www.elsevier.nlrlocateraqua-online
Bacterial additives that consistently enhance rotifer
growth under synxenic culture conditions
2. Use of single and multiple bacterial probiotics
P.A. Douillet
)The UniÕersity of Texas at Austin, Marine Science Institute, 1300 Port Street, Port Aransas, TX 78373, USA
Accepted 20 July 1999
Abstract
Axenic rotifers were cultured under synxenic conditions on a bacteria-free artificial diet. The cultures were inoculated either with one of four strains of cultured marine bacteria, or with mixtures of bacteria made up with the same four strains and differing only in the proportions of cells from each strain within the blends. Control cultures were inoculated with bacteria present in
Ž .
freshly collected samples of seawater SW . The lowest rotifer growth rates were observed in these control cultures. The highest growth rates were determined in cultures inoculated with either
Ž .
an Alteromonas strain or a mixture of strains M2 composed of a larger proportion of the
Alteromonas cells than any of the other microbial constituents. The largest variations in rotifer
growth rate between replicate cultures were determined in cultures seeded with SW bacteria, while the lowest were determined in cultures inoculated with either Alteromonas or two blends including mixture M2. Similar results were obtained with both species of rotifers tested,
Brachionus plicatilis Muller and B. rotundiformis Tschugunoff.¨ q2000 Elsevier Science B.V. All rights reserved.
Keywords: Bacterial additives; Rotifer growth; Synxenic culture conditions
1. Introduction
One of the major problems in cultures of rotifers is the poor reproducibility in terms
Ž .
of survival and growth between replicate cultures Skjermo and Vadstein, 1993 .
) 1692 Houghton Ct North, Dunwoody, GA 30338 USA. Tel.: q1-770-671-9393; E-mail:
philippe [email protected]–
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
Selected strains of bacteria have been found to play a major role in the productivity of
Ž
rotifer cultures Yasuda and Taga, 1980; Yu et al., 1988; Maeda and Hino, 1991;
.
Hagiwara et al., 1994 . In a previous study carried out under synxenic conditions, inoculation of cultures with bacteria present in seawater increased the variation between
Ž . Ž
replicate cultures without improving growth rate GR over axenic controls Douillet,
.
1999 . In that study, improvements in rotifer production were obtained with bacterial additives, and these improvements were larger with cultured bacterial strains than with commercial bacterial additives. Furthermore, variation between replicate cultures inocu-lated with selected cultured bacteria strains were significantly lower than in SW controls.
The possibility of using mixtures of strains made up with selected bacteria was considered in order to obtain both further increases in GR and reductions in variability within replicate cultures. Environmental conditions fluctuate in batch rotifer cultures
ŽYoshimura et al., 1997 , and bacterial strains have diverse, yet highly specific,.
Ž .
metabolic and environmental requirements Pearl and Pinckney, 1996 . Consequently, the seeding of rotifer cultures with blends of beneficial bacteria might increase the chances for successful colonization by beneficial microbes throughout the culture cycle. Furthermore, diverse bacterial mechanisms could be involved in the improvement of
Ž .
rotifer cultures Douillet, 1999 . The specificity of bacterial strains as food for grazers
Ž .
has long been reported Stuart et al., 1931; Curds and Vandyke, 1966 and nutritional
Ž .
supplementation is widely recognized Douillet, 1999 . Therefore, the addition of multiple strains might result in larger nutritional enhancement of rotifers than single
Ž
strain inoculations. Finally, biochemical processes i.e., bacterial degradation of organic
.
compounds and control of pathogenic microbes have been found to be more efficiently
Ž
carried out by multi-strain inoculations than by single-strain inoculations Lemanceau
.
and Alabouvette, 1993; Leeman et al., 1996; Serra and Villani, 1997 .
Preliminary rotifer growth experiments were carried out with blends of bacteria consisting of 2 to 12 different strains, including commercial products. All bacterial mixtures were added at same cell concentrations. Significant differences in rotifer production were determined between cultures seeded with these blends of different
Ž .
bacterial composition. However, addition to rotifer cultures of a mixture M1 prepared with four strains resulted in the best growth rates of all treatments. Not surprisingly, these four strains gave the highest growth rates of rotifers when added independently in
Ž .
the previous study Douillet, 1999 .
