Leaf secretion composition of the mangrove species A
6
icennia
germinans (L.) in relation to salinity: a case study by using
total-reflection X-ray fluorescence analysis
M .A. Sobrado
a,*, E.D . G reaves
baL aboratorio de Biologı´a A mbiental de Plantas, Departamento de Biologı´a de Organismos, Uni6ersidad S imo´n Bolı´6ar, A partado89000, Caracas1080A , V enezuela
bL aboratorio de Fı´sica N uclear, Uni6ersidad S imo´n Bolı´6ar, A partado89000, Caracas1080A , V enezuela R eceived 8 F ebruary 2000; received in revised form 9 M ay 2000; accepted 9 M ay 2000
Abstract
The aim here was to use total-reflection X-ray fluorescence (TXR F ) to determine the elemental composition (besides N a), and the relative contribution of each element in leaf secretion of the mangrove species A6icennia germinans, grown under contrasting
salinities (0 – 865 mol m−3N aCl). N a was determined by conventional atomic absorption spectrometry (AAS). Total secretion was
2.0090.28 mmol m−2per day in control plants (at 0 N aCl) and increased progressively up to 46.8797.14 mmol m−2per day
at 865 mol m−3N aCl. N a and Cl accounted for 85.9191.81% of the total secretion in control plants and about 96.3290.30%
in salt-treated plants. The excretion of N a exceeded that of Cl when salinity increased and this led to a progressive increase in N a/Cl ratios from 0.4690.02 in control plants up to 2.7590.42 (at 865 mol m−3N aCl). Other elements were also secreted in
sizeable amounts such as K , S, Ca, Br and Zn. H owever, the relative importance of these elements, in terms of total secretion, was considerable in control plants (15% of the total secretion) and declined significantly under salinity treatments (B5% of the total secretion). In conclusion, TXR F has been shown to be a powerful tool allowing quantitative determination of Cl (1.2 – 13 mmol m−2 per day), secreted in relatively large quantities, as well as other elements secreted in intermediate (S, Ca and K ;
0.07 – 1.00 mmol m−2per day) and in trace quantities (Br and Zn; 0.6 – 4mmol m−2per day). © 2000 Elsevier Science Ireland
Ltd. All rights reserved.
Keywords:A6icennia germinans; M angroves; Salinity; G land secretion; Total-reflection X-ray fluorescence; Trace element analysis
www.elsevier.com/locate/plantsci
1. Introduction
M angroves are halophytic trees thriving in inter-tidal zones found in tropical and subtropical cli-mates [1]. The ability to use seawater is one striking attribute of the mangrove species. Like other halophytes, they have mechanisms to pre-vent excessive build-up of N a and Cl [2]. There-fore, most of the salt in the external solution is excluded by the roots of these species and only a small fraction reaches the leaves [3]. H owever, mangrove species having leaves with secreting
glands, transport relatively more salt in the xylem [4,5]. Thus, excess salt carried to the leaves is maintained within physiologically acceptable levels by salt secretion [4,6]. Specificity of salt secretion in halophytes species is not well documented, but other ions may also be secreted with N a and Cl, such as K , Ca, R b, SO42− and Zn [7 – 9].
The black mangrove (A6icennia germinans) is
found along tropical and subtropical coastlines of the Atlantic-East Pacific hemisphere [1,10,11]. Ecophysiological processes play a key role in the mangrove forest structure [12]. In order to under-stand the response to the environment of these species, as well as interaction with other species, many physiological parameters have been assessed * Corresponding author. F ax:+58-2-9063064.
E -mail address:[email protected] (M .A. Sobrado).
in earlier studies, e.g. water relations [13 – 18], car-bon assimilation [15,19 – 21] and leaf solutes bal-ance [22]. The aim here is to document the elemental composition of the leaf secretion of A .
germinans, as well as the relative proportion of each
element in terms of total secretion in plants grown under contrasting salinities. We used total-reflec-tion X-ray fluorescence (TXR F ) to analyse gland secretion from the leaves of A . germinans. TXR F analysis of inorganic fluids can readily be done by adding an internal standard. The technique allows determinations in both materials from plants and animal species and is a useful tool because it provides multielemental analysis simultaneously [23,24].
