L
Journal of Experimental Marine Biology and Ecology 243 (2000) 169–184
www.elsevier.nl / locate / jembe
Effects of shell fit on the biology of the hermit crab Pagurus
longicarpus (Say)
* Jennifer E. Angel
Department of Biology, Tufts University, Medford, MA 02155, USA
Received 19 February 1999; received in revised form 5 July 1999; accepted 27 July 1999
Abstract
Most hermit crabs have a specialized lifestyle that requires them to occupy gastropod shells. The size of shells that hermit crabs inhabit relative to their body size affects their growth, survival and fecundity. In this study, the effects of shell fit on individual growth rate, risk of predation, feeding rate, and activity level were examined in the laboratory for hermit crabs Pagurus
longicarpus collected from Nahant, MA, USA. Feeding rate and general activity level of hermit
crabs confined to tightly fitting shells and hermit crabs occupying shells of preferred size were not significantly different. Hermit crabs confined to tightly fitting shells grew at significantly slower rates, and were significantly more susceptible to predation by a common North Atlantic rock crab,
Cancer irroratus. While this study provides further evidence of the negative effects of tight shell
fit on growth and survivorship of hermit crabs, a mechanistic explanation for the decreased growth rate of hermit crabs in tightly fitting shells is still sought. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Behavior; Growth rate; Hermit crab; Predation risk; Shell adequacy
1. Introduction
The dependence of hermit crabs on the shells they inhabit has long been a subject of fascination to ecologists. Ideally, a hermit crab continually switches to larger shells as it grows, thereby maintaining a shelter that adequately protects it from predators and provides enough space for a female hermit crab’s brood (Childress, 1972; Hazlett, 1981). The ability of hermit crabs to seek out and occupy shells that provide optimal fit
*Present address: c / o Jan A. Pechenik, Department of Biology, Dana Laboratory, Tufts University, Medford, MA 02155, USA. Tel.:11-617-627-3199; fax:11-617-627-3805.
E-mail address: [email protected] (J.E. Angel)
is well documented (Scully, 1979; McClintock, 1985; Wada et al., 1997). In reality, there are rarely enough shells of the right sizes for all the hermit crabs in a population (e.g., Vance, 1972a; Scully, 1979). Such shell limitation forces many hermit crabs to occupy shells that are the wrong size for them; often the shells are too small.
Shells of inadequate size can increase the risk of both desiccation and predation. Mortality from desiccation at low tide increased for pagurid hermit crabs in shells that were too small for them (Taylor, 1981). The hermit crab Pagurus granosimanus was significantly more vulnerable to predation by the brachyuran crab Cancer gracilis when the hermit crab was unable to retreat completely into its shell (Vance, 1972b). Whether inadequate shell fit increases risk of predation in other pagurid species has not been examined.
When confined to shells that were too small for them, the temperate hermit crabs
Pagurus bernhardus (Markham, 1968), Pagurus pollicaris (Fotheringham, 1976a), Pagurus longicarpus (Fotheringham, 1976a; Blackstone, 1985), and Clibanarius vittatus
(Fotheringham, 1976b) grew more slowly. Nothing is known of the mechanisms regulating the reduced growth rate observed in hermit crabs with tight shell fit. Possible mechanisms that could mediate growth rate declines include increased metabolic activity, decreased feeding activity, or decreased food assimilation efficiency.
While hunting or scavenging food, lobsters and true crabs must often leave shelter and expose themselves to potential predators. In contrast, the hermit crab, a scavenger and filter feeder, carries shelter with it at all times (Hazlett, 1981). However, a hermit crab’s chances of avoiding predation are only as good as its shell protection. This mobile protection is probably less effective if a hermit crab has outgrown its shell, and is unable to withdraw its body fully inside. Thus, for a hermit crab in a shell-limited environment, maximal growth (best achieved by maximal net energy intake) can be at odds with the need for protection. Because there is strong evidence of a behavioral component to the hermit crab’s assessment and improvement of shell fit (Elwood and Neil, 1992; Hazlett, 1996; Wada et al., 1997), it is likely that a quantifiable behavioral change contributes to any changes in growth rate the hermit crab experiences.