In this study, the strains that made up the blend M1 were evaluated for their effects on rotifers either independently or combined in blends made up with all four strains and differing only in the proportion of the microbial constituents. Rotifers were grown under synxenic culture conditions, and fed on an artificial diet.
2. Materials and methods
2.1. Preparation of rotifers
Ž .
Cysts of the rotifer Brachionus plicatilis Muller formerly called L-type B. plicatilis
¨
Ž .
Tschugunoff formerly called S-type B. plicatilis were either purchased from
Aquacul-Ž
ture Supply, or kindly provided by Dr. Terry W. Snell Georgia Institute of Technology,
.
Atlanta, GA . Axenic rotifers were obtained by sodium hypochlorite treatment and tested for microbial contamination according to the methods presented in Douillet
Ž1998 . The same experimental protocol was used to evaluate the effects of additions of.
Ž .
diverse bacterial additives as in Douillet 1999 .
2.2. Experimental protocol
B. plicatilis were used in Experiment 1, while B. rotundiformis were used in all other
experiments. Initial rotifer densities in the different experiments varied between 6 and 13.3 mly1. All rotifer cultures, including control treatments, were fed an artificial diet
Ž .
described in Douillet 1999 .
Bacterial additives tested in Experiment 1 included the individual strains
Al-Ž . Ž
teromonas sp. A.sp , and three unidentified marine Gram negative rods B3, B4 and
.
B5 . Further bacterial treatments included six blends of the previously described single strains. These blends were prepared by adding the strains in different proportions, but maintaining a final concentration in rotifer cultures of 2=107cells mly1. The composi-tion of the blends is presented in Table 1, and the numbers represent final cell concentrations in culture tubes. Control rotifer cultures were either kept axenic or were
Ž .
inoculated with bacteria present in freshly collected seawater SW .
Bacterial treatments tested in Experiment 2 included two blends of bacteria that enhanced GR of B. plicatilis while reducing coefficients of variation between replicate
Ž .
cultures in Experiment 1 M1 and M2 . Also included in this experiment was one of the bacterial components of the blend, the Alteromonas sp. strain, which was the single strain additive that provided the highest GR of rotifers in Experiment 1. Rotifer cultures inoculated with bacteria present in seawater were used as controls. This experiment was
Ž .
replicated three times to determine consistency of treatments Experiments 2, 3 and 4 .
2.3. Data collection and analysis
At the end of each experiment, rotifer GR in each culture tube was determined as in
Ž .
Douillet 1999 . GR in Experiment 1 were analyzed using one-way ANOVA, followed
Table 1
Composition of blends of bacteria evaluated in Experiment 1 with B. plicatilis. Cell numbers are final cell concentrations in culture tubes in cells mly1
Ž .
by Tukey’s range test T-method, Sokal and Rohlf, 1981 to determine differences between treatments at the 0.05 level of probability. GR data in Experiments 2, 3 and 4 were analyzed by two-way ANOVA, with experimental run and bacterial treatment as variables. Due to significant differences between experimental runs, the data for each run were analyzed by one-way ANOVA following a square transformation to satisfy homocedasticity requirements in Experiment 3. Differences between treatments were determined by Tukey’s range test at the 0.05 level of probability.
Ž U. Ž . U
Coefficients of variation V were also calculated as in Douillet 1999 . V under the different treatments in Experiments 2, 3 and 4 were analyzed by one-way ANOVA, followed by Tukey’s range test to determine differences between treatments at the 0.05 level of probability. VU
values were log-transformed to fulfill parametric assumptions.
Ž
All tests were performed with the computer program Statistix 2 NH Analytical
.
Software .
3. Results
Additions of single and multiple probiotics significantly affected rotifer GR in all
Ž .
experiments ANOVA, P-0.05 . In all four experiments, the lowest GR in cultures receiving bacterial additives resulted in cultures inoculated with bacteria present in
Ž .
freshly collected seawater SW .