2. Material and methods
2.1. Plant material and secretion collection
Seedlings of A . germinans (L.) were planted in pots with sand and nutrient solution in a glasshouse
under natural sunlight and photoperiod (12 h). Temperatures ranged from 25 to 35°C during the day and 15 to 20°C at night. A soil solution was prepared dissolving N aCl in 50% H oagland solu-tion. F ourteen plants per treatment were grown at salinities below that of seawater at 0, 171, and 428 mol m−3 and above seawater 856 mol m−3. The
plants were maintained under these salinities for 6 months. Branches and leaves used for measure-ments were young, fully expanded, and selected from the upper part of the plant canopy where irradiance exposure was maximal. Ten leaves from each of the four plants were randomly selected per treatment. They were washed with distilled water at dawn (06:00 h), allowed to excrete for 24 h, and then were washed again with 100 ml of distilled water. This procedure was followed for 2 successive days, and then the area of the leaves was measured. The solutions of secreted salt crystals were evapo-rated at 60°C in an oven. Afterwards, the residual salt was redissolved in 400ml of distilled water. The reliability of this procedure for recovering leaf secretion has been already tested in an earlier study [22].
2.2. S ample preparation and elemental analysis
In TXR F analysis, the ratio of intensity of a target element of unknown concentration to that of an internal standard of known concentration is related to the concentration of the target element [23]. We added an amount of 1159 mg ml−1 of
cobalt solution to obtain 10mg ml−1in each sample
as internal standard. Then, a drop of 10 ml of the specimen was deposited in the centre of a quartz reflector, dried under vacuum and placed in the TXR F analyser. The apparatus consisted of an X-ray generator coupled to a TXR F unit. Condi-tions for spectra collection were 40 K V, 20 mA and a counting time of 500 s. M ore detailed description of this instrument can be found elsewhere [25]. The relative elemental sensitivity of the spectrometer was determined by the analysis of a multielemental aqueous standard solution containing 10 mg ml−1
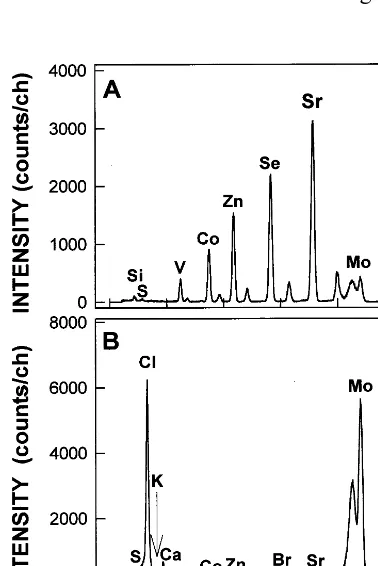
of each of V, Co, Zn, Se and Sr (F ig. 1A). Typical spectrum for leaf secretion of A . germinans is shown in F ig. 1B. This spectrum is in a linear scale and shows that Co internal standard signal seems small in comparison with the very large signal of other elements (e.g. Cl and Ca). H owever, Co signal had a total integral of 2020 counts, which is large
F ig. 1. (A) Spectrum of an aqueous multielement standard solution with approximately 10 mg ml−1 of each contained
element. (B) Spectrum of leaf secretion of A . germinans grown at 856 mol m−3 N aCl spiked with Co as internal standard
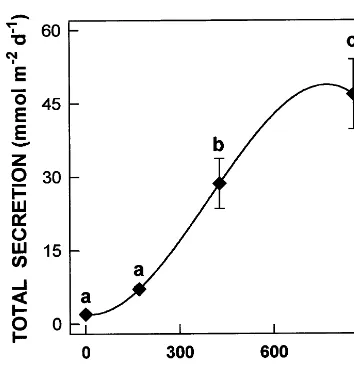
F ig. 2. Total leaf gland secretion as a function of external salinity. Values are the means of six observations and bars are standard errors. D ifferent letters on top of the symbols indi-cate statistically significant differences at PB0.05 for each parameter.
basis. Total secretion, as well as the relative contri-bution of each element was calculated. After-wards, results were subjected to a one-way AN OVA and then the least significant difference (LSD ) at PB0.05 was determined [27].
