Industrial Crops and Products 11 (2000) 237 – 242
Hydrolysis of sucrose in the presence of H-form zeolites
Claude Moreau
a,*, Robert Durand
a, Fre´de´ric Alie`s
b, Michel Cotillon
b,
Thierry Frutz
b, Marc-Andre´ The´oleyre
baLaboratoire de Mate´riaux Catalytiques et Catalyse en Chimie Organique,UMR5618ENSCM-CNRS, Ecole Nationale Supe´rieure de Chimie,8Rue de l’Ecole Normale,34296Montpellier Cedex5,France
bApplexion,264,A6enue de la Mauldre,78691Epoˆne Cedex,France
Accepted 8 October 1999
Abstract
Hydrolysis of sucrose was performed in aqueous medium in the presence of various H-form dealuminated zeolites (H-BEA, H-MFI, H-MOR, H-Y-FAU). For all catalysts, hydrolysis takes place with a high selectivity whatever the conversion is. The important feature to be noted is the capacity for those catalysts to act as specific adsorbents of colored by-products formed during the course of the reaction, 5-hydroxymethylfurfural (HMF) in particular. The amount of this by-product can be reduced to less than 100 ppm, thus offering an alternative route for the production of colorless invert sugars. In this way, H-Y FAU (Si/Al=15) was found to have the better balance between activity, selectivity and by-product amount. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Hydrolysis; Sucrose; Glucose; Fructose; 5-Hydroxymethylfurfural; Decoloration; Invert sugars; Zeolites; Faujasites www.elsevier.com/locate/indcrop
1. Introduction
Sucrose is widely used in the food industry as such or as precursor of invert sugar through its partial or total hydrolysis (Bussie`re et al., 1990) and in the non food industry, as for example, a starting material for the formation of furanic derivatives with high added value like 5-hydrox-ymethylfurfural (Kunz, 1993; Moreau et al., 1994, 1996) (Scheme 1). Enzymes are the catalysts the most often used on the industrial scale to
trans-form sucrose into invert sugar (Bussie`re et al., 1990). However, their use is restricted to the food industry as far as the products formed, glucose and fructose, inhibit the hydrolysis reaction (Hahn-Ha¨gerdal et al., 1983), with a conversion
of sucrose that does not exceed 95%. Strong H+
ion-exchange resins are also used and allow a complete conversion of sucrose in the temperature range compatible with their stability, but with a relatively high level of impurities (Satyanarayana and Varma, 1970; Hahn-Ha¨gerdal et al., 1983; Masroua et al., 1988). Therefore, aluminosilicates are particularly suitable catalysts for performing hydrolysis at low or high temperatures (Butter-sack and Laketic, 1994; Moreau et al., 1994, 1996, * Corresponding author. Tel.:+33-467-14-4320; fax:+
33-467-14-4349.
E-mail address:[email protected] (C. Moreau)
Scheme 1. Simplified reaction scheme for sucrose hydrolysis.
Table 1
Sucrose conversions and corresponding 5-hydroxymethylfurfural concentrations at different temperatures vs. reaction time; 800 g/l of sucrose, 700 rpm, 1 wt.% of catalyst
HMF concentration (mg/l) Sucrose conversion (%)
Time (min)
75°C 85°C 95°C
75°C 85°C 95°C
2.7 3.7
21.9 12.0
5.9 41.4
0
2.9 10.0
10 7.2 25.2 54.2 20.0
3.2 12.0 40.0
71.4
30 10.1 32.9
82.0
15.5 43.4 3.5 13.0 57.0
60
3.6 16.0 98.0
92.6
90 21.9 54.6
7.0 18.0 148.0
120 26.5 65.5 97.5
9.2 23.0 190.0
97.9
150 31.1 72.5
9.5
180 35.3 76.7 100.0 30.0 257.0
9.4 52.0 325.0
100.0 85.9
210 40.3
9.6
C.Moreau et al./Industrial Crops and Products11 (2000) 237 – 242 239
Table 2
Sucrose conversions and corresponding 5-hydroxymethylfurfural concentrations at different temperatures vs. reaction time; 800 g/l of sucrose, 700 rpm, 3 wt.% of catalyst
Time (min) Sucrose conversion (%) HMF concentration (mg/l)
85°C
75°C 95°C 75°C 85°C 95°C
45.4 96.7
0 19.8 3.8 30.0 138.0
20 32.2 89.5 99.5 6.5 72.5 227.5
40 39.9 94.2 100 8.0 75.0 296.0
96.3 100 10.0
50.0 77.5
60 369.0
66.9
100 99.5 100 12.0 405.0 700.0
100 100
120 72.5 17.0 685.0 825.0
Table 3
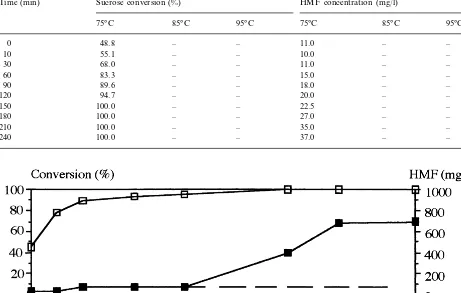
Sucrose conversions and corresponding 5-hydroxymethylfurfural concentrations at different temperatures versus reaction time; 800 g/l of sucrose, 700 rpm, 6 wt.% of catalyst
Time (min) Sucrose conversion (%) HMF concentration (mg/l)
85°C 95°C 75°C 85°C
75°C 95°C
– –
0 48.8 11.0 – –
10 55.1 – – 10.0 – –
– –
30 68.0 11.0 – –
– – 15.0
83.3 –
60 –
89.6
90 – – 18.0 – –
– – 20.0 –
120 94.7 –
– – 22.5
100.0 –
150 –
100.0
180 – – 27.0 – –
– – 35.0 – –
210 100.0
– – 37.0 –
100.0 –
240
Table 4
Influence of the temperature and of the catalyst weight on the amount of 5-hydroxymethylfurfural formed at :66% inver-sion; 800 g/l of sucrose, 700 rpm
aExtrapolated value from the results reported in Table 1.
