www.elsevier.com / locate / bres
Research report
Degree of immediate early gene induction in striatum by eticlopride
determines sensitivity to N-methyl-
D
-aspartate receptor blockade
a b ,
*
Amy C. Adams , Kristen A. Keefe
a
Department of Neurosurgery, University of Utah, Salt Lake City, UT 84112, USA
b
Department of Pharmacology and Toxicology, University of Utah, Salt Lake City, UT 84112, USA
Accepted 29 August 2000
Abstract
Cortical afferents excite striatal efferent neurons through activation of N-methyl-D-aspartate (NMDA) receptors, which can be
modulated by D2 dopamine receptors. It is suggested that activation of PKA by D2 receptor blockade leads to NMDA receptor phosphorylation in the dendrites or phosphorylation of transcription factors in the nucleus. Thus, the levels and cellular localization of activated PKA may determine if D2 antagonist-mediated gene expression is dependent on NMDA receptor activation. We have previously demonstrated that NMDA receptor antagonists block gene expression induced by a high dose of eticlopride in medial and central but not lateral striatum. Here, we examined the effects of NMDA receptor antagonists on striatal gene expression after administration of a low dose of eticlopride. The results showed that NMDA receptor antagonists blocked gene induction by eticlopride throughout striatum. Less PKA activation by the low dose of eticlopride might explain why the expression was more sensitive in the lateral striatum to NMDA receptor blockade than in our previous study. To increase levels of PKA activation to the extent that NMDA receptor blockade would have less effect on eticlopride-mediated gene induction in all regions of striatum, we administered the phosphodiesterase inhibitor IBMX to animals treated with eticlopride. The combined administration of IBMX and eticlopride induced gene expression that was only partially attenuated (c-fos) or unaffected (zif268 ) by NMDA receptor blockade. These data support the suggestion that the degree of second messenger activation by D2 receptor blockade determines whether D2 dopamine receptor antagonist-mediated gene expression is dependent on NMDA receptor activation. 2000 Elsevier Science B.V. All rights reserved.
Theme: Motor systems and sensorimotor integration
Topic: Basal ganglia
Keywords: Striatum; Eticlopride; NMDA receptor antagonist; IBMX; PKA; c-fos
1. Introduction tein) and the induction of the immediate early genes c-fos
and zif268 [3,5,31]. D2 dopamine receptors are negatively The striatum is the main input nucleus of the basal coupled to adenylyl cyclase, inhibiting the formation of ganglia and is involved in motor and cognitive functions. cAMP and activation of protein kinase A (PKA) [2,42]. The striatum receives afferent glutamate projections from Thus, D2 receptor blockade presumably facilitates the the cerebral cortex and thalamus, which act on N-methyl- activation of PKA. Additionally, it has been demonstrated D-aspartate (NMDA) and non-NMDA receptors [13,20– that D2 receptor antagonists, such as haloperidol, induce 22]. It also receives dopamine input from the substantia the expression of immediate early genes in striatum nigra pars compacta [16]. Calcium entry via the NMDA [6,12,19,43] and that this effect of haloperidol is complete-receptor and L-type channels presumably activates a kinase ly blocked in mice bearing a targeted disruption of thebII cascade that leads to the phosphorylation of the transcrip- regulatory subunit of PKA [1].
tion factor CREB (cAMP response element binding pro- Dopamine, acting through D1 and D2 subtypes of dopamine receptors, modulates NMDA receptor-mediated responses in striatal slices [9]. Recently, administration of
*Corresponding author. Tel.: 11-801-585-7989; fax: 1
1-801-585-haloperidol has been shown to result in phosphorylation of
5111.
E-mail address: [email protected] (K.A. Keefe). the NMDA receptor NR1 subunit [26]. In addition, NMDA
receptor blockade attenuates D2 dopamine receptor antago- neurons in that region. Presumably, less nuclear PKA was nist-mediated immediate early gene expression activated in the medial and central thirds of striatum, [6,12,19,43]. Such data suggest strongly that there are leading to a lesser degree of direct phosphorylation of intracellular interactions between these two transmitter CREB by PKA and greater dependence on NMDA re-receptor systems leading to the regulation of gene expres- ceptor activation [4,14,26,41]. By blocking the NMDA sion. However, the signaling pathways responsible for the receptor with CGS 19755 or MK-801, the D2 dopamine modulatory effects between these two neurotransmitter receptor-mediated induction of c-fos and zif268 in the systems have not yet been fully explored, particularly in medial and central striatum was therefore attenuated.