3. Results and discussion
Leaves from plants grown under 428 and 856 mol m−3 N aCl treatments excreted significantly
higher quantities compared to those grown at 171 mol m−3N aCl and control plants (F ig. 2). At the
highest salinities, secretion values were higher that those calculated from osmometric measurements, and assuming that all secretion was composed only for N a and Cl in comparable amounts [22]. N a and Cl represented a large proportion of the total secretion for leaves (F ig. 3). Secretion of N a in the control plants suggested that A . germinans had the capability of preferential N a uptake which was present in trace amounts in the growing solu-tion. The excretion of N a exceeded that of Cl when salinity increased and this led to a progres-sive increase in N a/Cl ratios from 0.46 in control plants up to 2.75 in plants grown at 856 mol m−3
N aCl (F ig. 3). An increase in N a/Cl with rising external N aCl concentration may be the result of high N a in the xylem of these species. Salinity causes changes in the composition of xylem fluids in halophytes. In particular, N a may increase [2,28]. The concentration of N a in the xylem sap of A . germinans is about 8 mol m−3 in plants
grown without N aCl and increases to 60 mol m−3
in plants grown at 856 mol m−3 N aCl (M .A.
Sobrado, unpublished). These values account for 20 and 60% of the total osmolality reported in control and salt-treated plants, respectively [22]. Enrichment of the secretion of N a with respect to Cl has also been found in other salt-secreting mangrove (A . marina), growing in both field and controlled conditions [5,6].
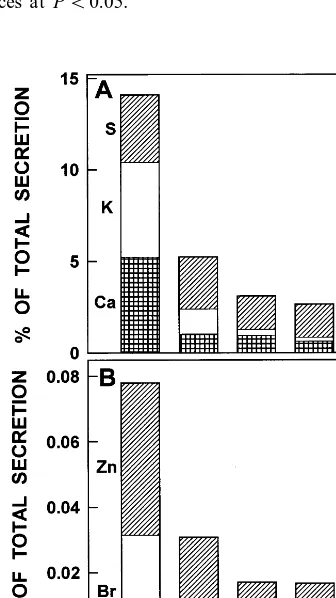
Even though mangroves mainly secrete N a and Cl, indicating plant control over the type of sub-stance secreted from their glands, other ions have been detected [6,7,9]. In A . germinans, N a and Cl accounted for 85.9191.81% of total secretion in control plants and about 96.3290.30% in salt-treated plants. H owever, other elements were also excreted in sizeable amounts such as S as SO42−,
Ca, Br and Zn (F ig. 4). Total excretion of S, Ca, Br and Zn tended to increase with salinity.
Ex-F ig. 3. Leaf gland secretion of N a and Cl, and N a/Cl ratios as a function of external salinity. Values are the means of six observations and bars are standard errors. D ifferent letters on top of the symbols indicate statistically significant differences at PB0.05 for each of the three parameters.
creted K remained about 100.8910.1 mmol m−2
per day in all the treatments. H owever, the relative contribution of these elements, in terms of the
total secretion, was important in control plants (15% of the total secretion) and declined signifi-cantly under salinity treatments (B5% of the total secretion; F ig. 5). The secretion of essential macronutrients (K , Ca, S) and micronutrients (Zn) may have significance in the ecological distribution of these species in areas with low availability of these nutrients.
Physiological significance of leaf glands for the ionic balance of the A6icennia species is of
paramount importance [3,29,30]. H owever, cation (N a, Ca, K and Zn) to anion (Cl and S mainly as SO42−) ratios increased with salinity from 0.709
0.02 per day in control plants up to 1.1090.05 (at 171 mol m−3N aCl), 1.9290.19 (at 428 mol m−3
N aCl) and 2.5190.31 (at 856 mol m−3 N aCl).
This suggested that high N aCl concentration may induce changes in the secretion mechanisms fa-vouring the secretion of cations. It may be possible that at high salinity, contribution of organic an-ions is enhanced to maintain the charge equi-librium [31].