In the summer of 1997, approximately a quarter of the individuals of P. longicarpus at Nahant occupied tightly fitting shells (personal observation), making research of effects of tight shell fit relevant. In this study, it was first determined whether growth rate decreased for hermit crabs confined to tightly fitting shells. Then, ultimate and proximate mechanisms that might influence growth rate were examined.
2. Methods
2.1. Animals and study site
2.2. Shell adequacy index
The methods used to determine the shell fit preferred by P. longicarpus at Nahant were pioneered by Vance (1972a). Fifty hermit crabs occupying shells of Littorina
littorea were collected from the field site in June, 1997. Shells of the periwinkle, L.
littorea, are the dominant shell type used by the study population (personal observation).
The hermit crabs were introduced to an aquarium filled with circulating seawater and 250 empty shells, making a total of 300 shells available for occupation. Empty shells were prepared by collecting live L. littorea from one rock surface at Nahant, boiling and removing the flesh, rinsing in seawater, and air-drying. The shells ranged in size from 5 to 22 mm in aperture length. The hermit crabs were allowed 48 h to choose new shells. The shells occupied at this time were presumed to be of preferred size since the crabs had ceased exploring and moving into new shells. Crabs were then removed from their chosen shells. Each crab was blotted of excess water and massed to the nearest 0.1 mg. The aperture length of the shell occupied by each crab was measured to the nearest 0.1 mm using calipers.
Measurements (mass of crab and shell aperture length) were log-transformed and plotted. The best fit line describing the relationship between crab mass and preferred
2
shell size was determined using least-squares regression (r 50.8625, P,0.0001).
log shell aperture length50.2623(log crab mass)10.4342 (1)
Shell adequacy index is defined for any crab-shell combination as the ratio of predicted crab mass to actual crab mass. Thus, a hermit crab that occupies a shell of preferred size (as predicted by Eq. 1) will have a shell adequacy index51.0. A 500 mg hermit crab that occupies a shell predicted to be the preferred size for a crab half its mass, a 250 mg crab, has a shell adequacy index of 0.5.
Throughout this study, shell fit of experimental hermit crabs was manipulated to yield a shell adequacy index of either 0.5 (tight fit) or 1.0 (preferred fit). When hermit crabs with a shell adequacy index of 0.5 retreated into the shell, they were unable to withdraw their chelae behind the shell aperture, allowing predatory crabs to grasp and pull these hermit crabs from the shell. Hermit crabs with a shell adequacy index of 1.0 could withdraw completely into their shells.
2.3. Growth rate
days when feeding rate was measured (described in Section 2.5.2). Final wet masses were measured after removing each hermit crab from its assigned shell after 89 days. Growth rate was calculated as the difference between final and initial wet mass divided by time (mg / day). One hermit crab died, thus data were available for 13 pairs.
2.4. Predation risk
Cancer irroratus (Say 1817), a common North Atlantic rock crab (family Cancridae)
coexists with the population of P. longicarpus at Nahant. C. irroratus is known to prey on pagurid hermit crabs in the New York Bight (Stehlik, 1993) and in kelp beds off Nova Scotia (Drummond-Davis et al., 1982).
Individuals of C. irroratus were collected from White Beach, Manchester-by-the-Sea, MA. Although C. irroratus inhabits the field site at Nahant, it was more abundant and easier to collect at this site 15 miles north of Nahant. Collections were made on May 29, June 15, and June 19, 1998. Eight males and three non-gravid females were collected. All predators were maintained in continuously flowing seawater provided via pipeline from surrounding ocean. Temperatures ranged from 9 to 188C, reflecting natural increases in field temperatures from late May to late June, 1998, and the salinity averaged 30 ppt. Predators were fed crushed blue mussel Mytilus edulis every 2–3 days. Prior to use in the predation experiments, the predators were isolated in mesh-walled plastic containers (32032303150 mm) and starved at least 3 days.
Twenty-two hermit crabs were collected from Nahant. Each hermit crab was pulled from its shell, massed to the nearest 10 mg, and paired by mass with another hermit crab. The hermit crabs were not sexed, but none was ovigerous. Each member of the pair was randomly assigned to receive one of two shell treatments, ‘preferred fit’ or ‘tight fit’, as described in Section 2.2.
