43
American Journal of Botany 88(1): 43–46. 2001.
CONTRIBUTIONS TO THE DEBATE ON WATER
TRANSPORT
1M
ARTINJ. C
ANNYResearch School of Biological Sciences, Australian National University, PO Box 475, Canberra 2601, Australia
The useful criticisms of my theory of water transport by Comstock (American Journal of Botany 86: 1077–1081) and by Stiller and Sperry (American Journal of Botany 86: 1082–1086) are acknowledged and reviewed. I make the following responses. (1) Tensile stresses to contain tissue pressure are kept within modest limits by the organization of vascular tissues into cylindrical bundles with small ratios of radius/boundary thickness. (2) The balance of pressures within tissues of a nontranspiring leaf is best understood by treating it as a single compartment containing several pressure-generating engines whose resultant is the pressure throughout the compartment. An error in the published notional balances for a transpiring leaf is corrected. (3) The argument against a valve in the transpiring leaf, which allows water out but not in, is not convincing. (4) The ‘‘robust and extremely consistent’’ cohesion theory gains this status by neglecting large bodies of experimental fact, once well known to plant physiologists. (5) The demonstration that living cells are not involved in the refilling of embolisms in birch stems is welcomed as an important advance. However, the major questions remain unresolved. (6) Proof is still needed that embolisms in vessels are not refilled by the collapse of gas bubbles under small positive pressures during conductance measurement. (7) The survival of unbroken water threads in vessels under centrifugal stress has still not been demonstrated. (8) Both questions 6 and 7 can be easily answered by direct observation of gas/liquid volumes in frozen stems in the cryo-scanning electron microscope.
Key words: cavitation; cohesion theory; compensating pressure theory; embolism; tissue pressure; water transport.
When I proposed a new theory for the ascent of sap (Canny,
1995), I knew well that it touched upon many topics in plant
biology wherein I had little expert knowledge. I wrote: ‘‘The
full implications of the Compensating-Pressure Theory, and a
just appreciation of its usefulness, must await the concerted
criticism of a wider community.’’ I am very pleased that this
criticism is beginning to be published, both theoretical
objec-tions (Comstock, 1999) and experimental tests (Stiller and
Sperry, 1999). I welcome the chance to respond to this
criti-cism and to develop further some of the theoretical and
ex-perimental aspects of the theory. All criticisms, even invalid
ones, help me to clarify my understanding of the theory and
to find clearer ways of explaining it and new ways of testing
it. They often show me how a whole new set of puzzle pieces
can be fitted in to the growing picture of the theory.
There is not space here to provide all the relevant arguments
and facts from the beginning, and I assume that readers are
familiar at least with the two articles quoted above and with
the paper that stimulated the writing of them (Canny, 1998).
They should consult the original statement (Canny, 1995) for
the discussion of its fig. 12 (p. 352). The two critical articles
(Comstock, 1999; Stiller and Sperry, 1999) will be discussed
separately, treating the main topics raised by each.
THEORETICAL OBJECTIONS OF COMSTOCK
Tensile strength of tissues—I have neglected the question of whether planttissues are in fact strong enough to contain the pressures predicted by the theory, and it is most valuable to have the question raised in quantitative form. For a cylinder, radius (R) containing pressure (P) by means of its outer 1Manuscript received 21 October 1999; revision accepted 31 March 2000. The author thanks Johnathan Comstock, Volker Stiller, and John Sperry for their important contributions to the debate, for showing me several errors I have made, for making me think and write more carefully, and for contrib-uting several more pieces that dovetail with, and enlarge, the coherent patches of the picture that are already in place. The work was supported by an NSERC operating grant.
wall (thickness T), Comstock’s Eq. 2 (Comstock, 1999) gives the tension in the wall (S) as
S5PR/T.