4. Conclusions
In conclusion, TXR F has been shown to be a powerful tool allowing quantitative determination of Cl, secreted in relatively large quantities, as well as other elements secreted in intermediate (S, Ca, K ) and in trace quantities (Br and Zn). Analyzing standard reference solutions, this method is seen to avoid differential dilutions for quantifying ele-ments in such contrasting proportions. M oreover, it allowed differentiating each treatment and the contribution of each element to the total secretion. In the biological context, it was found that leaf glands of the mangrove A . germinans, like other
A6icennia species, are largely adapted to exclude
N a and Cl (86 – 96%). Thus, these species would be better adapted to high N aCl and possibly excluded from sites with very low N aCl. M oreover, the loss of other elements, particularly when N aCl was absent or low in the soil solution, may exclude these species from nutrient-impoverished non-sa-line soils. U nder natural conditions, mangrove species distribute differentially in a banded zona-tion pattern oriented roughly parallel to shore, and A6icennia species occupy medium and high
intertidal areas where salinity is high and fluctuat-ing [1,10,11].
F ig. 4. Leaf gland secretion rates of (A) S, (B) Br, (C) Ca and (D ) Zn as a function of external salinity. Values are the means of six observations and bars are standard errors. D ifferent letters on top of the symbols indicate statistically significant differences at PB0.05.
Acknowledgements
F inancial support was provided for ‘F ondo de Trabajo-D ID -U SB’ to M . A. Sobrado. A TA pro-ject of the IAEA, Vienna, Austria supplied the TXR F spectrometer. Thoughtful comments of the referees are sincerely thanked. Technical assistance of Lic. A. C. M onteverde is sincerely appreciated.
References
[1] P.B. Tomlinson, The Botany of M angroves, Cambridge U niversity Press, London, 1986.
[2] R . M unns, Effect of high external N aCl concentrations on ion transport within shoot of L upinus alba. I. Ions on xylem sap, Plant Cell Environ. 11 (1988) 283 – 289. [3] P.B. Scholander, H ow mangrove desalinate seawater,
Physiol. Plant. 21 (1968) 251 – 261.
[4] M .C. Ball, Salinity tolerance in the mangroves A egiceras
corniculatum and A6icennia marina. I Water use in
rela-tion to growth, carbon partirela-tioning and salt balance, Aust. J. Plant Physiol. 15 (1988) 447 – 464.
[5] P.F . Scholander, H .T. H ammel, E.A. H emmingsen, W. G arey, Salt balance in mangroves, Plant Physiol. 37 (1962) 722 – 729.
[6] M .R . Atkinson, G .P. F indlay, A.B. H ope, M .G . Pitman, H .D . Saddler, K .R . West, Salt regulation in the man-groves R hizophora mucronata Lam. and A egialitis
annu-alata R .BR , Aust. J. Biol. Sci. 20 (1967) 589 – 599.
[7] P.I. Boon, W.G . Allaway, R ates of ionic specificity of salt secretion from excised leaves of the mangrove, A6icennia marina (F orsk.) Vierh, Aquat. Bot. 26 (1986) 143 – 153.
[8] U . Lu¨ttge, Structure and function of plant glands, Ann. R ev. Plant Physiol. 22 (1971) 23 – 44.
[9] G .R . M ac F arlane, M .D . Burchett, Zinc distribution and excretion in the leaves of the grey mangrove, A6icennia marina (F orsk.) Vierh, Environ. Exp. Bot. 41 (1999)
167 – 175.
[10] N .C. D uke, M .C. Ball, J.C. Ellison, F actors influencing biodiversity and distributional gradients in mangroves, G lobal Ecol Biogeogr. Letts. 7 (1998) 27 – 47.
[11] A.E. Lugo, S.C. Snedaker, The ecology of mangroves, Ann. R ev. Ecol. Syst. 5 (1974) 39 – 64.
[12] M .C. Ball, M .A. Sobrado, Ecophysiology of mangroves: challenges in linking physiological process with patterns in forest structure, in: M .C. Press, J.D . Scholes, M .G . Barker (Eds.), Advances in Plant Physiological Ecology, Blackwell Science, Oxford, 1999, pp. 331 – 346.