vivo. The present study was designed to further evaluate the
Konradi and colleagues [24,26,32] have proposed that hypothesis that the level of activation of signaling path-the levels of activated PKA may determine whepath-ther striatal ways by D2 receptor blockade determines whether eticlop-immediate early gene expression is dependent on NMDA ride-induced gene expression is dependent on NMDA receptor activation. In primary striatal cell culture, the receptor activation. We hypothesized that treatment with a NMDA receptor antagonist MK-801 had no effect on low dose of eticlopride (0.5 mg / kg) would render immedi-phosphorylation of CREB or the induction of c-fos after ate early gene expression susceptible to NMDA receptor the application of a high concentration of the cAMP blockade throughout the striatum due to lower levels of inducer forskolin [24,32]. However, MK-801 did block PKA activation, whereas a higher dose of eticlopride (1.0 CREB phosphorylation and c-fos expression induced by a mg / kg) had previously induced gene expression indepen-low concentration of forskolin [24,32]. The model pro- dent of NMDA receptor activation in the lateral striatum posed by Konradi and colleagues for D2 dopamine / [19]. In addition, we hypothesized that administration of NMDA receptor interactions in the regulation of gene the phosphodiesterase inhibitor IBMX to animals treated induction is thus based on the level of activation of PKA with eticlopride would increase the amount of PKA by the D2 / cAMP pathway [24,26]. Low levels of activated activation to the extent that NMDA receptor blockade PKA are thought to remain localized to the dendrites and would no longer have an effect on D2 antagonist-mediated to phosphorylate the NMDA receptor [26,32,36], leading gene induction in any region of striatum.
to greater influx of calcium and a strong depolarization of the dendritic spine. This depolarization is thought to promote opening of voltage-sensitive L-type calcium
chan-2. Materials and methods
nels. The resultant calcium influx is then thought to activate a signaling pathway that propagates to the nucleus,
2.1. Animals and housing leading to the phosphorylation of CREB and the induction
of c-fos [26,31]. Therefore, MK-801 is thought to
effec-Male Sprague-Dawley rats (Charles River Laboratories, tively block c-fos induction in response to low levels of
Wilmington, MA, USA) weighing 225 to 300 g were used
21
PKA activation by inhibiting Ca influx-mediated
activa-in all experiments. Rats were housed activa-in groups of four activa-in tion of signaling kinases. However, high levels of cAMP
hanging wire-mesh cages in a temperature-controlled room accumulation are proposed to activate a more widespread
on a 12:12 light:dark cycle. Rats had free access to food distribution of PKA throughout the neuron. In this case,
and water. All studies were approved by the Institutional PKA activated within the nucleus is thought to directly
Animal Care and Use Committee at the University of Utah phosphorylate CREB, leading to c-fos induction that is
and were performed in accordance with the National independent of NMDA receptor activation. Thus, this
Institutes of Health Guide for the Care and Use of effect is not blocked by NMDA receptor antagonist
Laboratory Animals.
administration [26,32].
We previously observed regional differences in striatal
immediate early gene expression after administration of the 2.2. Drugs D2 dopamine receptor antagonist eticlopride and the
2.3. Pharmacological manipulations RNA mix (final concentrations: 100mg / ml salmon sperm DNA; 250 mg / ml yeast total RNA; 250 mg / ml yeast 2.3.1. Determining the effects of MK-801, CGS 19755, tRNA). The mixture was heated to 658C for 5 min and then
and IBMX on eticlopride-induced gene expression cooled on wet ice for 1 min. Dithiothreitol (100 mM, final Rats were taken from their home cages and placed in concentration), sodium dodecyl sulfate (0.2% w / v, final plastic tub cages (four per cage). Rats were weighed and concentration), sodium thiosulphate (0.1% w / v, final con-then injected with either MK-801 (1 mg / kg), CGS 19755 centration), and hybridization buffer were added to the (10 mg / kg), IBMX (5.0–25.0 mg / kg), or the appropriate ribonucleotide mixture. The hybridization buffer contained vehicle solution. Sixty minutes (CGS 19755), 30 min (final concentrations): Tris buffer (23.8 mM, pH 7.4), (MK-801), or 15 min (IBMX) later, rats were injected EDTA (1.2 mM, pH 8.0), NaCl (357 mM), dextran sulfate with the D2 dopamine receptor antagonist eticlopride (0.5 (11.9%, w / v), Denhardt’s solution (1.23), and formamide mg / kg, i.p.). Doses and times between injections were (59.5%, v / v). Ninety microliters of probe in hybridization chosen based on previously published studies showing buffer was applied to each slide containing four sections. effective NMDA receptor blockade at these doses and Slides were coverslipped and hybridized overnight in within these time ranges [7,8,11,23]. Animals were sac- humid chambers at 558C. Slides were then washed at room rificed 40 min after the injection of eticlopride. Previous temperature four times in 13 saline-sodium citrate (SSC, studies have demonstrated that the expression of immedi- 0.15 M NaCl / 0.015 M sodium citrate, pH 7.2), incubated ate early genes in response to eticlopride administration is in ribonuclease A (RNase A; 5–20 mg / ml; Boehringer significant at this time point (H. Steiner, personal com- Mannheim) in buffer containing 0.5 M NaCl, 10 mM Tris munication). Control animals received two vehicle in- (pH 8.0), and 0.25 mM EDTA (pH 8.0) for 15 min at
jections. room temperature, and washed four times in 0.23 SSC at
608C. Slides were rinsed briefly in deionized water, air 2.3.2. Determining the effect of CGS 19755 on gene dried, and apposed to Kodak Biomax X-ray film (Kodak
expression induced by the combined administration of Biomax MR, Eastman Kodak Co., NY, USA) for 4 days to
eticlopride and IBMX 2 weeks to obtain film autoradiograms. Rats were taken from their home cages, placed in plastic
tub cages (four per cage), and weighed. Rats were injected 2.5. Data analysis with CGS 19755 (10 mg / kg) or vehicle 45 min prior to the
administration of IBMX (25 mg / kg) or vehicle. Fifteen Film autoradiograms were analyzed using the Macintosh-minutes later, eticlopride was administered (1.0 mg / kg). based image analysis program, Image (Wayne Rasband, Rats were sacrificed 40 min after the eticlopride injection. NIH). Images of brain sections were captured with a video camera, digitized, and stored on computer. Images were 2.4. In situ hybridization histochemistry captured under constant lighting conditions and within the linear range of the system response. Mean gray values Rats were euthanized by exposure to CO (1 min) and2 were analyzed in medial, central, and lateral thirds of the decapitated. The brains were rapidly removed and frozen right striatum from its dorsal aspect to the anterior in isopentane chilled on dry ice. Brains were stored at commissure ventrally. The average gray value of the white
2208C until they were cut into 12-mm thick sections in a matter was subtracted from the average gray value of the cryostat (Cryocut 1800, Cambridge Instruments, Germany) regions of interest to correct for background labeling. Data and thaw-mounted onto gelatin-chrome alum-subbed from film autoradiograms were analyzed with a one-way slides. Slides were stored at 2208C. Once all brains from analysis of variance for medial, central, and lateral thirds an experiment were sectioned, slides were postfixed in 4% of the striatum. Post hoc analysis was performed with the paraformaldehyde / 0.9% NaCl, acetylated in fresh 0.25% Tukey–Kramer test. Statistical significance was set at P#
acetic anhydride in 0.1 M triethanolamine / 0.9% NaCl (pH 0.05. 8.0), dehydrated in an ascending series of alcohols,
delipidated in chloroform, and rehydrated in a descending
series of alcohols. Slides were air-dried and stored at 3. Results 2708C.
For detection of c-fos and zif268 mRNAs, full-length 3.1. CGS 19755 and MK-801 block immediate early ribonucleotide probes complementary to the mRNAs for gene expression throughout striatum after administration
c-fos [10] and zif268 [27] were synthesized from the of a low dose of eticlopride
35
cDNAs using S-UTP and SP6 (c-fos) or T7 (zif268 )
RNA polymerase (Boehringer Mannheim, Indianapolis, As noted in the Introduction, the induction of c-fos and IN, USA). Labeled probes were diluted in hybridization zif268 in striatum by 1 mg / kg eticlopride was not blocked
6
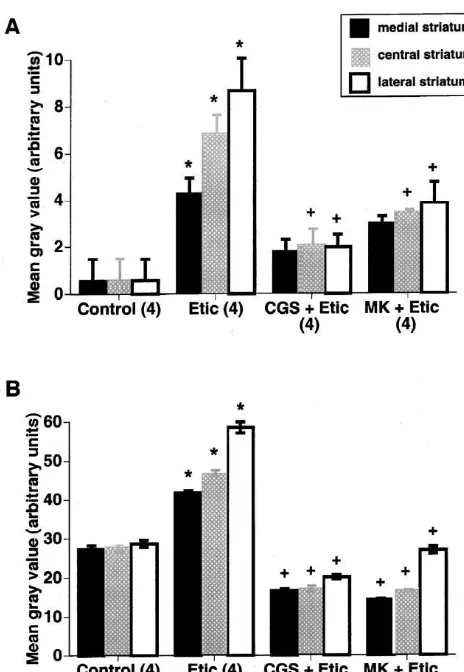
mg / kg) also induced c-fos and zif268 expression through-out the striatum, with greater expression in the lateral region. However, in this case the NMDA receptor antago-nists CGS 19755 (10 mg / kg) and MK-801 (1 mg / kg) effectively attenuated eticlopride-induced gene expression in all regions of the striatum (P,0.05) (Fig. 1).
