and with Sleep Deprivation in Premenstrual Dysphoric
Disorder and Normal Control Subjects
Barbara L. Parry, Suryabanu Javeed, Gail A. Laughlin,
Richard Hauger, and Paul Clopton
Background:In this study we extended previous work by examining whether disturbances in the circadian rhythms of cortisol during the menstrual cycle distinguish patients with premenstrual dysphoric disorder (PMDD) from nor-mal control (NC) subjects. In addition, we tested the differential response to the effects of early and late partial sleep deprivation on cortisol rhythms.
Methods:In 15 PMDD and 15 NC subjects we measured cortisol levels every 30 min from 6:00 PM to 9:00 AM during midfollicular (MF) and late luteal (LL) menstrual cycle phases and also during a randomized crossover trial of early (sleep 3:00 AM–7:00 AM) versus late (sleep 9:00 PM–1:00AM) partial sleep deprivation administered in two subsequent and separate luteal phases.
Results:In follicular versus luteal menstrual cycle phases we observed altered timing but not quantitative measures of cortisol secretion in PMDD subjects, compared with NC subjects: in the LL versus MF phase the cortisol acrophase was a mean of 1 hour earlier in NC subjects, but not in PMDD subjects. The effect of sleep deprivation on cortisol timing measures also differed for PMDD versus NC subjects: during late partial sleep deprivation (when subjects’ sleep was earlier), the cortisol acrophase was almost 2 hours earlier in PMDD subjects.
Conclusions:Timing rather than quantitative measures of cortisol secretion differentiated PMDD subjects from NC subjects both during the menstrual cycle and in response to early versus late sleep deprivation interventions. Biol Psychiatry 2000;48:920 –931 © 2000 Society of Biologi-cal Psychiatry
Key Words: Premenstrual dysphoric disorder, cortisol, circadian rhythms, women, sleep deprivation, menstrual cycle
Introduction
B
ecause of a growing body of evidence linking the clinical phenomenology and treatment response of premenstrual dysphoric disorder (PMDD) to major depres-sive disorder (MDD; American Psychiatric Association 1996, 317–394), PMDD was classified as a MDD, not otherwise specified, in DSM-IV (American Psychiatric Association 1994). Disturbances of hypothalamic–pitu-itary–adrenal (HPA) function characterize patients with MDD (Carroll et al 1976; Halbreich et al 1985; Krishnan et al 1990; Linkowski et al 1985a, 1985b; Pfohl et al 1985; Rubin et al 1987; Sachar et al 1973) and have been described in PMDD as well. Although most studies find that basal levels of cortisol and adrenocorticotropin hor-mone (ACTH) are normal in women with PMDD (Bloch et al 1998; Mortola et al 1989; Parry et al 1994; Rosenstein et al 1996; Rubinow et al 1988; Steiner et al 1984; Su et al 1997), we observed differences in timing measures of cortisol secretion (Parry et al 1994) in normal control (NC) subjects, but not PMDD subjects: a delay in the peak time of the cortisol rhythm occurred in the luteal menstrual cycle phase, as compared with the follicular phase. Dif-ferences in cortisol and ACTH responses to a variety of stimuli also have been reported in PMDD subjects, as compared with NC subjects: cortisol responses to cortico-tropin-releasing hormone are increased in women with PMDD (Facchinetti et al 1994; Rabin et al 1990). Abnor-malities, however, frequently are not confined to the symptomatic luteal phase. For example, a blunting of the cortisol and ACTH responses toL-tryptophan (Bancroft et al 1991) and to a serotonin agonist (Su et al 1997) occurs in both phases of the menstrual cycle. Moreover, the increase in cortisol induced by opioid blockade in NC women is almost absent in PMDD patients (Facchinetti et al 1994). Thus, women with PMDD display alterations in the timing of cortisol circadian rhythms and both hypo-and hyper-responsiveness in challenge studies of the HPA axis.Sleep deprivation improves mood in a majority of patients From the Department of Psychiatry, University of California, San Diego.
Address reprint requests to Barbara L. Parry, M.D., Department of Psychiatry MC 0804, 9500 Gilman Drive, La Jolla CA 92093-0804.
Received September 15, 1999; revised March 3, 2000; accepted March 3, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
with MDD (Gillin 1983; Kuhs and To¨lle 1991; Leibenluft and Wehr 1992; Van den Hoofdakker 1994; Wu and Bunney 1990). Critically timed sleep deprivation interventions also may have promise in providing alternative or adjunctive treatments for the management of PMDD: we previously reported the therapeutic effect of both total (Parry and Wehr 1987) and early (ESD) and late (LSD) partial sleep depriva-tion (Parry et al 1995) in PMDD. The therapeutic effect of sleep deprivation in PMDD, similar to its effect in MDD and in contrast to its effect in anxiety disorders (Roy-Byrne et al 1986b), further supports the link between PMDD and MDD. It was on the basis of this similarity in clinical phenomenol-ogy and treatment response that PMDD was classified as a depressive disorder in DSM-IV (American Psychiatric Asso-ciation 1994; see sourcebook [American Psychiatric Associ-ation 1996, 317–394] for review of database).
The mechanism for the therapeutic effect of sleep deprivation in depressive disorders is unknown. Yamagu-chi et al (1978) found that abnormal cortisol circadian rhythms were corrected in MDD subjects who responded to sleep deprivation, and Gerner et al (1979) and Baum-gartner et al (1990a, 1990b) reported a greater increase in cortisol levels in responders. According to the internal coincidence model for sleep deprivation and depression (Wehr and Wirz-Justice 1981), the alteration of the timing (phase) relationships between sleep and underlying corti-sol rhythms may be one mechanism by which sleep deprivation improves mood in PMDD patients.
Some reports have indicated that sleep deprivation late in the night is more effective than sleep deprivation early in the night (Gillin 1983; Kuhs and To¨lle 1991; Leibenluft et al 1993; Parry and Wehr 1987). Our design incorporated both ESD and LSD, allowing us to compare therapeutic efficacy to changes in both quantitative and circadian timing measures of cortisol secretion patterns. A description of the effects of sleep deprivation on mood ratings in PMDD is published in Parry et al (1995). In summary, patient mood ratings showed a significant reduction of depressive symptoms from baseline (without induction of mania) after recovery nights of sleep from both ESD and LSD, but not the day after ESD or LSD (Parry et al 1995). In this study we examine the effects of sleep deprivation on cortisol rhythms. Basing our hypothesis on our previous work, we hypothesize that 1) timing distur-bances in cortisol secretion would differentiate PMDD and NC subjects during the menstrual cycle and 2) patient and control groups would have different responses to sleep deprivation interventions as measured by cortisol parameters.