He applies this to a 1-cm diameter stem with an epidermis 20mm thick and an internal pressure of 2 MPa, giving a tensile stress in the epidermis of 500 MPa. He quotes values for the tensile strength of ‘‘simple steel’’ as 400 MPa, of wood (4–100 MPa), and of live carrot cell walls as 450 MPa. He makes the point that organs with a high ratio of R/T will be readily burst by internal pressure. I am grateful for this insight, because it explains for me the fact (noted by Strasburger, 1891) that all nonwoody plant organs have their vas-cular systems confined within sheaths (endodermis, fibrous sheaths, etc.). The pressure-containing elements of such organs are not primarily the outer sur-face, but the much smaller sheathed vascular bundles. They have smaller ratios of R/T, and so will have lower tensile stresses in their sheaths. Three of the four pressure-containing systems I illustrated in figs. 9–11 of Canny (1995) are of this kind. The fourth (figs. 7, 8) is wood, which is a much more complex solid—a coherent composite of radially oriented pressurizing plates (rays) and pressurized sheets of tracheary elements, which cannot be described by the La Place equation (above). Using measurements of the stele of the wheat root in the fig. 9, R/T57, so a pressure of 2 MPa within the stele would require a tensile strength of 14 MPa in the endodermis, which seems modest enough.
The balance of pressures/water potentials in Fig. 12 of Canny (1995)—
44
A
MERICANJ
OURNAL OFB
OTANY[Vol. 88
which uses the accepted terminology (Canny, 1999). Within the one com-partment there are several pressure-generating engines: osmotic pressure, wall pressure, tissue pressure, xylem pressure; and the resultant of all these forces is the pressure within the compartment. By arranging the appropriate signs, the resultant may be turned into a water potential. Comstock’s failure to treat the compartment as a whole has led him to misinterpret the part of fig. 12 which forms section A of his redrawn version (his fig. 1). The water potential of the composite system is zero, and all the fluid space within it is at a pressure of10.8 MPa, parenchyma and vessel sap. There are no gradients of pressure, and thus no movements of water, no changes of volume or concentration with time, and no matter or energy moving through it. It is indeed at equilibrium. It is not helpful to isolate parts of the system mentally and try to assign them water potentials. Dissecting the system will change the potential of all the components. For example, if a parenchyma cell is removed from its con-straints without change of volume, its water potential will go from zero to
20.8 MPa.
In section B of Comstock’s fig. 1 he is right and I am wrong. I see now, as I did not at the time I was devising fig. 12, that as work is done by the system the usable energy decreases and the unusable energy increases. Water moves into the cells, and their volume increases and their walls are further stretched. Tissue pressure decreases and wall pressure increases pari passu. This transfer between the two components was fully understood by Ho¨fler (1920) and is obvious from his fig. 3. In section B of Comstock’s fig. 1 I should have set wall pressure (WP)51.0 MPa. All the osmotic pressure is balanced by the wall pressure. In section B, the values of the variables are such that mental dissection of the parts of the system to seek individual water potentials of components is innocuous. Physical dissection does not alter the forces produced by the separate engines. This set of values of variables was chosen as the steady-state example for fig. 12 because they illustrate the lim-iting condition of usable tissue pressure. At stresses beyond this, the com-ponents of the tissue cease to interact with one another and the cohesion theory takes over.
Before leaving the discussion of fig. 12, I should remind the reader of a point that Comstock does not bring out, but which will be important when I come to discuss the article by Stiller and Sperry. The object of devising fig. 12 was to investigate what in fact the water potential of a tissue is a measure of. Having arrived at the conclusion that it was not a measure of tension in the xylem sap, I tried to imagine the changes in water contents, pressures, and volumes within a tissue during life, sample collection, and measurement. The conclusion was that the water potential measured that part of the turgor pressure that had been doing work in the intact plant—the compensating pres-sure.
The phase-change valve in the leaf—For my theory to work, water
move-ment into the pipes at the bottom and out of the pipes at the top needs to be independent of the pressure in the pipe. Comstock finds this impossible to imagine and focuses his disbelief on the top valve, where I claim that evap-orative water flux out of the leaf is not changed by changing the absolute pressure in the xylem. This is because the energy to effect the phase change from liquid water to water vapor is large compared with the pressures in the xylem. It is a valve also in the sense that the water movement through it cannot be reversed. Comstock has not convinced me that this argument is invalid. He justifies his disbelief in the valve by reference to an experiment in which the valve is nonexistent. ‘‘The lack of regulating valve anywhere in the leaf mesophyll is made particularly obvious by hydraulic conductance measurement techniques in which water is pushed into the xylem of a severed twig under positive pressure. Under these conditions, water readily moves through the leaf tissue, floods the normally gas-filled intercellular spaces, and drips as a liquid from each stomatal pore.’’ He has abolished the phase change (and therefore the valve). It is not sensible to argue from the behavior of water flow under positive pressure with no phase change to what might be expected with evaporation, where there is a phase change, and which gener-ates a negative pressure. The squeezing of water out of cells into intercellular spaces, noted by Comstock for the the leaf mesophyll, is minimal within vascular bundles. Their lack of intercellular spaces makes good sense when they are viewed as pressure-containing ducts, as discussed above. Within the
bundles, pressure pushes water into the main space available, the vessels, repairing embolisms.