3.2. The non-selective phosphodiesterase inhibitor IBMX
potentiates immediate early gene expression induced by eticlopride
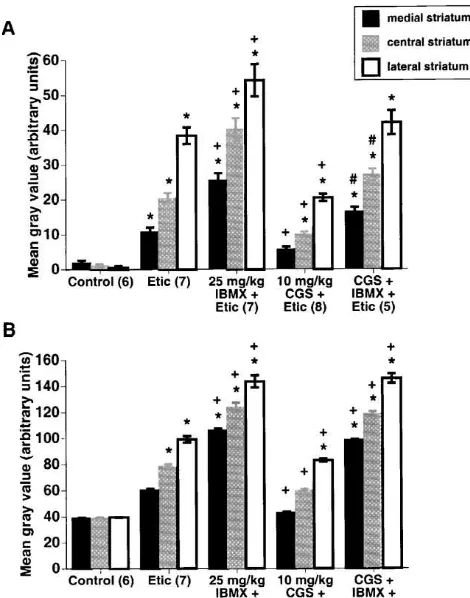
As is shown in Fig. 2, eticlopride (1 mg / kg) increased
Fig. 2. Effect of IBMX on (A) c-fos and (B) zif268 expression in striatum induced by a high dose of eticlopride. The non-selective phosphodiesterase inhibitor IBMX (5, 10, and 25 mg / kg) was adminis-tered intraperitoneally to animals treated with the D2 dopamine receptor antagonist eticlopride (‘Etic’; 1.0 mg / kg, i.p.). IBMX was administered 15 min before the administration of eticlopride. Animals receiving eticlopride alone received a vehicle injection 15 min prior to eticlopride administration. Control animals received two vehicle injections. Animals were sacrificed 40 min after the second injection. Values are mean gray values (6S.E.M.; arbitrary units) obtained from densitometric analysis of the medial, central, and lateral thirds of the mid-striatum (approximately 0.5 mm anterior to bregma). Numbers in parentheses indicate the number of animals per group. *Significantly different from control, P,0.05.
1Significantly different from eticlopride alone, P,0.05.
c-fos (A) and zif268 (B) expression in the striatum, with greater induction in the lateral striatum. Combined ad-ministration of the phosphodiesterase inhibitor IBMX with this dose of eticlopride increased the expression of both
Fig. 1. Effect of the NMDA receptor antagonists CGS 19755 and
MK-immediate early genes throughout all regions of the
801 on (A) c-fos and (B) zif268 expression in striatum induced by a low
striatum. This effect was significantly greater than the
dose of eticlopride. The NMDA receptor antagonists CGS 19755 (‘CGS’;
10 mg / kg) and MK-801 (‘MK’; 1 mg / kg) were administered intraperito- induction produced by eticlopride alone at the IBMX dose
neally to animals treated with the D2 dopamine receptor antagonist of 25 mg / kg (P,0.05, Fig. 2). eticlopride (‘Etic’; 0.5 mg / kg, i.p.). CGS 19755 was administered 1 h
before the administration of eticlopride. MK-801 was administered 30
3.3. CGS 19755 only partially attenuates c-fos
min before the injection of eticlopride. Animals receiving eticlopride
expression and does not affect zif268 expression after
alone received a vehicle injection either 1 h or 30 min before eticlopride
administration. Control animals received two vehicle injections. Animals the combined administration of eticlopride and IBMX
were sacrificed 40 min after the second injection. Values are mean gray
values (6S.E.M.; arbitrary units) obtained from densitometric analysis of As previously observed, the NMDA receptor antagonist the medial, central, and lateral thirds of the mid-striatum (approximately
CGS 19755 significantly reduced the induction of c-fos
0.5 mm anterior to bregma). Numbers in parentheses indicate the number
and zif268 by 1.0 mg / kg eticlopride alone (Fig. 3).
of animals per group. *Significantly different from control, P,0.05.