Methods and Materials
Subjects
Potential PMDD and NC subjects were referred by local profes-sionals or were recruited by advertisement for participation in
mood and sleep studies during the menstrual cycle. Screening procedures consisted of a structured menstrual assessment ques-tionnaire (adapted by Parry and Mostifi from the Menstrual Assessment Form described in Roy-Byrne et al 1986a), the Structured Clinical Interview for DSM-III-R (Spitzer et al 1990), a psychiatric interview, a physical examination, and laboratory tests, including chemistry panel, complete blood count, urinaly-sis, and measurements of thyroid indices. If the subject did not have other major medical, gynecologic, or psychiatric illness; had regular (26- to 32-day) menstrual cycles; reported recurrent premenstrual affective symptoms severe enough to disrupt social or occupational functioning; and was willing to endure the rigors of a long-term research study, she was admitted for a 2- to 3-month prospective evaluation for diagnostic assessment. A past but not recent (within the last year) history of affective illness was permitted for PMDD subjects but not for NC subjects. Normal control subjects had to be without a lifetime history of psychiatric illness (including alcohol abuse) and to have no active medical illnesses. Their first-degree relatives also had to be free of a lifetime history of psychiatric illness with the exception of alcohol abuse (we found that inclusion of the last criterion severely restricted recruitment).
During the 2-month evaluation, both potential PMDD and NC subjects completed twice-daily (morning and evening) mood ratings (100-mm visual analogue scales of depression, anxiety, irritability, fatigue, withdrawal, physical symptoms, and appe-tite) and visited the clinic weekly for interview-based (21-item Hamilton Rating Scale for Depression [HRSD; Hamilton 1967]) and self-report (Beck Depression Inventory [BDI; Beck et al 1961]) depression ratings. In addition, an addendum to the HRSD to assess atypical items of depression and a hypomania rating scale to determine if sleep deprivation induced manic or hypomanic symptoms were used in evaluation (Rosenthal and Heffernan 1986). On the basis of this examination, to be selected for the study PMDD subjects had to meet DSM-III-R (American Psychiatric Association 1987) criteria for late luteal phase dysphoric disorder and, retrospectively, DSM-IV (American Psychiatric Association 1994) criteria for PMDD. To meet impairment criteria, PMDD subjects had to have a mean score of 14 or more on the HRSD and 10 or more on the BDI; have a 30% increase in daily ratings in the late luteal phase (1 week before the onset of menses); and demonstrate a reduction in mean scores to 7 or less on the HRSD and 5 or less on the BDI, and less than 50 mm on daily ratings by the week after the cessation of menses. All of the PMDD subjects had debilitating affective symptoms that occurred during the late luteal phase of each menstrual cycle throughout the year (i.e., they did not have seasonal premenstrual symptoms). To be selected for the study, NC subjects had to have mean HRSD scores less than 7 and BDI ratings less than 5 at all menstrual cycle phases, and their daily ratings needed to show
,30% clinical variation in association with the menstrual cycle. Premenstrual dysphoric disorder and NC diagnosis were not determined until the end of the 2-month evaluation.
were ruled out by our obtaining urine toxicology screens before admissions. Subjects needed to be off oral contraceptives and to have not been smoking 3 months before entering the evaluation phase of the study, but their use before this time was not exclusionary.
The protocol was approved by the Human Subjects Committee of the University of California, San Diego (UCSD), and all subjects gave written informed consent after the procedures had been explained fully.
Procedures
Subjects underwent one night of baseline study during two menstrual cycle phases: 1) the midfollicular (MF; days 6 –10 after menses) and 2) late luteal (LL; 2– 4 days before the onset of menses based on the midcycle luteinizing hormone (LH) surge, assuming a 14-day luteal phase). The timing of the midcycle LH surge was determined by a colorimetric urinary immunoassay (Ovustick, Irvine, CA). Subjects also were studied during a randomized crossover trial of ESD (sleep 3:00 AM–7:00 AM) versus LSD (sleep 9:00 PM–1:00 AM). Each night of sleep deprivation was followed by a night of recovery sleep (ESD-R, LSD-R; sleep 10:30 PM– 6:30 AM) in which we primarily ob-tained sleep electroencephalograms (EEGs) but not hormonal measures (Parry et al 1999). Early and late sleep interventions and their respective nights of recovery sleep were administered in the hospital during the premenstrual (late luteal) phase of separate menstrual cycles.
During each night of study, subjects were admitted to the General Clinical Research Center of the UCSD Medical Center from 4:30 PM to 9:00 AM. A physical examination, screening laboratory tests (chemistry panel, thyroid indices, complete blood count, and urinalysis) were obtained on each admission to rule out the development of other medical conditions that might affect results. Baseline levels of reproductive hormones (estra-diol, progesterone, follicle-stimulating hormone, and LH) were measured at 6:00AMand 6:00PMon each admission to document menstrual cycle phase and the effect of these hormones on outcome measures. Trained nurses inserted an intravenous cath-eter at 5:00PMand drew samples for the cortisol hormone assay every 30 min from 6:00PMto 9:00AMin dim light (,100 lux). So as not to disturb sleep, they obtained samples between 10:30 PM and 6:30 AM by a catheter that was threaded through a porthole to an adjoining room. Subjects adhered to their habitual bedtimes and awakening times (except when determined by the sleep deprivation schedules) and slept in rooms by themselves. Blood was drawn for the baseline studies in the MF and LL phases of the menstrual cycle and during ESD and LSD nights in the luteal phase of subsequent menstrual cycles.
Cortisol Assay
Plasma cortisol concentrations were measured by radioimmuno-assay kits obtained from the Diagnostic Products Corporation (Los Angeles). The intra- and interassay coefficients of variation were 4% and 6%, respectively. Assay sensitivity was 0.3mg/dL.
Statistical Analyses
Cosine analyses of the concentration series of cortisol were performed on each of the 30-min interval data sets for each individual by fitting the general cosine function, CS(t)5M1A cos (vt1 f), where CS(t) is the hormone concentration at time t, M is the mesor (midline value), A is the absolute amplitude, andvand fare the angular frequency and acrophase, respec-tively (Nelson et al 1979). As described by Klemfuss and Clopton (1993), this method is one of the most reliable for biological rhythm analyses. The three parameters defining the cosine rhythm are 1) acrophase, the time at which the maxima of the cosine curve occurs; 2) amplitude, half the difference between the highest and lowest value; and 3) mesor, the concentration about which the oscillation occurs. The peak concentration, peak time, nadir concentration, and nadir time (noncosinor functions) also were derived to account for less than 24 hour data. Timing measures (acrophase, peak time, and nadir time) were fixed to 24-hour clock time. To confirm the validity of using cosine rhythmometry analysis for hormone profiles of less than 24 hour duration, 24 hour (obtained at 30-min intervals) cortisol data sets from a previous study (Laughlin and Yen 1996) were analyzed twice— once over the entire 24 hours and once for cortisol values from 6:00PM to 9:00AM, corresponding to the sampling interval for this study. The correlation coefficients for the 24-hour versus the 16-hour analyses were .91 for mesor, .91 for amplitude, and .93 for acrophase (all p, .0001).