Each hermit crab was isolated in a 100 ml plastic cup filled with beach sand to a depth of 20 mm and covered with nylon mesh, then submerged in seawater. Like the predators, they were held in a sea table receiving continuous water flow. All the hermit crabs received a diet of crushed blue mussel and shrimp pellets, and were fed every 2–3 days. Each pair of hermit crabs adjusted to the shell treatments at least 4 days before being offered to a predator.
Each predator was moved to a glass aquarium (40032103250 mm) receiving continuous seawater flow. Two to 3 days later, a pair of hermit crabs was introduced into the aquarium. All interactions between predator and hermit crabs were recorded. Observations were continued until the predator wounded at least one of the hermit crabs; only the eleventh trial was not continuously observed.
The dependence of hermit crab mortality on shell adequacy index was analyzed using Fisher’s exact test (Ghent, 1996). Each predator was used only once, and surviving hermit crabs were not used again.
2.5. Feeding rate
2.5.1. Assay development
To determine feeding rates in P. longicarpus, amount of food eaten over time was measured. Imitation crab meat (as described in Section 2.3), which neither gained mass in seawater nor crumbled in seawater, was the food used in the assay. At the end of the feeding period, food fragments not eaten by the hermit crabs were easily collected from the glass dishes housing the hermit crabs, and then re-massed.
In a preliminary experiment, feeding rates as a function of hermit crab size were measured weekly for 3 weeks. As in the growth rate experiment, hermit crabs were housed in glass dishes in 30 ppt salinity seawater, this time at 238C. Each hermit crab was starved for 2 days, then given a piece of meat of a mass between 250.0 and 500.0 mg (mass was measured to the nearest 0.1 mg after the food soaked 15 min in seawater). After hermit crabs fed for 5 h, the food was removed and massed. To determine feeding rates (mg / h), final food mass was subtracted from initial food mass and divided by assay duration.
Hermit crab size was assessed by measuring the anterior carapace length (defined by Markham, 1968 as the length of the ‘hard portion of carapace’) to the nearest 0.1 mm at 123 power using a Zeiss dissecting microscope fitted with an ocular micrometer.
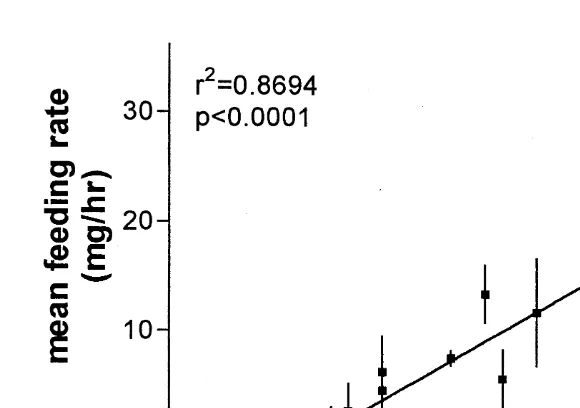
Feeding rates varied from barely detectable in the smallest crabs to one record of 35 mg / h in the largest crab (Fig. 1). The mean feeding rate (of three trials) increased
2
linearly with crab size (r 50.8694, P,0.0001). These results are in agreement with feeding rates reported for Pagurus acadianus, a larger relative of P. longicarpus. Scarratt and Godin (1992) reported feeding rates of 160 mg over 8 h, or 20 mg / h, for P.
acadianus feeding on haddock. This was for hermit crabs of a mass of approximately 1.5
g, which is about 20–30% larger than the average P. longicarpus used for assay development.
This preliminary result gave me confidence in my methods. I used this protocol in all subsequent feeding assays.
2.5.2. Feeding rate experiment 1
During the 12 weeks that growth rates were monitored for 14 hermit crab pairs (methods above), feeding rates were measured in weeks 2, 3, and 5 (each time after hermit crabs were starved 2 days.) Hermit crabs were given pieces of food with initial wet masses of 600.0–750.0 mg, and were allowed to feed for an average of 5 h. To determine shell treatment effects (‘preferred fit’ and ‘tight fit’) on feeding rate, a t statistic was calculated.
2.5.3. Feeding rate experiment 2
Fig. 1. Feeding rate as function of size for hermit crab P. longicarpus. Mean feeding rate6S.D. is shown as a function of anterior carapace length. Each point represents the mean of three measurements. n514 hermit crabs.
withdraw their chelae into their shells. Each crab was isolated in a glass dish of 30 ppt salinity seawater at 188C, and starved for 2 days before each assay. The hermit crabs were fed only during the assays. For each individual, feeding rates were measured three to five times in the first 2 weeks of captivity.