mg / kg) in the medial, but not the lateral aspect of the striatum. This was presumably due to greater numbers of D2 receptors and a more widespread activation of PKA in the lateral striatum. The results presented here demonstrate the susceptibility of c-fos and zif268 expression to NMDA receptor blockade throughout striatum after the administra-tion of a lower dose of eticlopride (0.5 mg / kg). We propose that the lower dose of eticlopride activated less nuclear PKA than did the higher dose, rendering gene expression dependent on NMDA receptor activation even in the lateral third of the striatum. These data are consistent with the recently published findings of Leveque et al. [26]. In this study, we have expanded these findings, showing that the non-selective phosphodiesterase inhibitor IBMX (25 mg / kg) potentiated eticlopride-induced gene expres-sion to such an extent that NMDA receptor blockade no longer affected the expression of zif268 and only partially suppressed c-fos expression. Although these studies do not directly assess the role of PKA in D2 / NMDA receptor interactions, they do indirectly support the model proposed by Konradi and colleagues [24,26] that the levels of activated PKA determine whether striatal gene expression is dependent on NMDA receptor activation.
It has been demonstrated that D2 dopamine receptor stimulation leads to the activation of an inhibitory G protein (G ) [38], presumably inhibiting cAMP formationi Fig. 3. Effect of CGS 19755 on (A) c-fos and (B) zif268 expression and the activation of PKA. Thus, D2 receptor blockade induced in striatum by the combined administration of eticlopride and
should disinhibit cAMP formation and PKA activation.
IBMX. The NMDA receptor antagonist CGS 19755 (‘CGS’; 10 mg / kg)
PKA can directly phosphorylate CREB, a transcription
and the non-selective phosphodiesterase inhibitor IBMX (25 mg / kg)
factor bound to the cAMP response element (CRE) in the
were administered intraperitoneally to animals treated with the D2
dopamine receptor antagonist eticlopride (‘Etic’; 1.0 mg / kg, i.p.). CGS promoter region of genes such as c-fos and zif268
19755 or vehicle was administered 45 min prior to IBMX or vehicle, [18,25,29]. It has also been demonstrated that PKA which was administered 15 min before the administration of eticlopride.
phosphorylates the NMDA receptor in striatal neurons
Animals receiving eticlopride alone received two vehicle injections, 45
[32,36], and administration of the D2 dopamine receptor
min and 15 min prior to eticlopride administration. Control animals
antagonist haloperidol results in NMDA receptor subunit
received three vehicle injections. Animals were sacrificed 40 min after the
last injection. Values are mean gray values (6S.E.M.; arbitrary units) phosphorylation [26]. NMDA receptor activation mediates
21
obtained from densitometric analysis of the medial, central, and lateral influx of Ca , strongly depolarizing the postsynaptic thirds of the mid-striatum (approximately 0.5 mm anterior to bregma).
membrane. This depolarization can trigger the opening of
Numbers in parentheses indicate the number of animals per group.
L-type voltage-sensitive calcium channels [31]. Calcium
*Significantly different from control, P,0.05. 1Significantly different
entry via the NMDA receptor and L-type channels then
from eticlopride alone, P,0.05.[Significantly different from IBMX1
eticlopride, P,0.05. presumably activates a kinase cascade that leads to the phosphorylation of CREB and the induction of c-fos in striatal neurons [31].
the combined administration of eticlopride and IBMX (Fig. The induction of immediate early genes by D2 receptor 3). The expression of c-fos was only partially attenuated blockade has been shown to be attenuated by NMDA by CGS 19755 in the medial and central regions of the receptor antagonists [6,12,19,43], suggesting a modulatory striatum, whereas zif268 expression was completely unaf- interaction between these two receptors. For example, fected by NMDA receptor blockade with CGS 19755 in all induction of c-fos or Fos protein by the antipsychotic
regions of the striatum. haloperidol is blocked by administration of the NMDA
receptor antagonists MK-801 and CPP [6,12,26,43]. Boeg-man and Vincent [6] showed a 68% blockade by MK-801
4. Discussion of Fos-positive immunoreactivity in the dorsolateral region
induced by a low dose of eticlopride not only in the lateral genes can be induced through activation of various sig-striatum, but also in the medial and central regions. The naling pathways and thus may be differentially regulated expression of zif268 also was completely blocked by by intracellular second messengers [3,14]. Furthermore, administration of MK-801 and CGS 19755 throughout the number and types of transcriptional regulatory ele-striatum after administration of this lower dose of eticlop- ments within the promoter regions differ between c-fos and ride. Furthermore, our findings extend those of previous zif268 genes [3,28,34]. Therefore, the expression of c-fos reports in that we have shown that D2 dopamine receptor may be more dependent on activation of the NMDA antagonist-mediated gene expression is differentially sus- receptor, whereas zif268 expression is more heavily depen-ceptible to NMDA receptor blockade depending on the dent on activation of other intracellular signals. Further dose of antagonist administered and the region of striatum experiments are necessary to address this possibility. examined (see Fig. 1). This is presumably due to greater However, our data strongly suggest that the degree of gene activation of PKA in the lateral striatum after administra- induction and, presumably, intracellular signaling activity tion of the higher dose of eticlopride. Interestingly, ad- by the combined administration of eticlopride and IBMX ministration of a higher dose of haloperidol (1 mg / kg) in determines the extent to which D2 antagonist-induced gene the Boegman and Vincent study [6] had a greater effect on expression is dependent on NMDA receptors, especially in the number of Fos-positive nuclei than did the lower dose, the case of zif268 expression.