Each plasma cortisol profile was plotted and examined visu-ally by an investigator with extensive experience in the analyses of cortisol rhythms (GAL). For three of the 60 profiles (one PMDD and one NC subject during the luteal phase and one NC during the follicular phase), abrupt and exaggerated increases in cortisol occurred for the duration of three samples. Nurses’ notes indicated that these excursions were coincident with reinsertion of the intravenous catheter. These data were smoothed by interpolation before further analyses. Overall repeated-measures analyses of variance (ANOVAs) were used to test main effects of group (PMDD vs. NC), condition (MF, LL, ESD, and LSD), and their interaction for each of the cortisol measures. When signif-icant main effects or interactions were found, post hoc repeated-measures ANOVAs testing the effect of menstrual cycle phase (MF vs. LL) and the effects of sleep deprivation compared with the baseline LL phase (LL vs. ESD vs. LSD) were performed. Significant interactions for sleep deprivation were delineated further by comparing the delta from the LL baseline to ESD to that from the LL baseline to LSD by repeated-measures ANOVA. Delta acrophase was derived by subtracting ESD or LSD values from baseline values so that, according to conven-tion, phase advances of the cortisol rhythm were calculated as positive values and phase delays as negative values. The signif-icance of deltas for each group was tested by single sample t
tests.
Pearson correlation coefficient. As previous studies indicated effects of, particularly, sleep onset time and sleep latency with cortisol parameters, these sleep variables were correlated with cortisol parameters in NC and PMDD subjects during baseline luteal and ESD and LSD nights. To ascertain effects of repro-ductive hormonal change on cortisol outcome measures, we examined correlations between the mean of 6:00PMand 6:00AM estradiol and progesterone levels and cortisol measures during baseline follicular and luteal menstrual cycle phases. To account for multiple comparisons in these correlations, only results in whichp#.01 were considered statistically significant. To test whether order of treatment (ESD or LSD first) affected cortisol outcome measures, we compared order of treatment (between-group factor) in a repeated-measures ANOVA for ESD and LSD. Finally, we performed a between-group ANOVA to test whether a personal or family history of depression affected cortisol rhythms in PMDD subjects.
For other than the correlational analyses listed above, statisti-cal significance was set at a probability level ofp,.05. Data are presented as means6SDs.
Results
Subjects were recruited from the community by question-naire: Of 2002 questionnaires sent to interested applicants, 779 were returned; from questionnaires received, 216 women were deemed appropriate for further screening; 103 subjects were evaluated; 41 subjects entered the study—23 subjects with PMDD and 18 NC subjects. Reasons for exclusion were use of medications (including birth control pills), not having regular menstrual cycles, not meeting diagnostic criteria, and not being able to meet the demands of a long-term research study. Three NC subjects and eight PMDD subjects dropped from the study, once entered, because of other time commitments (work or family), anemia, pregnancy, failure to ovulate, or because we were not able to obtain complete or reliable data on them (for further description, see Parry et al 1995). The data from 15 NC and 15 PMDD subjects from whom we were able to obtain complete cortisol profiles are pre-sented in the following analyses.
Subject Demographics
The mean age for PMDD subjects (n 5 15) was 36.06 4.1 years (range 29 – 43); for NC subjects (n5 15) it was 37.265.8 years (range 24 – 45). Premenstrual dysphoric disorder and NC subjects did not differ significantly by age, education, marital status, or parity.
Mood Rating Scales
The description of mood rating scales, the nature and severity of depressive symptoms, and treatment response are published in Parry et al (1995). In summary, patient
mood ratings showed a significant reduction of depressive symptoms from baseline (without induction of mania) after recovery nights of sleep from both ESD and LSD, but not the day after ESD or LSD (Parry et al 1995).
Cortisol Rhythm Analyses
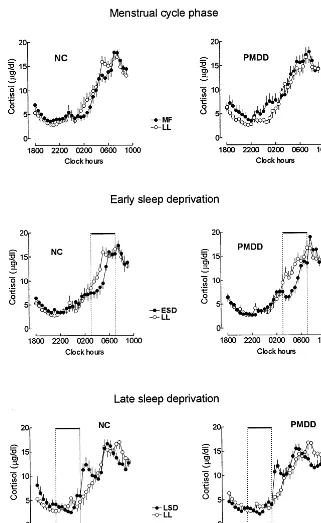
Mean cortisol profiles for PMDD and NC subjects during baseline MF and LL menstrual cycle phases and during nights of ESD and LSD are shown in Figure 1. Results of cortisol rhythm analyses are presented in Table 1.
OVERALL ANOVA. In an overall ANOVA to test for
main effects of group (PMDD and NC), condition (MF, LL, ESD, and LSD) and their interaction on cortisol rhythm parameters, we found significant main effects of condition for acrophase [F(3,84) 5 14.87, p, .001], peak time [F(3,84) 5 9.42, p , .01], mesor [F(3,84) 5 6.25, p , .001], amplitude [F(3,84) 5 7.51, p , .0001], peak concentration [F(3,84) 5 3.28, p , .03], and nadir concentration [F(3,84) 5 3.70,p,.02] and a significant interaction of group and condition for acrophase [F(3,84) 5 2.89, p , .04]. There were no statistically significant effects of condition for nadir time and no statistically significant main effects of group for any parameter.
FOLLICULAR VERSUS LUTEAL MENSTRUAL CYCLE PHASE. Mesor, amplitude, and peak concentration
(quantitative measures) were not significantly affected by menstrual cycle phase. A statistically significant effect of menstrual cycle phase was seen for acrophase [ANOVA, F(1,28)5 4.48,p, .04], with a significant group by phase interaction [F(1,28) 5 4.97, p , .04]. The cortisol acrophase occurred a mean of 1.0061.22 hours earlier in the LL menstrual cycle phase as compared with the MF phase for NC subjects, but not for PMDD subjects. The nadir value was higher [F(1,28) 5 5.405, p , .028] during the follicular menstrual cycle phase for both NC and PMDD groups.
LATE LUTEAL PHASE BASELINE VERSUS EARLY AND LATE SLEEP DEPRIVATION. In a repeated-measures
baseline LL phase and ESD, the mesor and amplitude of the cortisol rhythm were lower and the acrophase and peak time were earlier. The nadir concentration was higher during ESD than during the LL baseline and LSD.
POST HOC ANALYSES. For the comparison of LL and
ESD there were significant effects of condition for
ac-rophase [F(1,28) 5 4.13, p , .05] and peak time [F(1,28)58.92,p, .006]. For the comparison of LL and LSD there were significant effects of condition for amplitude [F(1,28) 5 18.81, p , .001], acrophase [F(1,28)5 18.40, p, .001], and mesor [F(1,28)5 0.035, p , .04] and significant interaction effects for acrophase [F(1,28) 5 5.40, p , .03]. For the
comparison of ESD and LSD there were significant main effects of condition for amplitude [F(1,28) 5 14.90, p, .001], acrophase [F(1,28) 5 29.54, p , .001], mesor [F(1,28) 5 5.08, p , .03], peak time [F(1,28) 5 28.00, p , .001], and nadir [F(1,28) 5 7.35,p,.01]; significant effects of group for peak time [F(1,28) 5 4.72,p, .04]; and significant interaction effects for acrophase [F(1,28) 5 4.60, p , .04].