After 2 weeks, 17 of the crabs were each offered a choice of three larger shells to move into. All of them switched into larger shells (shell switchers). The other 20 crabs were denied the opportunity to switch (control). Feeding rates were then re-measured three to five times for each hermit crab in the two treatments (control and shell switchers) during the third and fourth weeks of captivity. To determine shell treatment effects on feeding rate, a t statistic was calculated.
2.6. Activity level
Activity was monitored for 1 h intervals using a Sony Hi 8 mm video camcorder. The two hermit crabs in each pair were placed on either side (assignment to sides was random) of a glass aquarium (40032103250 mm) divided down the middle by a perforated, opaque plastic tank divider (Penn-Plax, New York). A black-on-white paper grid of 25 mm squares was affixed to the outside of the tank’s bottom surface. There were an equal number of squares marked out on each side of the divider. Seawater (248C, 30 ppt) covered the crabs, and was replaced for each new pair of crabs observed. The hermit crabs acclimated to their new environment for 1 h.
Each pair of hermit crabs was then recorded for 1 h. The recording room was free of visual and auditory distractions, such as human movement. Both hermit crabs in each pair received the same light intensity (approximately 350 lux) from an overhead fluorescent lamp, and because the tank divider was perforated, the paired crabs shared the same seawater environment.
To analyze the data, the videotape was reviewed at high speed. Activity level was defined as the number of squares on the grid crossed by each hermit crab during the 1 h of observation. To determine shell treatment effects on activity level, a t statistic was calculated.
3. Results
3.1. Growth rate
Shell fit significantly affected crab growth rate (df512, t53.316, P50.0062). For 11 of 13 pairs, the crab in the tightly fitting shell grew at a slower rate. Mean growth rate6S.E.M. for 13 hermit crabs in tight shells (shell adequacy index50.5) was 0.060.1 mg / day. Mean growth rate6S.E.M. for 13 hermit crabs in shells of preferred fit (shell adequacy index51.0) was 0.460.1 mg / day. The mean difference in growth rates between shell treatments was significant.
3.2. Predation risk
During the observation periods, predators handled both hermit crabs in seven out of the 10 trials observed. (In the eleventh trial, there was no observation period.) In these seven cases where both hermit crabs were handled, all the hermit crabs with tight shell fit were wounded, while none of the hermit crabs with preferred shell fit were harmed. In the three cases in which only the hermit crab with tight shell fit was attacked during the observation period, all three were wounded.
Table 1
a Status of hermit crabs after 481h exposure to predators Shell adequacy index Hermit crab status
Dead Alive
0.5 (tight fit) 11 0
1.0 (preferred fit) 1 10
a
Each of 11 pairs of hermit crabs P. longicarpus, one with shell adequacy index 1.0 (preferred fit), and the other a shell adequacy index of 0.5 (tight fit), was exposed to a distinct predator (C. irroratus). The category ‘Dead’ includes hermit crabs that were eaten or fatally wounded. P,0.0001, Fisher exact test.
3.3. Feeding rate
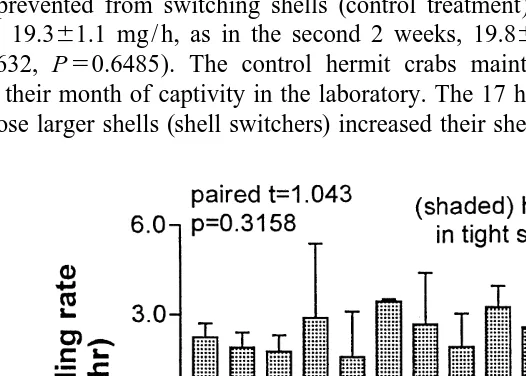
There was no significant difference in feeding rate between the paired hermit crabs in the two shell treatments of the first feeding rate experiment (Fig. 2; df513, t51.043,
P50.3158). The mean feeding rate6S.E.M. of 14 hermit crabs in shells of preferred fit (shell adequacy index51.0) was 2.760.2 mg / h. The mean feeding rate6S.E.M. of 14 hermit crabs in tightly fitting shells (shell adequacy index50.5) was 2.360.2 mg / h.