I am disappointed that Comstock did not help me with the operation of the hypothesized lower valve (pump) in the roots. That pump is much more com-plicated and interesting than the leaf valve, and relies on more esoteric and less familiar principles. Since publishing an account of it I have been ex-pecting someone to point out some fatal error or misconception in my rea-soning, or some experimental evidence against it. The experimental evidence we have collected ourselves is consistent with it (Enns et al., 1998, 2000).
Measuring the usefulness of a theory—Comstock’s confidence in the
co-hesion theory, that it has proved ‘‘itself robust and extremely consistent to a wide range of experimental and technical approaches’’ is clearly legitimate, in the sense that the water relations of plants have been comfortably studied within its constraints for 35 yr. However, this has been possible only because of a tacit agreement to ignore large bodies of experimental fact about water transport that were once well known and widely believed to be essential. To take a single example, Haberlandt (1914), after a long discussion of the known facts of water transport and the tissues that carry the stream, makes a sum-mary, which he starts with what he presumably thought was the most impor-tant fact.
‘‘At this stage it will be desirable to summarize in half a dozen sentences those facts concerning the conduction of water which may be regarded as definitely established: (1.) Vessels and tracheides normally contain both air and water, the relative amounts of the two substances varying according to the season and the time of day.’’ He means here, not just that wood contains gas as well as water, but that individual vessels and tracheids contain simult-neously gas and liquid. The body of evidence supporting this statement is vast and various, and spans 200 yr. But because it is not possible to believe simultaneously this statement and the cohesion theory, that evidence and that fact are never discussed. Now that independent evidence of the fact is ap-pearing, collected by modern techniques (Canny, 1997a, b; McCully, Huang, and Ling, 1998; Buchard, McCully, and Canny, 1998; McCully, 1999; Pate and Canny, 1999; Shane and McCully, 1999), resistance to its publication is fierce (see McCully et al., 2000).
I am frequently asked, how do you tell whether one theory is better than another? My answer is very simple. The usefulness of a theory is measured by the range and diversity of the phenomena that it explains, the number of pieces in the completed puzzle-picture. A theory that the World is flat explains most of what is necessary to be known by a community living in the same territory for many generations. It does not suffice for a group that sets out resolutely to find the edge of the World. The cohesion theory satisfies those who are content not to look beyond ‘‘the wide range of experimental and technical approaches’’ within which it works. I find the compensating pressure theory more useful, because it explains that range and much else besides.
PRACTICAL TESTS OF STILLER AND SPERRY
There are three questions at issue here, only one of which
is answered by the experiments of Stiller and Sperry (1999).
1) The fundamental question (not addressed) is whether the
water potential is a measurement of tension in the liquid in
the xylem vessels. There are two derivative questions.
2) Whether embolized vessels can refill during measurement
of the hydraulic conductance of a plant organ when this is
assessed by the rate of flow of solution through it down a
pressure gradient (addressed but not answered satisfactorily).
3) If the proposed refilling does occur during conductance
measurement, whether this is due to the activity of living cells
in the xylem (addressed and answered for woody
tissue—liv-ing cells are not necessarily involved).
an-January 2001]
C
ANNY—D
EBATE ON WATER TRANSPORT45
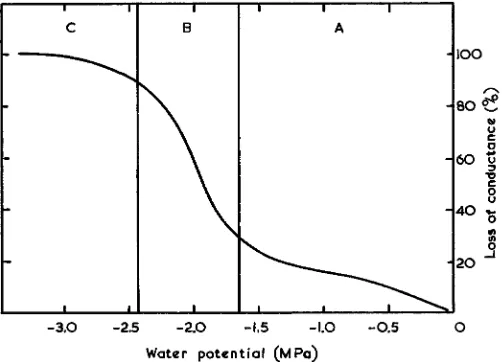
Fig. 1. ‘‘Vulnerability curve’’ for loss of hydraulic conductance of a plant stem at different levels of drying stress, measured as water potential. The contents of the tracheary elements in regions A, B, and C are discussed in the text. Redrawn from fig. 3 of Stiller and Sperry (1999).
swer to the fundamental question. I objected at the time that
the demonstration was invalid because the presence of
contin-uous liquid threads under tension in the vessels was not
proved. All that was proved was that three different drying
stresses (as measured by the change in water potential)
changed the hydraulic conductance along a sigmoid path. One
interpretation of this was what Pockman, Sperry, and O’Leary
(1995) said, that the drying stresses produced stretched liquid,
which broke at the point of inflection of the curve. I pointed
out that there were other interpretations that would produce
similar curves, but that the crucial test was to show that the
liquid was there up to the inflection, and was not there beyond
the inflection. This has still not been shown.