but data were not reported for the effect of MK-801 on Fos In conclusion, our data support the model proposed by induction by the high dose of haloperidol. Konradi and colleagues [24,26] that the degree of activa-The model of Konradi and colleagues [24,26] suggests tion of intracellular signaling pathways determines whether that the intracellular kinase mediating the interactions D2 dopamine receptor antagonist-mediated immediate between NMDA and dopamine receptors is PKA. There- early gene expression is dependent on NMDA receptor fore, we administered the non-selective phosphodiesterase activation. It is important to note, however, that the inhibitor IBMX to animals treated with eticlopride in an contribution of PKA to the induction of immediate early attempt to potentiate the eticlopride-induced gene expres- genes by eticlopride has not been directly tested in our sion, presumably through an increase in PKA activation. experiments. Therefore, it will be necessary to examine the The combined administration of IBMX plus eticlopride effects of PKA inhibition on eticlopride-induced c-fos and produced significantly greater gene induction than did zif268 expression in vivo.
eticlopride alone. Pretreatment with the NMDA receptor antagonist CGS 19755 had no effect on the zif268
expres-sion induced by the combined IBMX and eticlopride Acknowledgements administration, whereas c-fos expression was partially
attenuated. This work was supported by grant NS 35579 (K.A.K.).
Although IBMX potentiated eticlopride-induced imme-diate early gene expression, the mechanism for this potentiation is unknown. It has been previously
demon-References
strated that IBMX administration alone can lead to the expression of c-fos in the striatum [37]. However, the
[1] M.R. Adams, E.P. Brandon, E.H. Chartoff, R.L. Idzerda, D.M.
induction by IBMX in that study was attributed to the
Dorsa, G.S. McKnight, Loss of haloperidol induced gene expression
antagonistic effect of IBMX on the adenosine A2a receptor and catalepsy in protein kinase A-deficient mice, Proc. Natl. Acad. [15], due to the lack of effect of other selective phospho- Sci. USA 94 (1997) 12157–12161.
[2] P.R. Albert, K.A. Neve, J.R. Bunzow, O. Civelli, Coupling of a
diesterase inhibitors on striatal c-fos expression [37].
cloned rat dopamine D2 receptor to inhibition of adenylyl cyclase
Adenosine A2a receptors are colocalized on striatopallidal
and prolactin secretion, J. Biol. Chem. 265 (1990) 2098–2104.
neurons with D2 dopamine receptors [35] and are
positive-[3] H. Bading, D.D. Ginty, M.E. Greenberg, Regulation of gene
ly coupled to adenylyl cyclase [30,39]. However, it has expression in hippocampal neurons by distinct signaling pathways, been demonstrated that A2a receptor antagonist, rather Science 260 (1993) 181–186.
than agonist, administration leads to the induction of [4] H. Bito, K. Deisseroth, R.W. Tsien, CREB phosphorylation and
21
dephosphorylation: a Ca - and stimulus duration-dependent switch
immediate early genes in striatum, presumably by
promot-for hippocampal gene expression, Cell 87 (1996) 1203–1214.
ing the release of calcium from microsomes of the 21
[5] H. Bito, K. Deisseroth, R.W. Tsien, Ca -dependent regulation in
endoplasmic reticulum [37,40]. Therefore, we cannot neuronal gene expression, Curr. Opin. Neurobiol. 7 (1997) 419– absolutely attribute the increase in immediate early gene 429.
expression reported herein to the phosphodiesterase prop- [6] R.J. Boegman, S.R. Vincent, Involvement of adenosine and gluta-mate receptors in the induction of c-fos in the striatum by
halo-erties of IBMX. Further experiments are necessary to
peridol, Synapse 22 (1996) 70–77.
differentiate these two possibilities.