trations up to 800 g/l of water, catalyst weights
from 1 to 6 wt.% with respect to the feed, and agitation speeds over 700 rpm, those operating conditions avoid external and internal diffusional limitations. In the flow mode, the sucrose solution is passed through a tubular reactor fed with 7 g of preformed zeolite and flow rates of 0.15 – 0.50
ml/min. Analyses were followed by high
perfor-mance liquid chromatography using UV and/or
RI detectors.
Coloration tests were performed by determining
the concentration in 5-hydroxymethylfurfural
from absorbance measurements at 280 nm and at 420 nm in order to have the corresponding ICUMSA color scale. In this way, it was found that a concentration in 5-hydroxymethylfurfural
of 10 mg/l corresponds to 20 ICUMSA.
3. Results and discussion
3.1. Batch reactor
Hydrolysis of sucrose was firstly performed in a batch mode in the presence of commercially avail-able H-form zeolites, H-BEA, H-MFI, H-MOR, H-Y-FAU, with different structures and dealumi-nation extents. After a classical screening of the different zeolites used, the H-Y faujasite with a
Si/Al ratio of 15 was chosen in order to obtain a
maximum acidity, and at temperatures ranging from 75 to 100°C, to maximize hydrolysis of sucrose as well as to minimize the formation of 5-hydroxymethylfurfural.
Experimental results obtained for the hydrolysis of sucrose in the presence of the H-Y faujasite with a Si/Al ratio of 15 as a function of reaction time and at temperatures from 75 to 95°C are Table 5
Influence of the temperature and of the catalyst weight on the amount of 5-hydroxymethylfurfural formed at :95% inver-sion; 800 g/l of sucrose, 700 rpm
1997), and particularly in the direct production of 5-hydroxymethylfurfural (Moreau et al., 1994, 1996). In all cases, very specific properties for the catalytic systems are required, acidity, shape selec-tivity, microporous vs. mesoporous volumes and sorption properties. The aim of this work was thus to emphasize on this last aspect that is particularly important to consider for the produc-tion of colorless invert sugars.
2. Experimental
Hydrolysis reactions were performed in a batch or in a flow reactor. In the batch mode, for temperatures from 75 to 100°C, sucrose
concen-Table 6
Sucrose conversion, amounts of invert sugar formed and concentration in 5-hydroxymethylfurfural at 100°C; sucrose: 500 g/l; flowrate: 0.15 ml/min; 7 g of HY (Si/Al=15) catalyst
HMF concentration (mg/l) Time (h) Sucrose conversion (%) Amount of invert sugar (g)
C.Moreau et al./Industrial Crops and Products11 (2000) 237 – 242 241
reported in Table 1 for 1 wt.% of catalyst, in Table 2 for 3 wt.% of catalyst, and in Table 3 for 6 wt.% of catalyst.
From these different tables, it can be seen that partial or total conversion of sucrose is easily controlled by the amount of catalyst or by temperature.
Nevertheless, 5-hydroxymethylfurfural levels
may be different at those temperatures. For a given sucrose conversion, this level is weaker at lower temperatures. It is also worth noting that 5-hydrox-ymethylfurfural appears late in the course of the reaction, as illustrated in Fig. 1. The low amount of by-products may result from the better ad-sorbing capacity of faujasites compared to resins. It may also result from differences in the structures between catalyst families, the formation of by-products being less important in microporous zeo-lites than in macroporous resins (Moreau et al., 1994, 1996, 1997). Indeed, in spite of its relatively important molecular size, sucrose was reported to be flexible in solution (Poppe and van Halbeek, 1992), and is then capable of entering the dealumi-nated Y zeolites (Buttersack and Laketic, 1994). In that way, this leads to intracrystalline catalysis and to a reduced amount of by-products, thus ensuring a better control of the formation of colored impu-rities non acceptable in food industry.