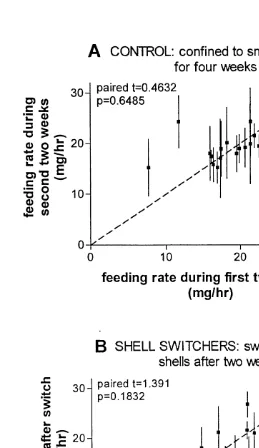
In the second feeding rate experiment, the mean feeding rate6S.E.M. of the 20 hermit crabs prevented from switching shells (control treatment) was the same in the first 2 weeks, 19.361.1 mg / h, as in the second 2 weeks, 19.860.6 mg / h (Fig. 3A, df519,
t50.4632, P50.6485). The control hermit crabs maintained constant feeding rates during their month of captivity in the laboratory. The 17 hermit crabs that were allowed to choose larger shells (shell switchers) increased their shell size by an average6S.E.M.
of 19.360.9% over the original shell size. However, there was again no significant effect of shell treatment on mean feeding rate; mean feeding rate6S.E.M. pre-switch5 16.861.1 mg / h, and post-switch515.161.4 mg / h (Fig. 3B, df516, t51.391, P5 0.1832).
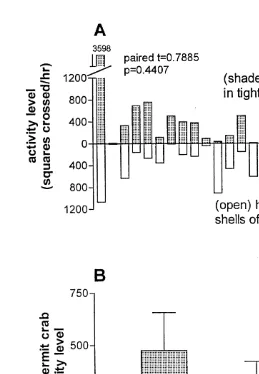
3.4. Activity level
Activity levels for the hermit crabs ranged from 6 to 3598 squares crossed in 1 h. In 10 out of 19 pairs, the hermit crab in the smaller shell was more active; in seven cases, the hermit crab in the shell of preferred fit was more active; in two cases, the hermit crabs were equally active (Fig. 4A). The mean activity level6S.E.M. for hermit crabs in tightly fitting shells was 4746181 squares crossed / h. The mean activity level6S.E.M. for hermit crabs occupying shells of preferred fit was 346675 squares crossed / h. This difference in mean activity level was not significant (Fig. 4B; df518, t50.7885,
P50.4407).
4. Discussion
4.1. Growth rate
Forcing P. longicarpus to occupy shells too small for them significantly decreased their growth rate to near zero. This result is in agreement with laboratory studies of at least four hermit crab species, including P. longicarpus from populations in Texas (Fotheringham, 1976a), Connecticut, and North Carolina (Blackstone, 1985). Zero or negative growth is rare in most crustaceans (Hartnoll, 1982), although females may stop growing during the reproductive season. Females of the Japanese species Diogenes
nitidimanus occupied tightly fitting shells in the field (shell adequacy index less than
1.0) more often than males, and frequently showed zero or negative growth at molt (Asakura, 1992).
4.2. Predation risk
Inadequate shell fit for P. longicarpus from Nahant significantly increased the risk of mortality to a natural predator, C. irroratus, in the laboratory. This result strengthens the results of Vance (1972b), who studied the mortality of a congener, P. granosimanus, on the North American west coast, but may have confounded his analysis by using the same predators in multiple trials. Several shell characteristics have been shown to affect hermit crab mortality in the field and laboratory (presence of sea anemones on shell: Brooks, 1988; size and species of shell, and shell damage: Kuhlmann, 1992; presence of hydroid epibionts: Buckley and Ebersole, 1994). However, none of these studies assessed hermit crab vulnerability as a function of relative shell fit. Vance’s results have not been replicated until now.
Fig. 4. Activity level as function of shell fit for hermit crab P. longicarpus. (A) Each bar above the line shows the activity level for a hermit crab having a shell adequacy index of 0.5; each bar below the line shows the activity level for its partner, a hermit crab having a shell adequacy index of 1.0. n519 pairs. (B) Overall mean activity level6S.E.M. for all hermit crabs having shell adequacy index of 0.5 (tight fit), and for all hermit crabs having shell adequacy index of 1.0 (preferred fit).
with fine chelae, and octopuses. Some other brachyuran crabs known to prey on P.
longicarpus are the blue crab Callinectes sapidus (observed attacking P. longicarpus in Florida by Kuhlmann, 1992) and the green crab Carcinus maenas (gut content analyses by Ropes (1968) and Elner (1981) revealed Pagurus spp.)
hermit crabs occupying small shells may therefore be advantageous, at least in the short term. However, if hermit crabs cannot recoup this lost growth over the long term, then shell fit-related decreases in growth rate could reduce individual fecundity. This is because most of the variation in reproductive output can be attributed to differences in body size (Wilber, 1989).