[7] D. Cain, D. Saucier, F. Boon, Testing hypotheses of spatial learning:
At present, the basis for the differential effect of CGS the role of NMDA receptors and NMDA-mediated long-term 19755 on c-fos and zif268 expression induced by IBMX potentiation, Behav. Brain Res. 84 (1997) 179–193.
82.0715 as cerebral antiischemic agents. III. Evidence for antago- Barczak, S. Kang, X.-M. Li, J.T. Coyle, R.L. Huganir, S. Heckers, nistic effects at the polyamine modulatory site within the N-methyl- C. Konradi, Intracellular modulation of NMDA receptor function by
D-aspartate receptor complex, J. Pharmacol. Exp. Ther. 253 (1990) antipsychotic drugs, J. Neurosci. 20 (2000) 4011–4020.
475–482. [27] J. Milbrandt, A nerve growth factor-induced gene encodes a possible [9] C. Cepeda, N.A. Buchwald, M.S. Levine, Neuromodulatory actions transcriptional regulatory factor, Science 238 (1987) 797–799.
of dopamine in the neostriatum are dependent upon the excitatory [28] C.K. Miranti, D.D. Ginty, G. Huang, T. Chatila, M.E. Greenberg, amino acid receptor subtypes activated, Proc. Natl. Acad. Sci. USA Calcium activates serum response factor-dependent transcription by
21 90 (1993) 9576–9580. a ras- and Elk-1-independent mechanisms that involves a Ca / [10] T. Curran, M.B. Gordon, K.L. Rubino, L.C. Sambucetti, Isolation calmodulin-dependent kinase, Mol. Cell. Biol. 13 (1995) 5195–
and characterization of the c-fos (rat) cDNA and analysis of post- 5205.
translational modification in vitro, Oncogene 2 (1987) 79–84. [29] M.R. Montminy, L.M. Bilezikjian, Binding of a nuclear protein to [11] G. DeSarro, A. DeSarro, Anticonvulsant properties of non-competi- the cyclic-AMP response element of the somatostatin gene, Nature
tive antagonists of the N-methyl-D-aspartate receptor in genetically 328 (1987) 175–178.
epilepsy-prone rats: comparison with CPPene, Neuropharmacology [30] J. Premont, M. Perez, G. Blanc, J.P. Tassin, A.M. Thierry, D. Herve, 32 (1993) 51–58. J. Bockaert, Adenosine-sensitive adenylate cyclase in rat brain [12] M. Dragunow, G.S. Robertson, R.L. Faull, H.A. Robertson, K. homogenates: kinetic characteristics, specificity, topographical, sub-Jansen, D2 dopamine receptor antagonists induce fos and related cellular, and cellular distribution, Mol. Pharmacol. 16 (1979) 790– proteins in rat striatal neurons, Neuroscience 37 (1990) 287–294. 804.
[13] L. Dube, A.D. Smith, J.P. Bolam, Identification of synaptic terminals [31] A. Rajadhyaksha, A. Barczak, W. Macias, J.-C. Leveque, S.E.
21
of thalamic or cortical origin in contact with distinct medium-size Lewis, C. Konradi, L-Type Ca channels are essential for glu-spiny neurons in the rat neostriatum, J. Comp. Neurol. 267 (1988) tamate-mediated CREB phosphorylation and c-fos gene expression
455–471. in striatal neurons, J. Neurosci. 19 (1999) 6348–6359.
[14] H. Enslen, T.R. Soderling, Roles of calmodulin-dependent protein [32] A. Rajadhyaksha, J.C. Leveque, W. Macias, A. Barczak, C. Konradi, kinases and phosphatase in calcium-dependent transcription of Molecular components of striatal plasticity: the various routes of immediate early genes, J. Biol. Chem. 269 (1994) 20872–20877. cyclic AMP pathways, Dev. Neurosci. 20 (1998) 204–215. [15] B.B. Fredholm, B. Bergman, J. Fastbom, B. Jonzon, The role of [33] G. Robertson, H. Fibiger, Neuroleptics increase c-fos expression in
adenosine in the actions of xanthines on the central nervous system, the forebrain: contrasting effects of haloperidol and clozapine, in: K.E. Andersson, C.A. Persson (Eds.), Anti-asthma Xanthines and Neuroscience 46 (1992) 315–328.
Adenosine, Excerpta Medica, Amsterdam, 1985, pp. 291–308. [34] L.M. Robertson, T.K. Kerpolla, M. Vendrell, D. Luk, R.J. Smeyne, [16] O. Hornykiewicz, H.-J. Lisch, A. Springer, Homovanillic acid in C. Bocchiara, J.I. Morgan, T. Curran, Regulation of c-fos expression different regions of the human brain: attempt at localizing central in transgenic mice requires multiple interdependent transcription dopamine fibers, Brain Res. 11 (1968) 662–671. control elements, Neuron 14 (1995) 214–252.