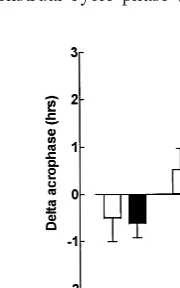
DELTA CALCULATIONS. We calculated change
scores for cortisol parameters between the LL baseline and ESD (LL-ESD) and between the LL baseline and LSD (LL-LSD). In a comparison of these two conditions (LL-ESD vs. LL-LSD) we found for cortisol acrophase a statistically significant main effect of condition [F(1,28) 5 29.5, p , .0001] and a significant group by condition interaction [F(1,28) 5 4.599, p 5 .04]: the cortisol acrophase was delayed for PMDD (delta: 20.63 6 1.18 hours, p 5 .06) and NC subjects (delta: 20.51 6 1.82 hours, ns) during ESD (mean delta: 20.5761.51 hours,p5 .05) as compared with the LL baseline. In contrast, during LSD the cortisol acrophase was advanced an average of 1.75 6 1.09 hours (p , .001) for PMDD subjects, but was not significantly shifted for NC subjects (0.5261.74 hours, ns; Figure 2).
ANALYSES FOR MEAN CORTISOL VALUES FROM 11:00PMTO 7:00AM. In an overall ANOVA, mean levels
of cortisol from 11:00 PM to 7:00 AM were compared
between groups (PMDD vs. NC subjects) and for the four conditions (MF, LL, ESD, and LSD). There were statisti-cally significant main effects of condition [F(3,26) 5 7.93,p, .001] but no significant main effects of group or group by condition interaction. Post hoc ANOVAs comparing menstrual cycle phase (MF vs. LL) showed a
Figure 2. Mean6SD values for delta acrophase comparing the late luteal baseline for each treatment condition (early sleep deprivation [ESD] and late sleep deprivation [LSD]) derived from a fitted curve of the cortisol circadian rhythm, obtained every 30 min from 6:00 AM to 9:00 AM in 15 premenstrual dysphoric disorder (PMDD) subjects and 15 normal control (NC) subjects during the midfollicular and late luteal phases of the menstrual cycle and also during ESD and LSD (administered in separate late luteal menstrual cycle phases). *Statistically signif-icant (p,.001) difference between PMDD and NC subjects for delta acrophase during LSD.
Table 1. Mean6SD Values for Cortisol Rhythm Characteristics for 15 Premenstrual Dysphoric Disorder (PMDD) and 15 Normal Control (NC) Subjects during Midfollicular (MF) and Late Luteal (LL) Menstrual Cycle Phases and during Early and Late Partial Sleep Deprivations (ESD and LSD; Administered during Separate LL Menstrual Cycle Phases)
MF LL ESD LSD
Mesor (mg/dL)
PMDD 10.6862.81 9.7061.88 9.4461.63 8.2061.90
NC 10.2062.25 9.4862.85 9.6862.46 9.2163.06
Amplitude (mg/dL)
PMDD 6.5861.77 7.1161.64 7.2361.42 6.1061.25
NC 7.3061.51 7.0861.39 6.9162.28 5.3861.39
Acrophasea
PMDD 9:23AM61 hour 35 min 9:25AM61 hour 9 min 10:04AM61 hour 30 min 7:41AM61 hour 26 min NC 9:58AM61 hour 17 min 8:58AM61 hour 25 min 9:29AM61 hour 50 min 8:28AM656 min Peak (mg/dL)
PMDD 22.5063.66 20.7263.37 21.9064.40 18.9862.74
NC 22.2664.45 21.3564.93 20.3765.02 20.5766.00
Peak timea
PMDD 6:30AM61 hour 39 min 6:14AM61 hour 53 min 7:34AM639 min 5:19AM61 hour 32 min NC 6:24AM61 hour 19 min 5:54AM61 hour 30 min 6:25AM61 hour 7 min 4:58AM61 hour 48 min Nadir (mg/dL)
PMDD 2.5561.78 1.6260.69 1.8960.63 1.6760.44
NC 2.0861.02 1.9560.94 2.2660.85 1.9261.00
Nadir timea
PMDD 10:56PM62 hours 43 min 10:50PM62 hours 8 min 10:34PM62 hours 1 min 10:34PM61 hour 46 min NC 11:48PM62 hours 14 min 10:56PM61 hour 53 min 11:38PM61 hour 53 min 11:28PM61 hour 2 min
significant effect of group by phase interaction [F(1,28) 5 5.06, p , .04] but no significant main effects of group or phase: the mean value of cortisol for NC subjects increased in the LL phase (9.764.0mg/dL), as compared with the EF phase (8.56 6 1.9 mg/dL), whereas in PMDD subjects the mean value decreased in the LL phase (8.962.18mg/dL), as compared with the EF phase (10.276 3.01mg/dL).
In an ANOVA comparing LL, ESD, and LSD, there were significant main effects of condition [F(2,27) 5 13.57, p , .001] but no significant main effects of group or group by condition interactions. In post hoc comparisons of LL and ESD, and ESD and LSD, there were main effects of condition [F(1,28) 5 11.02, p , .003 and F(1,28) 5 24.52, p , .001, respectively]. The mean cortisol levels from 11:00PMto 7:00AMwere highest during LSD (10.126 2.05 mg/dL), as compared with ESD (7.8262.76mg/dL) or the LL baseline (9.326 3.18mg/dL).
Correlation of Mood Ratings and Cortisol Parameters
As noted in Methods and Materials, to account for multiple comparisons in these correlational analyses we only considered ps # .01 to be statistically significant. With these criteria, there were no statistically significant correlations between baseline cortisol parameters and HRSD or BDI scores in the luteal phase in PMDD subjects and no statistically significant correlations between the change in HDRS or BDI scores and the change in cortisol measures between the LL baseline and the night of ESD or LSD. There was a trend for a negative correlation between the change in BDI ratings and the change in cortisol amplitude between the LL baseline and ESD (r5 2.52, n 5 15, p # .05). Normal control subjects were not included in these analyses, as they did not have elevated or significant changes in mood ratings.
Reproductive hormones
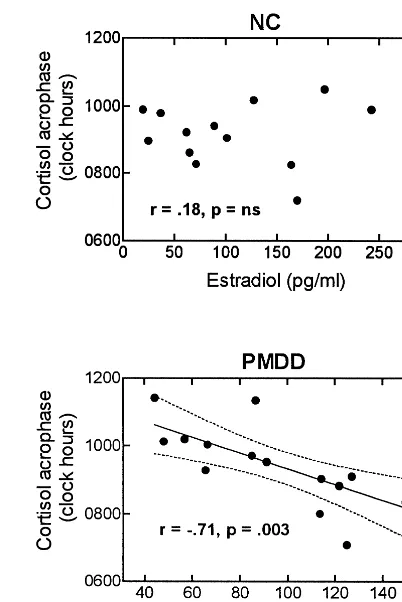
Baseline levels of reproductive hormones did not differ between groups, as reported by Parry et al (1996). Corre-lations were made between the mean levels of estradiol and progesterone at 6:00 AM and 6:00 PM and cortisol parameters during the baseline follicular and luteal phases. There was a statistically significant inverse correlation between estradiol levels and cortisol acrophase in the luteal phase, but not the follicular, in PMDD subjects, but not NC subjects (r 5 2.71, n5 15, p5 .003; Figure 3). Levels of estradiol and progesterone were not signifi-cantly correlated with other cortisol parameters.