4.3. Feeding rate
Manipulations of shell fit for P. longicarpus did not significantly affect their feeding rates in the first laboratory experiment. The second feeding rate experiment also showed that allowing the crabs to switch into larger shells did not affect mean feeding rate, yet many of the hermit crabs in this treatment did change their feeding rates substantially after moving into larger shells. Some fed at faster rates, and some at slower rates. This suggests that feeding rate was affected by the change in shell fit, but not in a consistent direction. Feeding rates under the experimental conditions, where hermit crabs were fed ad libitum and were exempted from having to find or compete for food, may not reflect foraging efficiency in the field. However, the decrease in growth rate for hermit crabs in tightly fitting shells (shown here and in several previous studies noted above) was documented under laboratory conditions. Therefore, a mechanism hypothesized to affect growth rate, e.g., feeding rate, should be observed under laboratory conditions if it is truly in effect.
Despite the negative laboratory result, there may still be interesting effects of shell fit on the foraging biology of hermit crabs in the field. Using video surveillance, Ramsay and Hughes (1997) found intense competition among hermit crabs P. bernhardus in the Irish Sea when there was a limited food resource (i.e., small patches of food). The corresponding reduction in feeding success was related to hermit crab size, with the smallest crabs suffering the greatest loss of feeding opportunity. Whether shell fit affects competitive success for limited food resources is not known.
Besides the influence of competitive interactions on foraging success and ultimate net energy intake, there is also the question of foraging effort. Foraging effort may be reduced for hermit crabs occupying suboptimal shells (e.g., shells too small) if it puts them at greater risk to predators. This risk of predation may be immediate, as shown, or it may be a future risk that is magnified by continued growth in the current shell. Feeding less, thus growing at a slowed rate until a larger shell is obtained, may result from reducing foraging effort in the field.
suspended organic particles available in their shelters, and only actively forage for higher-energy food as adults (Wahle, 1992). Similarly, smaller crayfish, to a greater extent than larger crayfish, reduce foraging in the presence of predators, and increase defensive behaviors such as displaying chelae and burrowing in the sediment (Stein and Magnuson, 1976). It is therefore apparent that predation threat will affect how some animals forage; when risks of predation are great, maximizing energy intake may be maladaptive. Further studies are needed to track hermit crab foraging effort in the field as a function of shell fit.
4.4. Activity level
Increased activity level is most likely correlated with increased energy expenditure. Therefore, the growth rate differences observed could be explained if hermit crabs in tightly fitting shells were more active, and expending energy at a faster rate. Assuming increased activity in the laboratory reflected increased activity in the field, high activity in the field would increase the chances of shell encounters, hence improving the likelihood that a hermit crab in a tightly fitting shell would be able to secure a better-fitting shell. However, shell fit was not found to significantly affect activity levels in hermit crabs P. longicarpus matched by size and observed over the same hour in the laboratory. There was a trend for the hermit crabs in tightly fitting shells to be more active, but the difference was not significant. Increasing the sample size, or repeatedly observing the same pairs over multiple hours on different days may aid detection of differences in activity level should they exist.
In fact, there is evidence from other studies that supports the hypothesis that hermit crabs occupying shells too small for them are more active. Elwood et al. (1998) measured the ‘startle response’ of hermit crabs P. bernhardus: the time they spent withdrawn in their shells after being startled with a standardized stimulus. In each trial of their experiment, two hermit crabs were startled as they fought for possession of a shell. Those hermit crabs that stood to gain a lot from the fight (because they currently occupied shells of tight fit) startled for significantly less time than crabs in shells of preferred fit. This indicates an increased motivation of hermit crabs experiencing tight shell fit to acquire new shells.
