Hering agonists on neurons receiving aortic depressor nerve inputs within reflex requires excitatory amino acid neurotransmission in a discrete the nucleus of the solitary tract, J. Autonom. Nerv. Syst. 73 (1998) region of the nucleus tractus solitarius, in: D. Speck, M. Dekin, W. 170–181.
Revelette, D. Frazier (Eds.), Respiratory Control: Central and [45] Y. Zheng, J.C. Barillot, A.L. Bianchi, Patterns of membrane Peripheral Mechanisms, University Press, Lexington, KY, 1993, pp. potentials and distributions of the medullary respiratory neurons in
86–90. the decerebrate rat, Brain Res. 546 (1991) 261–270.