Correlation between Cortisol and Sleep Parameters
Correlations were made between sleep latency and sleep onset time with cortisol parameters during the baseline luteal phase and during ESD and LSD in NC and PMDD subjects. Statistically significant (p # .01) correlations were found during ESD between sleep latency and the cortisol nadir concentration (r 5 .51, n 5 15, p 5 .004) and between sleep onset time and the cortisol peak time (r 5 2.45, n 5 15, p 5 .01).
Other Analyses
There were no statistically significant effects of order of treatment on cortisol parameters, and there were no differences in PMDD subjects between groups who had a personal (three of 15) or family (six of 15) history of depression on baseline cortisol parameters. In PMDD patients with a personal history of depression, the cortisol
amplitude was significantly higher during LSD [F(1,13) 5 9.04,p , .01]. In PMDD patients with a positive family history for depression the cortisol peak time was later during LSD [F(1,13) 5 5.82,p, .03].
Discussion
In this study of 15 PMDD and 15 NC subjects during the MF and LL phases of the menstrual cycle and during ESD and LSD in subsequent luteal phases, we found that menstrual cycle phase differentially affected primarily the timing of cortisol secretion, but not the quantitative measures of it, in PMDD and NC subjects: the cortisol acrophase was phase advanced a mean of 1 hour in NC subjects in the LL phase of the menstrual cycle, as compared with the MF phase. This menstrual cycle phase-dependent alteration was absent in PMDD subjects. The effect of sleep deprivation also differed for PMDD and NC subjects primarily in timing rather than in quantitative measures of cortisol secretion: the cortisol acrophase was phase advanced an average of almost 2 hours during LSD (when subjects slept earlier) in PMDD subjects, but not in NC subjects. This finding is in contrast to similar phase delays of about 30 min observed for both PMDD and NC subjects during ESD (when subjects slept later). Thus, both our first hypothesis, that timing disturbances in cortisol secretion would differentiate PMDD and NC subjects during the menstrual cycle, and our second hypothesis, that patient and control groups would have different responses to sleep deprivation interventions as measured by cortisol parameters, were supported.
The normal baseline cortisol levels in PMDD patients seen in this study during both menstrual cycle phases are consistent with our previous report (Parry et al 1994) and most, but not all, other studies: Rabin et al (1990) found low evening cortisol levels in patients with premenstrual syndrome, as compared with NC subjects, as did we when we examined cortisol levels during the nocturnal quiescent hours (11:00 PM–7:00 AM). In a study by Bloch et al (1998), however, examination of morning cortisol levels every 2–3 days failed to identify differences in cortisol at any part of the menstrual cycle. In addition, in frequent (every 20 –30 min) sampling studies conducted over ex-tended (18- to 24-hour) time periods, cortisol levels and the circadian amplitude of cortisol (Mortola et al 1989; Parry et al 1994; Steiner et al 1984) were unaltered in women with PMDD. Normal levels of ACTH in PMDD also are seen in most studies (Bloch et al 1998; Rabin et al 1990; Rosenstein et al 1996; Su et al 1997), although Redei and Freeman (1993) found that baseline plasma ACTH levels, but not cortisol levels, were lower in PMS subjects as compared with control subjects. Perhaps the hypercortisolism that often characterizes patients with
MDD may be attributed in part to the chronicity of the illness (Halbreich et al 1982). In contrast, PMDD is a more transient disorder and may not be of sufficient duration to elicit a sustained increase in cortisol levels.
In contrast to the lack of substantial differences in quantitative measures of cortisol secretion observed be-tween patient and control groups, we have noted, as in this study, altered timing measures in PMDD and NC subjects during the menstrual cycle: the cortisol peak was phase delayed in the luteal compared with the follicular men-strual cycle phase in NC subjects but not in PMDD subjects (Parry et al 1994). These findings suggest that PMDD subjects may not respond to the hormonal changes of the luteal phase (characterized by the secretion of progesterone) in the same way as do NC subjects. As Young (1999) noted in studies of menstrual cycle phase effects on LH pulsatility in depressed and healthy women, there was a resistance to the frequency-modulating effects of progesterone on LH pulse frequency in depressed women. The fact that in our previous study cortisol peak was phase delayed in the luteal versus follicular phase in NC subjects and that in this study the cortisol acrophase was phase advanced in the luteal versus follicular phase in NC subjects may reflect more a dysregulation of timing functions of cortisol secretion in patients, as compared with control subjects, in the luteal phase, rather than a specific abnormality of direction per se (Siever and Davis 1985).
Other menstrual cycle phase-dependent changes that have been reported to occur (although not differentially in PMDD and NC subjects) include dexamethasone suppres-sion of cortisol and type II glucocorticoid receptor mes-senger RNA expression in lymphocytes: they are reduced in the luteal phase of the menstrual cycle, as compared with the follicular phase (Altemus et al 1997). As sug-gested by the authors, premenstrual mood changes may be related to reduced glucocorticoid feedback restraint of central stress response systems during the luteal phase.
Although menstrual cycle phase did affect the nadir value (higher in the follicular phase than in the luteal), it did not have differential effects in PMDD and NC sub-jects. We attribute this finding to the enhancing effects of estrogen on cortisol secretion (Grant et al 1965) that occur in the follicular phase and that are consistent with our previous findings (Parry et al 1991).
subjects, phase advance their cortisol circadian rhythm with LSD (when they advance their sleep to 9:00PM). The fact that in the luteal phase baseline the cortisol acrophase of PMDD subjects did not advance as it did in NC subjects, but that it did advance with the challenge of LSD (when sleep is advanced), suggests that the resistance to a phase advance of the cortisol rhythm in PMDD subjects in the luteal phase may be corrected by LSD in that it restores the normal phase (timing) relationships between sleep and cortisol circadian rhythms. The baseline differences in cortisol rhythms are not very robust between the two subject groups. With challenge studies using ESD and LSD interventions, however, these cortisol circadian rhythm alterations become more pronounced. Previous studies on the effect of sleep deprivation on cortisol rhythms also suggest that sleep deprivation can affect the timing of the cortisol rhythm: Davidson et al (1991) found that sleep deprivation altered the timing (the nocturnal rise was earlier), but not the average 24-hour levels. Saletu et al (1986) found that partial sleep deprivation late in the night (1:30 – 6:00AM), timing similar to LSD in our study, also was associated with an earlier rise in cortisol secre-tion. Bouhuys et al (1990) found that, in 16 depressed patients, total sleep deprivation (TSD) advanced the time of the maximal cortisol excretion, changes not observed in NC subjects. The time shift, however, was not related to the mood response to TSD in the depressed group. Yamaguchi et al (1978) noted that with 20 depressed patients who underwent one night of TSD, in responders after sleep deprivation, the normal circadian rhythm of cortisol, not evident the preceding day, was restored to that of the NC subjects.