A diagram is necessary to clarify the arguments (Fig. 1).
This is a simplified version of fig. 3 of Stiller and Sperry
(1999), a plot of percentage loss of hydraulic conductance vs.
a stress produced by centrifugation. The abscissa of the
orig-inal figure by Pockman, Sperry, and O’Leary (1995) was a
generalized drying stress, which included drying on the bench,
and drying by forcing pressurized air through the stem, as well
as the centrifugal stress. All three stresses were translated into
values of water potential (MPa) and produced similar sigmoid
curves like that in Fig. 1. We all agree that the conductance
decreases along such a sigmoid path, that there is little change
in conductance in the region A to B, a sudden loss of
ductance in the region of B, and near complete loss of
con-ductance in the region C.
Sperry’s interpretation is that the continuous water threads
in the vessels cohere in the region A, their tension equaling
the drying stress. The tension reaches a critical value near B,
the threads break, the vessels fill with gas, and the conductance
falls to zero in region C.
My interpretation is that the water threads break and are
interrupted by gas even in region A. But the conductance is
little changed because the process of perfusing the xylem with
solution dissolves the gas. The greater the stress the more gas
is produced, but it is not until the gas content of the vessels
reaches a critical volume around B that this refilling ceases to
be significant, the measured conductance falls, and the vessels
contain mostly gas. The crucial test of my interpretation is to
study the gas/liquid content of the vessels in region A, first
before conductance measurement, and then after conductance
measurement.
One mechanism by which this refilling might happen
pre-occupied me because it derived from my proposal (explained
above) that the compensating pressure could be a major
com-ponent of the water potential, and because it made easily tested
predictions (Canny, 1998). This mechanism depended on
liv-ing cells to provide sufficient pressure to dissolve the
embo-lisms. This is the interpretation (Question 3) that Stiller and
Sperry have tested by doing two of the four tests I asked for
on young stems of birch. Their advance is important, and a
valuable contribution to the derivative question. My fanciful
interpretation of water potential as measuring compensating
pressure in these particular experiments was clearly wrong.
They have shown that neither the turgor of the xylem
paren-chyma cells, nor the live state of the xylem parenparen-chyma,
in-fluences the shape of the curve in this woody tissue.
They also attempted a partial answer to Question 2 by
test-ing the effect of livtest-ing/dead cells on the conductance
mea-surement (their fig. 4). But they chose to do this, not in the
critical region A where I suggest refilling happens, but in the
region B to C where, in my interpretation, no refilling would
be expected. The drying stress in this experiment was not
cen-trifuging, but a perfusion of the xylem with air for 10 min. A
cursory reading suggests that this was a fairly mild stress, but
in their fig. 4 the ordinate is percentage conductance. The
stressed stems had 30% of the control conductance, i.e., 70%
loss of conductance. This is the level of stress (Fig. 1) beyond
the inflection of the sigmoid curve where I would expect no
refilling during measurement, whatever the mechanism may
be.
I am grateful to Stiller and Sperry for resolving the question
of the role of living cells, but I am not satisfied that they have
answered Question 2, that my interpretation of the region A
to B is wrong. Having been proved wrong in one of my
in-terpretations of ‘‘vulnerability curves,’’ I am not shy to
pro-pose another. Even with no activity of living cells, during the
conductance measurement small bubbles of gas mixed with
liquid in the vessels would be subjected by the technique to a
small positive pressure (approximately
1
5 kPa). Small
bub-bles of gas in water at atmospheric pressure are inherently
unstable and collapse. As Sperry has already shown himself
(Alder et al., 1997: Fig. 6), centrifuged birch stems can take
up water and increase their conductance by 50% in 10 min. I
need more evidence to convince me that bubbles of gas are
not there in region A of Fig. 1, and that they do not partially
dissolve during conductance measurement. Even if living cells
of woody stems do not contribute to changes in xylem
con-ductance during these manipulations, herbaceous organs,
whose structural rigidity relies strongly on tissue turgor, should
also be tested. It seems quite possible that in them living cells
may provide the forces that, in wood, are provided by
imbi-bition and surface tension.
con-46
A
MERICANJ
OURNAL OFB
OTANY[Vol. 88
ductance measurement. I issue this public invitation to Sperry
to take advantage of our microscope and expertise to answer
the question. We can arrange that he freezes the stems in his
laboratory, transports them frozen to our laboratory, and
ex-amines the liquid and gas content of the frozen vessels.