In another experiment, the persistence of investigation of a new shell (unobtainable because the shell was sealed with dental cement) was significantly higher for hermit crabs currently occupying shells of tight fit than for crabs in shells of preferred fit (Elwood, 1995). Both experiments show that, for P. bernhardus, motivation to obtain a new shell is affected by current shell fit. Energy expenditure was not measured directly, but increased motivational effort expended in pursuing a shell may indeed require more energy.
fit. If hermit crabs occupying shells too small for them are more agitated in the presence of new shells, they might also be more active in their search for new shells to explore, or other hermit crabs to fight for possession of a shell. Increased locomotion might aid these hermit crabs in their search for new shells, and also affect growth rates in the field by raising energy consumption.
4.5. Alternative hypotheses
While neither feeding rate nor activity level in the laboratory were significantly affected by shell fit for hermit crab P. longicarpus, growth rate was significantly reduced for captive hermit crabs occupying shells that were too small. Increased basal respiration rates or decreased assimilation efficiencies are two alternate mechanisms that might be influencing growth rate. Respiration rates have been measured in terrestrial hermit crabs put on treadmills (Herreid and Full, 1986), but not for aquatic hermit crabs such as P.
longicarpus. Assimilation rates have not been measured in hermit crabs, but probably
could be, given that hermit crab feces are robust in seawater, and could be collected and analyzed. Exploring these alternative hypotheses may shed more light on the explanation for the growth rate decline observed.
5. Conclusion
Growth rate decreased for hermit crabs P. longicarpus confined to tightly fitting shells. This result replicated the previous results of others for P. longicarpus and other hermit crab species studied at multiple locations. There was a negative effect of tight shell fit on hermit crab survivorship under threat of predation by natural predator, C. irroratus, suggesting a possible advantage to slowing growth rate when the shell occupied is too small. No significant differences were found in feeding rate or activity level for hermit crabs confined to tightly fitting shells compared with hermit crabs occupying shells of preferred size. Therefore, neither of the two proximate mechanisms tested was found to account for the negative effect of tight shell fit on hermit crab growth rate.
Acknowledgements
The author acknowledges encouragement and advice received from her M.S. thesis advisor at Tufts University, J.A. Pechenik. N. Milburn, C.M. Orians, S.M. Lewis, and M. Gaudette of Tufts University and two anonymous reviewers made helpful criticisms of the manuscript. D. Smith of Northeastern University kindly donated sea table space at the Marine Science Center (Nahant, Massachusetts) for the predation risk experiment. [AU]
References
Asakura, A., 1992. Population ecology of the sand-dwelling hermit crab Diogenes nitidimanus terao. 5. Ecological implications in the pattern of molting. J. Crustacean Biol. 12 (4), 537–545.
Begon, M., Harper, J.L., Townsend, C.R., 1990. In: Ecology: Individuals, Populations, and Communities, Blackwell Scientific, Oxford.
Blackstone, N.W., 1985. The effects of shell size and shape on growth and form in the hermit crab Pagurus longicarpus. Biol. Bull. 168, 75–90.
Brooks, W.R., 1988. The influence of the location and abundance of the sea anemone Calliactis tricolor (Le Sueur) in protecting hermit crabs from octopus predators. J. Exp. Mar. Biol. Ecol. 116, 15–21. Buckley, W.J., Ebersole, J.P., 1994. Symbiotic organisms increase the vulnerability of a hermit crab to
predation. J. Exp. Mar. Biol. Ecol. 182, 49–64.
Childress, J.R., 1972. Behavioral ecology and fitness theory in a tropical hermit crab. Ecology 53 (5), 960–964.
Drummond-Davis, N.C., Mann, K.H., Pottle, R.A., 1982. Some estimates of population density and feeding habits of the rock crab, Cancer irroratus, in a kelp bed in Nova Scotia. Can. J. Fish. Aquat. Sci. 39, 636–639.
Elner, R.W., 1981. Diet of green crab Carcinus maenas (L.) from Port Hebert, Southwestern Nova Scotia. J. Shellfish Res. 1 (1), 89–94.
Elwood, R.W., 1995. Motivational changes during resource assessment by hermit crabs. J. Exp. Mar. Biol. Ecol. 193, 41–55.
Elwood, R.W., Neil, S.J., 1992. In: Assessments and Decisions: A Study of Information Gathering by Hermit Crabs, Chapman and Hall, London.