[13] E. M edina, M . F rancisco, Osmolality and d13C of leaf
tissue of mangrove species from environments of con-trasting rainfall and salinity, Estuarine Coast Shelf. Sci. 45 (1997) 337 – 344.
[14] F . R ada, G . G oldstein, A. Orozco, M . M ontilla, O. Zabala, A. Azo´car, Osmotic and turgor relations of three mangrove species, Aust. J. Plant Physiol. 16 (1989) 477 – 486.
[15] J.A.C. Smith, M . Popp, U . Lu¨ttge, W.J. Cram, M . D iaz, H . G riffith, H .S.J. Lee, E. M edina, C. Scha¨fer, K .H .
Stimmel, B. Thonke, Ecophysiology of xerophytic and halophytic vegetation of a coastal alluvial plain in north-ern Venezuela. VI. Water relations and gas exchange of mangroves, N ew Phytol. 11 (1989) 293 – 307.
[16] M .A. Sobrado, R elation of water transport to leaf gas exchange properties in three mangrove species, Trees 14 (2000) 258 – 262.
[17] N . Sua´rez, M .A. Sobrado, E. M edina, Salinity effects on the leaf water relations components and ion accumula-tion patterns in A6icennia germinans (L.) seedlings,
Oe-cologia 114 (1998) 299 – 304.
[18] N . Sua´rez, M .A. Sobrado, Adjustments in leaf water relations of the mangrove, A6icennia germinans (L.),
grown in a salinity gradient, Tree Physiol. 20 (2000) 277 – 282.
[19] A. Azo´car, F . R ada, A. Orozco, Water relations and gas exchange in two mangrove species with contrasting mechanisms of salt regulation, Ecotro´picos 5 (1992) 11 – 19.
[20] M .A. Sobrado, D rought effect on photosynthesis of the mangrove A6icennia germinans under contrasting
salini-ties, Trees 13 (1999) 125 – 130.
[21] M .A. Sobrado, Leaf photosynthesis of the mangrove
A6icennia germinans as affected by N aCl,
Photosynthet-ica 36 (1999) 547 – 555.
[22] M .A. Sobrado, Effect of high external N aCl concentra-tion on the osmolality of xylem sap, leaf tissue and leaf glands secretion of the mangrove A6icennia germinans
(L.), F lora 195 (2000) in press.
[23] E.D . G reaves, G . Bernasconi, P. Wobrauschek, C. Streli, D irect total-reflection X-ray fluorescence trace element analysis of organic matrix with a semiempirical standard, Spectrochim. Acta 52 (1997) 923 – 933.
[24] E.D . G reaves, H eavy elements in health-related organic matrix materials by total reflection X-ray fluorescence, Adv. X-R ay Anal (2000) in press.
[25] P. Wobrauscherk, P. K regsamer, Total X-ray fluores-cence analysis with polarized X-rays, a compact attach-ment unit, and high energy X-rays, Spectrochim. Acta 44B (1989) 453 – 460.
[26] IAEA, QXAS, Quantitative X-ray analysis system Ver-sion 3.5, International Atomic Energy Agency, Vienna, Austria, 1996.
[27] R .R . Sokal, F .J. R ohlf, Biometry, W.H . F reeman Com-pany, San F rancisco, 1969, pp. 271 – 321.
[28] R . M unns, N a+, K+ and Cl−in xylem sap flowing to
shoots of N aCl-treated barley, J. Exp. Bot. 36 (1985) 1032 – 1042.
[29] B.F . Clough, T.J. Andrew, I.R . Cowan, Physiological processes in mangroves, in: B.D . Clough (Ed.), M an-grove Ecosystems in Australia, Structure, F unction and M anagement, Australian N ational U niversity Press, Can-berra, Australia, 1982, pp. 193 – 210.
[30] M .C. Ball, Comparative ecophysiology of mangrove forest and tropical lowland moist rainforest, in: S.S. M ulkey, R .L. Chazdon, A.P. Smith (Eds.), Tropical F orest Plant Ecophysiology, Chapman & H all, N ew York, 1996, pp. 461 – 496.
[31] J. H agemeyer, Y. Waisel, Excretion of ions (Cd2+, Li+,
N a+, and Cl−) by T amarix aphylla, Physiol. Plant. 73