In order to have a better idea of the influence of the temperature and of the weight of catalyst, the experimental results reported in the different tables will be compared for the two industrial inversion rates, i.e. 66% (Table 4) and 95% (Table 5).
For a given weight of catalyst and at sucrose isoconversion, the concentration in 5-hydrox-ymethylfurfural, and consequently the coloration, increases in a significant manner. The coloration is, of course, less at a sucrose conversion of 66% than at 95% (Tables 4 and 5).
On the other hand, the concentration in 5-hy-droxymethylfurfural is not affected by an increase in the catalyst weight when the reaction is per-formed at 75°C. However, the coloration tends to increase at higher temperatures (Tables 4 and 5). The important feature to be noted is that a larger amount of catalyst does not increase the reaction rate but retards the appearance of 5-hydrox-ymethylfurfural (Fig. 1, dashed line). This excess of
catalyst, or another chemically inert solid (Durand et al., 1997) is therefore acting as an adsorbent. In a continuous process, 5-hydroxymethylfurfural will then be removed with the catalyst and the catalyst easily regenerated by thermal treatment under air flowing at 773 K. The amount of 5-hydroxymethyl-furfural is reduced by a factor of ten in comparison with what occurs in the presence of macroporous catalysts like sulfonic ion-exchange resins under similar experimental conditions.
3.2. Flow reactor
The experimental results obtained in a fixed bed reactor at 100°C, and at nearly complete conver-sion of sucrose, are reported in Table 6. Consider-ing both the high reaction temperature and the high level of sucrose conversion, the amount of 5-hy-droxymethylfurfural formed after 25 h on stream is kept at a low level, as mainly due to the sorption properties of the catalyst. However, after satura-tion of the catalyst, and in the absence of another selective adsorbent, the amount of 5-hydrox-ymethylfurfural becomes higher and tends to stabi-lize around 2500 mg/l. It should also be noted from Table 6 that no deactivation of the catalyst occurs over a 7-day period, and, as already mentioned, that 5-hydroxymethylfurfural can be removed with the catalyst and the catalyst easily regenerated by thermal treatment.
4. Conclusion
Acidic zeolites allow formation of invert sugar in mild operating conditions for high concentrations in the starting sucrose and with an efficient control of the degree of colored materials as due to their adsorbent properties. Their use in food industry can thus be taken into consideration since they are easily regenerated and recycled.
Acknowledgements
References
Bussie`re, G., Nowak, P., Cotillon, M., 1990. Les sucres inver-tis, I.A.A., 645 – 649.
Buttersack, C., Laketic, D., 1994. Hydrolysis of sucrose by dealuminated Y-zeolites. J. Mol. Catal. 94, L283 – L290. Durand, R., Faugeras, P., Laporte, F., Moreau, C., Neau,
M.C., Roux, G., 1997. Proce´de´ de fabrication d’une solu-tion pure de sucres simples par hydrolyse d’au moins un sucre compose´ en pre´sence d’un adsorbant se´lectif, EP c
0797686, Agrichimie.
Hahn-Ha¨gerdal, B., Skoog, K., Mattiasson, B., 1983. The utilization of solid superacids for hydrolysis of glycosidic bonds in di- and polysaccharides: a model study on cel-lobiose, sucrose, and starch. Eur. J. Microbiol. Biotechnol. 17, 344 – 348.
Kunz, M., 1993. Hydroxymethylfurfural, a possible basic chemical for industrial intermediates. In: Fuchs, A. (Ed.), Inulin and Inulin-Containing Crops. Elsevier, Amsterdam, pp. 149 – 160.
Masroua, A., Revillon, A., Martin, J.C., Guyot, A., Descotes, G., 1988. Hydrolyse d’oligo et polysaccharides en pre´sence de re´sines e´changeuses d’ions et de polyme`res hydrosolu-bles. Bull. Soc. Chim. Fr. 3, 561 – 566.
Moreau, C., Durand, R., Pourcheron, C., Razigade, S., 1994. Preparation of 5-hydroxymethylfurfural from fructose over H-form zeolites. Ind. Crops Prod. 3, 85 – 90.
Moreau, C., Durand, R., Razigade, S., Duhamet, J., Faugeras, P., Rivalier, P., Ros, P., Avignon, G., 1996. Dehydration of fructose to 5-hydroxymethylfurfural over H-mordenites. Appl. Catal. A: Gen. 145, 211 – 224.
Moreau, C., Durand, R., Duhamet, J., Rivalier, P., 1997. Hydrolysis of fructose and glucose precursors in the pres-ence of H-form zeolites. J. Carbohydr. Chem. 16, 709 – 714.
Poppe, L., van Halbeek, H., 1992. The rigidity of sucrose: just an illusion? J. Am. Chem. Soc. 114, 1092 – 1094. Satyanarayana, B., Varma, Y.B.G., 1970. Heterogeneous
catalysis of inversion of sucrose. Indian J. Technol. 8, 58 – 61.