Elwood, R.W., Wood, K.E., Gallagher, M.B., Dick, J.T.A., 1998. Probing motivational state during agonistic encounters in animals. Nature 393, 66–68.
Fotheringham, N., 1976a. Effects of shell stress on the growth of hermit crabs. J. Exp. Mar. Biol. Ecol. 23, 299–305.
Fotheringham, N., 1976b. Population consequences of shell utilization by hermit crabs. Ecology 57, 570–578. Ghent, A.W., 1996. In: Introduction to Quantitative Biology, University of Illinois, Champaign-Urbana, IL,
Unpublished lecture notes.
Hartnoll, R.G., 1982. Growth. In: Abele, L.G. (Ed.), The Biology of Crustacea, Vol. vol. 2, Academic Press, New York, pp. 111–196.
Hazlett, B.A., 1981. The behavioral ecology of hermit crabs. Annu. Rev. Ecol. Syst. 12, 1–22.
Hazlett, B.A., 1996. Assessments during shell exchanges by the hermit crab Clibanarius vittatus: the complete negotiator. Anim. Behav. 51, 567–573.
Herreid, C.F., Full, R.J., 1986. Energetics of hermit crabs during locomotion: the cost of carrying a shell. J. Exp. Biol. 120, 297–308.
Kuhlmann, M.L., 1992. Behavioral avoidance of predation in an intertidal hermit crab. J. Exp. Mar. Biol. Ecol. 157, 143–158.
Markham, J.C., 1968. Notes on growth patterns and shell utilization in Pagurus bernhardus. Ophelia 5, 189–205.
McClintock, T.S., 1985. Effects of shell condition and size upon the shell choice behavior of a hermit crab. J. Exp. Mar. Biol. Ecol. 88, 271–285.
Ramsay, K., Hughes, R.N., 1997. A field study of intraspecific competition for food in hermit crabs (Pagurus bernhardus). Estuar. Coastal Shelf Sci. 44, 213–220.
Rittschof, D., Sarrica, J., Rubenstein, D., 1995. Shell dynamics and microhabitat selection by striped legged hermit crabs, Clibanarius vittatus (Bosc). J. Exp. Mar. Biol. Ecol. 192, 157–172.
Rochette, R., Himmelman, J.H., 1996. Does vulnerability influence trade-offs made by whelks between predation risk and feeding opportunities? Anim. Behav. 52, 783–794.
Ropes, J.W., 1968. The feeding habits of the green crab, Carcinus maenas (L.). Fish. Bull. 67 (2), 183–203. Scarratt, A.M., Godin, J.-G.J., 1992. Foraging and antipredator decisions in the hermit crab Pagurus acadianus
(Benedict). J. Exp. Mar. Biol. Ecol. 156, 225–238.
Scully, E.P., 1979. The effects of gastropod shell availability and habitat characteristics on shell utilization by the intertidal hermit crab Pagurus longicarpus Say. J. Exp. Mar. Biol. Ecol. 37, 139–152.
Stehlik, L.L., 1993. Diets of the brachyuran crabs Cancer irroratus, C. borealis, and Ovalipes ocellatus in the New York Bight. J. Crustacean Biol. 13 (4), 723–735.
Stein, R.A., Magnuson, J.J., 1976. Behavioral response of a crayfish to a fish predator. Ecology 57, 751–761. Taylor, P.R., 1981. Hermit crab fitness: the effect of shell condition and behavioral adaptations on
environmental resistance. J. Exp. Mar. Biol. Ecol. 52, 205–218.
Vance, R.R., 1972a. Competition and mechanism of coexistence in three sympatric species of intertidal hermit crabs. Ecology 53 (6), 1062–1074.
Vance, R.R., 1972b. The role of shell adequacy in behavioral interactions involving hermit crabs. Ecology 53 (6), 1075–1083.
Wada, S., Ohmori, H., Goshima, S., Nakao, S., 1997. Shell size preference of hermit crabs depends on their growth rate. Anim. Behav. 54, 1–8.
Wahle, R.A., 1992. Body-size dependent anti-predator mechanisms of the American lobster. Oikos 65, 52–60. Wilber, Jr. T.P., 1989. Associations between gastropod characteristics and egg production in the hermit crab
Pagurus longicarpus. Oecologia 81, 6–15.