The lower mesor and amplitude of cortisol during LSD probably reflects that with an earlier (phase advanced) sleep onset time, cortisol secretion is decreased. Studies in normal healthy men show that there is a decrease of cortisol during slow-wave sleep (that tends to occur earlier in the night) and an increase during rapid eye movement sleep (that tends to occur later in the night; Weitzman et al 1981). Studies by Pietrosky et al (1994), Weibel et al (1995), and Spath-Schwalbe et al (1993) also suggest that sleep, particularly slow-wave sleep, that occurs during the first hours of nocturnal sleep is associated with decreased cortisol release. The differential effect of ESD and LSD on cortisol during the night (11:00PM–7:00AManalyses) also reflects the different times of waking, which increase cortisol levels, during this interval.
Other factors, such as sex, age, genetic factors, stress, and sleep latency, may affect cortisol secretion, and result in differences in study results: studies by Van Cauter et al (1996) showed that in normal premenopausal women the cortisol amplitude is lower than that of men in the same age group. The study by Van Cauter further showed that
these younger normal women had a phase delay in their cortisol circadian rhythm in the morning, due to their slower response to the circadian signal, compared with men of the same age group. Thus, our results may differ from those of studies in which men were exclusively or primarily studied, or women of a different age range. Twin studies done by Linkowski et al (1993) showed that apart from environmental factors like meals and sleep, the acrophase and especially the nadir of the cortisol rhythm are genetically controlled. The acrophase, however, is not as robust a marker of the circadian rhythm as the nadir (Linkowski et al 1993). In our study, in patients with a family history of depression the cortisol peak occurred later during LSD. According to Stoney et al (1990), the magnitude of stress responses during three different men-strual phases did not affect cortisol rhythms. If primarily stress factors were involved, we might expect the PMDD patients to have higher cortisol levels rather than lower ones during the quiescent nocturnal hours. Those patients, however, with a personal history of depression did have higher amplitudes of cortisol during LSD, a finding correlated with response to sleep deprivation in previous studies (Baumgartner et al 1990a, 1990b; Gerner et al 1979). Fehm and Born (1991), however, found that it was individual differences in sleep onset latencies that affected the cortisol rise. In our findings, during ESD the later the sleep onset time, the earlier the cortisol peak time, and the shorter the sleep latency, the lower the cortisol nadir concentration.
The strengths of this study include adherence to the rigorous DSM-IV criteria for PMDD: we observed both PMDD and NC subjects for a 2-month period with daily ratings and weekly clinic visits to screen subjects, to exclude subjects who did not meet the criteria for PMDD and to document in a comparison group of NC subjects a lack of clinically significant cyclical mood changes linked to the menstrual cycle. We also excluded a family history of depression in the NC subjects, and the PMDD subjects, although they could have a history of MDD, could not have such an illness in the last year. This approach eliminates confounding our study with women with cur-rent MDDs as PMDD subjects. A history of MDD differentiated treatment response, but not baseline cortisol parameters, in PMDD subjects. Other strengths include the frequent sampling of cortisol measures during the 24-hour cycle, the baseline measures in women in symptomatic and asymptomatic menstrual cycle phases, and the mea-sures obtained during challenge paradigms of ESD and LSD that were designed to improve mood in symptomatic subjects during the luteal phase.
with a larger number of subjects to be able to compare the findings with studies done with other depressive disorders. Also, the effects of stress, diet, sleep, and activity, which can affect cortisol secretion, were not minimized by using constant routine conditions. Another limitation of this study is the lack of 24-hour profiles for cosinor analyses. The maximum number of hours needed for a fitted curve has not been determined. Rough estimates of circadian parameters, however, can be gained from samples being drawn overnight from 6:00PM to 9:00AM.
From our study’s data, and similar MDD studies by Yamaguchi et al (1978), we find that the primary abnor-mality in PMDD subjects is the timing of the cortisol circadian rhythm and not the quantity of cortisol secreted. In support of the internal coincidence model for the basis of affective disorders (Wehr and Wirz-Justice 1981), cortisol circadian rhythms may have an abnormal phase position with respect to the timing of sleep in PMDD that is corrected by advancing sleep with LSD. Thus one mechanism by which sleep deprivation may exert its therapeutic effects is by realigning cortisol circadian rhythms with sleep in women with PMDD, with improve-ments in mood observed the day after recovery nights of sleep (Parry et al 1995). The fact that ESD (in which sleep is delayed until 3:00AM) also improved mood in PMDD subjects the following night, and was associated with a small delay in the cortisol acrophase (that was not differ-ent from that of NC subjects), suggests, however, that it may be the effect of sleep deprivation itself and not its timing that is related to therapeutic efficacy. Although most studies find that improvement of mood occurs after the night of sleep deprivation (day 1 responders; Gillin 1983; Wu and Bunney 1990), other investigators have observed that improvement of mood may not be noted until after at least a partial night of recovery sleep (day 2 responders; Matussek et al 1974; Sack et al 1988; Wirz-Justice et al 1976). Matussek et al (1974) hypothesize that day 1 responders primarily have a serotonergic dysfunc-tion and day 2 responders a noradrenergic dysfuncdysfunc-tion. Additional studies are warranted examining the effect of ESD and LSD with respect to the timing relationships between cortisol, sleep, and other circadian rhythms dur-ing nights of both sleep deprivation and recovery sleep to address these questions further.
This work was supported by National Institute of Mental Health Grant No. MH-42831, CRC Grant No. MH-30914, and National Institute of Health Research Center (CRC) Grant No. M01-RR-00827.
References
Altemus M, Redwine L, Leong YM, Yoshikawa T, Yehuda R, Detera-Wadleigh S, et al (1997): Reduced sensitivity to glucocorticoid feedback and reduced glucocorticoid receptor
mRNA expression in the luteal phase of the menstrual cycle.
Neuropsychopharmacology17:100 –109.
American Psychiatric Association (1987):Diagnostic and Sta-tistical Manual of Mental Disorders, 3rd ed rev. Washington, DC: American Psychiatric Press.
American Psychiatric Association (1994):DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Wash-ington, DC: American Psychiatric Press.
American Psychiatric Association (1996):DSM-IV Sourcebook, Vol 2. Washington, DC: American Psychiatric Association. Bancroft J, Cook A, Davidson D, Bennie J, Goodwin G (1991):
Blunting of neuroendocrine responses to infusion of L-tryptophan in women with perimenstrual mood change. Psy-chol Med21:305–312.
Baumgartner A, Graf KJ, Kurten I, Meinhold H, Scholz P (1990a): Neuroendocrinological investigations during sleep deprivation in depression I. Early morning levels of thyro-tropin, TH, cortisol, prolactin, LH, FSH, estradiol, testoster-one.Biol Psychiatry28:556 –568.
Baumgartner A, Riemann D, Berger M (1990b): Neuroendocri-nological investigations during sleep deprivation in depres-sion II. Longitudinal measurement of thyrotropin, TSH, cortisol, prolactin, GH, and LH during sleep and sleep deprivation.Biol Psychiatry28:569 –587.
Beck AT, Ward CH, Mendelson M, Mock JE, Erbuch J (1961): Inventory for measuring depression. Arch Gen Psychiatry
4:561–571.
Bloch M, Schmidt PJ, Tung-Ping S, Tobin MB, Rubinow DR (1998): Pituitary-adrenal hormones and testosterone across the menstrual cycle in women with premenstrual syndrome and controls.Biol Psychiatry43:897–903.