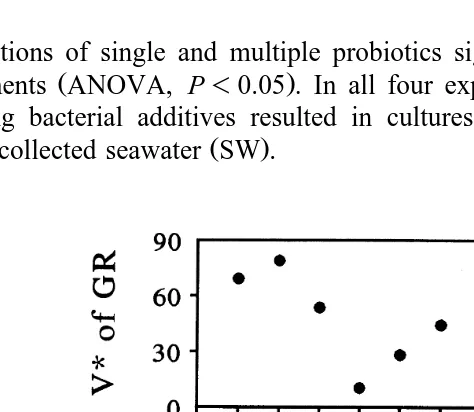
Ž .
Fig. 1. Growth rate GR of B. plicatilis cultured under synxenic conditions with single or multiple strains of bacteria, and VU
of growth rate under the different treatments in Experiment 1. Control rotifer treatments were
Ž .
either cultured axenically or were inoculated with bacteria present in freshly collected seawater SW .
Ž .
Table 2
Two-way ANOVA of growth rates of B. rotundiformis cultured with four different bacterial additives in three experimental runs
Source of variation df Sum of squares Mean squares F-ratio P
Ž .
Experiment A 2 0.32538 0.16269 12.52 0.0001
Ž .
Treatment B 3 1.1313 0.3771 29.02 0.0000
Ž .
Interaction A=B 6 0.095848 0.015975 1.23 0.3144
Ž .
Replicates C
Ž .
Residual A=B=C 36 0.46782 0.012995
Total 47 2.0204
In Experiment 1, no difference in GR was determined between axenic controls and cultures inoculated with SW bacteria. The enhancement of rotifer GR caused by mixtures of strains were either higher or lower than those observed with the individual
Ž .
strains that made up the mixtures Fig. 1 . Addition of mixtures M1, M2, M3 and M4 as well as strains Alteromonas sp., and B3 resulted in significantly higher GRs than in SW
Ž . U
controls Tukey’s, P-0.05 . V values in mixtures were either higher or lower than those observed with the single strains that made up the mixtures. The highest VU
was determined in cultures inoculated with SW bacteria. The lowest VU
values were
Ž .
Fig. 2. Growth rate GR of B. rotundiformis cultured under synxenic conditions with either Alteromonas sp.
ŽA.sp. , or with mixtures of bacteria strains M1 and M2 in Experiment 2 a , Experiment 3 b and. Ž . Ž . Ž .
Ž . Ž .
Experiment 4 c . Control cultures were inoculated with bacteria present in freshly collected seawater SW .
Ž .
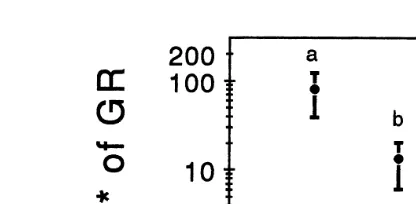
Ž U. Ž .
Fig. 3. Coefficients of variation V of growth rate GR of B. rotundiformis under the different treatments in Experiments 2, 3 and 4. Scatter plots with different letters are significantly different from each other
ŽTukey’s, P-0.05 . See Fig. 2 legend for abbreviations..
determined in cultures inoculated with either the Alteromonas strain or blends M1 and M2.
Significant differences in GR were determined between Experiments 2, 3 and 4
Žtwo-way ANOVA, P-0.05. ŽTable 2 . Therefore, data sets for each experiment were.
analyzed by one-way ANOVA to determine differences between bacterial treatments. In all three experiments, addition of Alteromonas sp. and blend M2 significantly enhanced
Ž . Ž .
rotifer GRs over SW controls Tukey’s, P-0.05 Fig. 2a,b,c . The highest GRs in all experiments were observed in cultures inoculated with mixture M2; however, no statistical difference in GR was determined in any one of the experiments between this blend and the Alteromonas treatment. In contrast, mixture M1 gave intermediate GR values between the SW control and the Alteromonas treatment. GR under M1 was always higher than the SW control but the difference was significative only in
Ž .