Without going to all this trouble, a very simple test to decide
between the two interpretations would be to measure (e.g., by
weighing) how much water comes out of the ends of the stems
at different radial accelerations. In Sperry’s interpretation little
water should come out in region A, but when region B is
reached there should be a sudden loss of water over a small
range of increasing acceleration. In my interpretation there
would be a linear relation between water loss and radial
ac-celeration over the whole range of A to C.
LITERATURE CITED
ALDER, N. N., W. T. POCKMAN, J. S. SPERRY,ANDS. NUISMAR. 1997. Use of centrifugal force in the study of xylem cavitation. Journal of Exper-imental Botany 48: 665–674.
BUCHARD, C. J. A., M. E. MCCULLY,ANDM. J. CANNY. 1999. Daily em-bolism and refilling of root xylem vessels in three dicotyledonous crop plants. Agronomie 19: 97–106.
CANNY, M. J. 1995. A new theory for the ascent of sap. Cohesion supported by tissue pressure. American Journal of Botany 75: 343–357.
———. 1997a. Vessel contents after excision—a test of Scholander’s as-sumption. American Journal of Botany 84: 1217–1222.
———. 1997b. Vessel contents during transpiration—embolisms and refill-ing. American Journal of Botany 84: 1223–1230.
———. 1998. Applications of the compensating pressure theory of water transport. American Journal of Botany 85: 897–909.
———. 1999. The forgotten component of plant water potential. Plant Bi-ology 1: 595–597.
COMSTOCK, J. P. 1999. Why Canny’s theory doesn’t hold water. American Journal of Botany 86: 1077–1081.
ENNS, L. C., M. J. CANNY,ANDM. E. MCCULLY. 2000. An investigation of the role of solutes in the xylem sap and in the xylem parenchyma as the source of root pressure. Protoplasma 22: 506–515.
———, M. E. MCCULLY,ANDM. J. CANNY. 1998. Solute concentrations in xylem sap along vessels of maize primary roots at high root pressure. Journal of Experimental Botany 49: 1539–1544.
HABERLANDT, G. 1914. Physiological plant anatomy. Translated by M. Drummond, Macmillan, London, UK.
HO¨ FLER, K. 1920. Ein Schema fu¨r die osmotische Leistung der Pflanzenzelle. Berichte der deutschen botanischen Gesellschaft 38: 288–298. MCCULLY, M. E. 1999. Root xylem embolisms and refilling. Relation to
water potentials of soil, roots and leaves and osmotic potentials of root xylem sap. Plant Physiology 119: 1001–1008.
———, C. X. HUANG,ANDL. E. C. LING. 1998. Daily embolism and re-filling of xylem vessels in the roots of field-grown maize. New Phytol-ogist 138: 327–342.
———, M. W. SHANE, A. N. BAKER, C. X. HUANG, L. E. C. LING,ANDM. J. CANNY. 2000. The reliability of cryoSEM for the observation and quantification of xylem embolisms and quantitative analysis of xylem sap in situ. Journal of Microscopy 198: 24–33.
PATE, J. S.,ANDM. J. CANNY. 1999. Quantification of vessel embolisms by direct observation: a comparison of two methods. New Phytologist 141: 33–44.
POCKMAN, W. T., J. S. SPERRY,ANDJ. W. O’LEARY. 1995. Sustained and significant negative water pressure in xylem. Nature 378: 715–716. SHANE, M. W.,ANDM. E. MCCULLY. 1999. Root xylem embolisms:
impli-cations for water flow to the shoot in large, field-grown maize plants with only one root. Australian Journal of Plant Physiology 26: 107–114. STILLER, V.,ANDJ. S. SPERRY. 1999. Canny’s compensating pressure theory
fails a test. American Journal of Botany 86: 1082–1086.