Bouhuys AL, Flentge F, Van den Hookdakker RH (1990): Effects of total sleep deprivation on urinary cortisol, self-rated arousal and mood in depressed patients.Psychiatry Res
34:149 –162.
Carroll BJ, Curtis GC, Mendels J (1976): Neuroendocrine regulation in depression I. Limbic system-adrenocorticol dysfunction.Arch Gen Psychiatry33:1039 –1044.
Davidson JR, Moldofsky H, Lue FA (1991): Growth hormone and cortisol secretion in relation to sleep and wakefulness.
J Psychiatry Neurosci16:96 –102.
Facchinetti F, Fioroni L, Martignoni E, Sances G, Costa A, Genazzani AR (1994): Changes of opioid modulation of the hypothalamo-pituitary-adrenal axis in patients with severe premenstrual syndrome.Psychosom Med56:418 – 422. Fehm HL, Born J (1991): Evidence for entrainment of nocturnal
cortisol release in relation to sleep structure.Sleep15:21–27. Follenius M, Brandenberger G, Bandesapt JJ, Libert JP, Ehrhart J (1992): Nocturnal cortisol release in relation to sleep structure.Sleep15:21–27.
Gerner RH, Post RM, Gillin JC, Bunney WE (1979): Biological and behavioral effects of one night’s sleep deprivation in depressed patients and normals.J Psychiatr Res15:21– 40. Gillin JC (1983): The sleep therapies of depression. Prog
Neuropsychopharmacol Biol Psychiatry7:351–364. Grant S, Pavlotos F, Forsham P (1965): Effect of estrogen
therapy on cortisol metabolism. J Clin Endocrinol Metab
25:1057–1066.
(1985): Cortisol secretion in endogenous depression. Arch Gen Psychiatry42:909 –914.
Halbreich U, Zumoff B, Kream J, Fukushima DK (1982): The mean 1300 –1600 h plasma cortisol concentration as a diag-nostic test for hypercortisolism. J Clin Endocrinol Metab
54:1262–1264.
Hamilton M (1967): Development of a rating scale for primary depressive illness.Br J Soc Clin Psychol6:278 –296. Holsboer F (1989): Psychiatric implications of altered
limbic-hypothalamic-pituitary-adrenocortical activityEur Arch Psy-chiatry Neurol Sci138:302–322.
Holsboer-Trachsler E, Ernst K (1986): Sustained antidepressive effect of repeated partial sleep deprivation.Psychopathology
19(suppl 2):172–176.
Hurt SW, Schnurr PP, Severino SK, Freeman EW, Gise LH, Rivera-Tovar A, et al (1992): Late luteal phase dysphoric disorder in 670 women evaluated for premenstrual com-plaints.Am J Psychiatry149:525–530.
Klemfuss H, Clopton PL (1993): Seeking tau: A comparison of six methods.J Interdisciplinary Cycle Res24:1–16. Krishnan KRRR, Ritchie JC, Saunders W, Wilson W, Nemeroff
CB, Carroll BJ (1990): Nocturnal and early morning secretion of ACTH and cortisol in humans.Biol Psychiatry28:47–57. Kuhs H, Farber D, Tolle R (1996): Serum prolactin, growth hormone, total corticoids, thyroid hormones and thyrotropin during serial therapeutic sleep deprivation.Biol Psychiatry
39:857– 864.
Kuhs H, To¨lle R (1991): Sleep deprivation therapy.Biol Psychi-atry29:1129 –1148.
Laughlin GA, Yen SSC (1996): Nutritional and endocrine-metabolic aberrations in amenorrheic athletes.J Clin Endo-crinol Metab81:4301– 4309.
Leibenluft E, Moul DE, Schwartz PJ, Madden A, Wehr TA (1993): A clinical trial of sleep deprivation in combination with antidepressant medication.Psychiatry Res46:213–227. Leibenluft E, Wehr TA (1992): Is sleep deprivation useful in the
treatment of depression?Am J Psychiatry149:159 –168. Linkowski P, Mendelwicz J, LeClercq R, Brasseur M, Hubain P,
Goldstein J, et al (1985a): The 24 hour profile of ACTH and cortisol in major depressive illness.J Clin Endocrinol Metab
61:429 – 438.
Linkowski P, Onderbergen AV, Kerkhofs M, Bosson D, Men-dlewicz J, Van Cauter E (1993): Twin study of the 24-h cortisol profile: Evidence for genetic control of the human circadian clock.Am J Physiol264:E173–E181.
Linkowski P, Van Cauter E, Leclercq R, Desmedt D, Brasseur M, Golstein J, et al (1985b): ACTH, cortisol and growth hormone 24-hour profiles in major depressive illness.Acta Psychiatr Belg85:615– 623.
Matussek N, Ackenheil M, Athen D, Beckmann H, Benkert O, Dittmer T, et al (1974): Catecholamine metabolism under sleep deprivation therapy of improved and not improved depressed patients. Contributions to biochemistry. Neuropsy-chopharmakologie7:108 –114.
Mortola JF, Girton L, Yen SSC (1989): Depressive episodes in premenstrual syndrome. Am J Obstet Gynecol 161:1682– 1687.
Nelson W, Tong YL, Lee JK, Halberg F (1979): Methods for cosinor-rhythmometry.Chronobiologia6:305–323.
O’Toole SM, Sekula LK, Rubin RT (1997): Pituitary-adrenal cortical axis measures as predictors of sustained remission in major depression.Biol Psychiatry42:85– 89.
Parry BL, Berga SL, Kripke DF, Klauber MR, Laughlin GA, Yen SSC, Gillin JC (1990): Altered waveform of plasma nocturnal melatonin secretion in premenstrual depression. Arch Gen Psychiatry47:1139 –1146.
Parry BL, Cover H, Mostofi N, LeVeau B, Sependa PA, Resnick MA, et al (1995): Early versus late partial sleep deprivation in patients with premenstrual dysphoric disorder and normal comparison subjects.Am J Psychiatry152:404 – 412. Parry BL, Gerner RH, Wilkins JN, Halaris AE, Carlson HE,
Hershman JM, et al (1991): CSF and endocrine studies of premenstrual syndrome. Am Coll Neuropsychopharmacol
5(20):127–137.
Parry BL, Hauger R, LeVeau B, Mostofi N, Cover H, Clopton P, Gillin JC (1996): Circadian rhythms of prolactin and thyroid stimulating hormone during the menstrual cycle and early vs. late sleep deprivation in premenstrual dysphoric disorder.
Psychiatry Res62:147–160.
Parry BL, Hauger R, Lin E, LeVeau B, Mostofi N, Clopton PL, Gillin JC (1994): Neuroendocrine effects of light therapy in late luteal phase dysphoric disorder.Biol Psychiatry36:356 – 364.
Parry BL, Mostofi N, LeVeau B, Nahum HC, Golshan S, Laughlin GA, et al (1999): Sleep EEG studies during early and late partial sleep deprivation in premenstrual dysphoric and normal control subjects.Psychiatry Res85:127–143. Parry BL, Wehr TA (1987): Therapeutic effects of sleep
depri-vation in patients with premenstrual syndrome.Am J Psychi-atry144:808 – 810.