[17] J.N. Joyce, S.K. Loeschen, J.F. Marshall, Dopamine D2 receptors in [35] S.N. Schiffman, O.P. Jacobs, J.-J. Vanderhaeghen, Striatal restricted rat caudate-putamen: the lateral to medial gradient does not corre- adenosine A2 receptor (RDC8) is expressed by enkephalin but not spond to dopaminergic innervation, Brain Res. 338 (1985) 209–218. by substance P neurons: an in situ hybridization histochemistry [18] B.L. Kee, J. Arias, M.R. Montminy, Adaptor-mediated recruitment study, J. Neurochem. 57 (1991) 1062–1067.
of RNA polymerase II to a signal-dependent activator, J. Biol. [36] G.L. Snyder, A.A. Fienberg, R.L. Huganir, P. Greengard, A Chem. 271 (1996) 2373–2375. dopamine / D1 receptor / protein kinase A / dopamine- and cAMP-[19] K.A. Keefe, A.C. Adams, Differential effects of NMDA receptor regulated phosphoprotein (M 32 kDa) / protein phosphatase-1 path-r
blockade on eticlopride-induced immediate early gene expression in way regulates dephosphorylation of the NMDA receptor, J. Neuro-the medial and lateral striatum, J. Pharmacol. Exp. Ther. 287 (1998) sci. 18 (1998) 10297–10303.
1076–1083. [37] P. Svenningsson, B. Johansson, B.B. Fredholm, Effect of different [20] J.M. Kemp, T.P. Powell, The termination of fibres from the cerebral xanthines and phosphodiesterase inhibitors on c-fos expression in rat
cortex and thalamus upon dendritic spines in the caudate nucleus: a striatum, Acta Physiol. Scand. 154 (1995) 17–24.
study with the Golgi method, Philos. Trans. R. Soc. London: Biol. [38] L. Vallar, J. Meldolsi, Mechanisms of signal transduction at the Sci. 262 (1971) 429–439. dopamine D2 receptor, Trends Pharmacol. Sci. 10 (1989) 74–77. [21] J.S. Kim, R. Hassler, P. Haug, K.-S. Paik, Effect of frontal cortex [39] D. Van Calker, M. Muller, B. Hamprecht, Adenosine regulates via
ablation on striatal glutamic acid level in rat, Brain Res. 132 (1977) two different types of receptors, the accumulation of cyclic AMP in
370–374. cultured brain cells, J. Neurochem. 33 (1979) 999–1005.
[22] S.T. Kitai, J.D. Kocsis, J. Wood, Origin and characteristics of the [40] A. Verma, D.J. Hirsch, S.H. Snyder, Calcium pools mobilized by cortico-caudate afferents: an anatomical and electrophysiological calcium or inositol 1,4,5-triphosphate are differentially localized in study, Brain Res. 118 (1976) 137–141. rat heart and brain, Mol. Biol. Cell 3 (1992) 621–631.
[23] C. Koerner, L. Danober, A. Boehrer, C. Marescaux, M. Vergnes, [41] S.R. Vincent, M. Sebben, A. Dumuis, J. Bockaert, Neurotransmitter Thalamic NMDA transmission in a genetic model of absence regulation of MAP kinase signaling in striatal neurons in primary epilepsy in rats, Epilepsy Res. 25 (1996) 11–19. culture, Synapse 29 (1998) 29–36.
[24] C. Konradi, The molecular basis of dopamine and glutamate [42] S. Weiss, M. Serben, J.A. Garcia-Sainz, J. Bockaert, D2-dopamine interactions in the striatum, Adv. Pharmacol. 42 (1998) 729–733. receptor-mediated inhibition of cyclic AMP formation in striatal [25] R.P. Kwok, J.R. Lundblad, J.C. Chrivia, J.P. Richards, H.P. Baching- neurons in primary culture, Mol. Pharmacol. 27 (1985) 595–599.
´ ¨
er, R.G. Brennan, S.G. Roberts, M.R. Green, R.H. Goodman, [43] B. Ziolkowska, V. Hollt, The NMDA receptor antagonist MK-801 Nuclear protein CBP is a coactivator for the transcription factor markedly reduces the induction of c-fos gene by haloperidol in the CREB, Nature 370 (1994) 223–226. mouse striatum, Neurosci. Lett. 156 (1993) 39–42.