Pfohl B, Sherman B, Schlecte J, Stone R (1985): Pituitary/ adrenal axis rhythm disturbances in psychiatric patients.Arch Gen Psychiatry42:897–903.
Pietrowsky R, Meyrer R, Kern W, Born J, Fehm HL (1994): Effects of diurnal sleep on secretion of cortisol, luteining hormone and growth hormone in man. J Clin Endocrinol Metab78:683– 687.
Rabin DS, Schmidt PJ, Campbell G, Gold PW, Jensvold M, Rubinow DR, Chrousos P (1990): Hypothalamo-pituitary-adrenal function in patients with the premenstrual syndrome.
J Clin Endocrinol Metab71:1158 –1162.
Redei E, Freeman EW (1993): Preliminary evidence for plasma adrenocorticotropin levels as biological correlates of premen-strual symptoms.Acta Endocrinol (Copenh)128:536 –542. Rivera-Tovar AD, Frank E (1990): Late luteal phase dysphoric
disorder in young women.Am J Psychiatry147:1634 –1636. Rosenstein DL, Kalogeras KT, Kalafut M, Malley J, Rubinow DR (1996): Peripheral measures of arginine vasopressin, atrial natriuretic peptide and adrenocorticotropic hormone in premenstrual syndrome.Psychoneuroendocrinology21:347– 359.
Rosenthal NE, Heffernan MM (1986): Bulimia, carbohydrate craving and depression: A central connection? In: Wurtman RJ, Wurtman JJ, editors.Nutrition and the Brain,Vol 7. New York: Raven Press, 139 –166.
Roy-Byrne PP, Uhde TW, Post RM (1986b): Effects of one night’s sleep deprivation on mood and behavior in panic disorder.Arch Gen Psychiatry43:895– 899.
Rubin RT, Poland RE, Winston RA, Blodgett ALN, Lesser IM (1987): Neuroendocrine aspects of primary endogenous de-pression.Arch Gen Psychiatry49:558 –565.
Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy-Byrne P, Andersen R, Merriam GR (1988): Changes in plasma hormones across the menstrual cycle in patients with men-strually related mood disorder and in control subjects.Am J Obstet Gynecol158:5–11.
Sachar EJ, Hellman L, Roffwarg HP, Halpern FS, Fukush DK, Gallagher TF (1973): Disrupted 24 hour patterns of cortisol secretion in psychotic depressives. Arch Gen Psychiatry
28:19 –24.
Sack DA, Duncan W, Rosenthal NE, Mendelson WE, Wehr TA (1988): The timing and duration of sleep in partial sleep deprivation therapy of depression. Acta Psychiatr Scand
77:219 –224.
Saletu B, Dietzel M, Lesch OM, Musalek M, Walter H, Grun-berger J (1986): Effect of biologically active light and partial sleep deprivation on sleep, awakening and circadian rhythms in normals.Eur Neurol25(suppl 2):82–92.
Schuckit MA, Daly V, Herrman G, Hineman S (1975): Premen-strual symptoms and depression in a university population.
Dis Nerv Syst36:516 –517.
Siever LJ, Davis KL (1985): Overview: Toward a dysregulation hypothesis of depression.Am J Psychiatry142:1017–1031. Spath-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL
(1991): Sleep disruption alters nocturnal ACTH and cortisol secretory patterns.Biol Psychiatry29:575–584.
Spath-Schwalbe E, Uthgenannt D, Voget G, Kern W, Born J, Fehm HL (1993): Corticotropin-releasing hormone-induced adrenocorticotropin and cortisol secretion depends on sleep and wakefulness.J Clin Endocrinol Metab77:1170 –1173. Spitzer RL, Williams JBW, Gibbon M, First MB (1990):
Structured Clinical Interview for DSM-III-R Patient Version 1.0 (SCID-P).Washington, DC: American Psychiatric Press. Steiner M, Haskett RF, Carroll BJ, Hays SE, Rubin RT (1984): Circadian hormone secretory profiles in women with severe premenstrual tension syndrome. Br J Obstet Gynaecol 91: 466 – 471.
Stoney CM, Owens JF, Matthews KA, Davis MC, Caggiula A (1990): Influences of the normal menstrual cycle on physio-logic functioning during behavioral stress.Psychophysiology
27:125–135.
Su T-P, Schmidt PJ, Danaceau M, Murphy DL, Rubinow DR (1997): Effect of menstrual cycle phase on neuroendocrine and behavioral responses to the serotonin agonist
m-chloro-phenylpiperazine in women with premenstrual syndrome and controls.J Clin Endocrinol Metab82:1220 –1228.
Van Cauter E, Leproult R, Kupfer DJ (1996): Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol.J Clin Endocrinol Metab81:2468 –2473.
Van den Hoofdakker RH (1994): Chronobiological theories of nonseasonal affective disorders and its implications for treat-ment.J Biol Rhythms9:157–183.
Watts FF, Butt WR, Edwards L, Holder G (1985): Hormonal studies in women with premenstrual tension. Br J Obstet Gynaecol92:247–255.
Wehr TA, Wirz-Justice A (1981): Internal coincidence model for sleep deprivation and depression. In: Koella WP, editor.Sleep 1980.Basel, Switzerland: Karger, 26.
Weibel L, Follenius M, Spiegel K, Ehrhart J, Brandenberger G (1995): Comparative effects of night and daytime sleep on the 24-hour cortisol secretory profile.Sleep18:549 –556. Weitzman ED, Czeisler CA, Zimmerman JC, Ronda JM (1981):
The sleep-wake pattern of cortisol and growth hormone secretion during non-entrained (free-running) conditions in man. In: Van Cauter E, Copinschi G, editors.Human Pitu-itary Hormones: Circadian and Episodic Variations: A work-shop Symposium Held in Brussels: Belgium.The Hague: M. Nijhoff, 29 –30.
Weitzman ED, Zimmerman JC, Czeisler CA, Ronda J (1983): Cortisol secretion is inhibited during sleep in normal man.
J Clin Endocrinol Metab56:352–358.
Weizman A, Mark M, Gil-Ad I, Tyano S, Laron Z (1988): Plasma cortisol, prolactin, growth hormone, and immunore-active beta endorphin response to fenfluramine challenge in depressed patients.Clin Neuropharmacol11:250 –256. Wetzel RD, Reich T, McClure JN Jr, Wald JA (1975):
Premen-strual affective syndrome and affective disorder.Br J Psychi-atry127:219 –221.
Wirz-Justice A, Puhringer W, Hole Q (1976): Sleep deprivation and clomipramine in endogenous depression.Lancet23:912. Wu JC, Bunney WE (1990): The biological basis of an antide-pressant response to sleep deprivation and relapse: Review and hypothesis.Am J Psychiatry147:14 –21.
Yamaguchi N, Maeda K, Kuromaru S (1978): The effects of sleep deprivation on circadian rhythm of plasma cortisol levels in depressive patients.Folia Psychiatric Neurol Jpn
32:479 – 487.
Young EA (1999): Pulsatile analysis of reproductive hormones in depressed women.Biol Psychiatry45:S109 –S110. Zahradnik R, Brennan G, Hutchinson JS, Odell WD (1989):