Experiment 1 Tukey’s, P-0.05 . GRs under the mixture M1 were always lower than those determined under the Alteromonas treatment, but there were no statistical
Ž . U
differences between both treatments Tukey’s, P)0.05 . V values under the SW treatment were significantly higher than those determined under the other three
treat-Ž . Ž . U
ments Tukey’s, P-0.05 Fig. 3 . The lowest V values were observed in cultures inoculated with blend M2, followed by those determined in cultures treated with
Alteromonas sp. There were no statistical difference between VU values under the
Alteromonas sp., M1 and M2 treatments.
4. Discussion
Inoculation of either individual strains or mixtures of strains into rotifer cultures resulted in improvements in GRs of B. plicatilis over control cultures seeded with
Ž .
naturally occurring marine bacteria SW . Addition of these mixtures of strains to rotifer cultures either increased or decreased rotifer GRs with respect to cultures that have been inoculated with the individual strains that made up the mixtures. Considering that,
Ž
individually, all four strains making the mixtures were beneficial for rotifers Douillet,
.
1999 , a surprisingly high variation in GR resulted from adding mixtures of strains
Ž
differing only in the proportion of their microbial constituents i.e., mixtures M6 and M2
.
Additions of Alteromonas sp. and blend M2 resulted in a consistent enhancement of rotifer cultures over SW control cultures. It should be pointed out that this M2 blend is composed of a larger proportion of Alteromonas cells than any of the other microbial constituents. This strain was previously found to consistently enhance cultures of oyster
Ž . Ž .
larvae Douillet and Langdon, 1993, 1994 and rotifers Douillet, 1999 . The improve-ments in GR caused by additions of either Alteromonas sp. or blend M2 were demonstrated with both rotifer strains tested, B. plicatilis and B. rotundiformis.
Poor reproducibility in terms of survival and growth between production batches is one of the major problems culturing rotifers. One method to evaluate this variability consists in comparing VU
. VU
values have been used as indexes of culture stability in
Ž . U
rotifer cultures Walz et al., 1997 . In Experiment 1, a reduction in V in B. plicatilis cultures resulted from seeding cultures with Alteromonas or blends M1 and M2, compared with SW controls. A statistical analysis of VU values for B. rotundiformis in Experiments 2, 3, and 4 demonstrated a significant reduction in variation within replicates in cultures seeded with either Alteromonas or blends M1 and M2 compared with SW controls.
Several mechanisms of bacterial enhancement of rotifer cultures have been proposed
Ž
or demonstrated by diverse authors as summarized in a previous manuscript Douillet,
.
1999 . The mechanisms by which the strains used in this research enhance rotifer growth are still unknown; however, the fact that there is a slight but consistent increase in rotifer GR and reduction in VU by using blend M2 over the individual components of the blend, added all at the same final concentration, indicates a synergistic effect. This synergistic enhancement was specific to M2 and was not observed in this study with
Ž .
other mixtures. In a similar type of experiment, Gatesoupe 1991 added Streptococcus
thermophilus and Lactobacillus helÕeticus to xenic rotifer cultures either independently
or in combination. Control cultures were not supplemented with bacteria. Rotifer concentrations were higher than in controls under treatments receiving the individual strains and the blend; however, the magnitude of the enhancement under the blend
Ž
treatment was intermediary between those recorded with the single strains Gatesoupe,
.
1991; Table 3 .
In summary, the seeding of rotifer cultures with an Alteromonas strain, or a blend of strains consisting in a large proportion of this Alteromonas strain resulted in a consistent enhancement of rotifer cultures, and a reduction in variation between cultures compared with cultures seeded with SW bacteria. Mixtures of strains, all beneficial for rotifers, and differing only in the proportion of their constituents led to significantly different results; thus, revealing the complexity of microbial ecology processes. Micro-bial contaminants were eliminated from the culture systems, so under these synxenic culture conditions, the beneficial effects described above can be ascribed directly to either the strain or the blend of bacteria added.
Acknowledgements
This work was supported by Grant aNA56RG0388 from the National Oceanic and
views expressed herein are those of the author and do not necessarily reflect the views
Ž
of NOAA or any of its sub-agencies. I am indebted to Dr. Ron Benner The University
.
of Texas at Austin, Marine Science Institute for the use of his epifluorescence microscope.
References
Curds, C.R., Vandyke, J.M., 1966. The feeding habits and growth rates of some fresh-water ciliates found in activated-sludge plants. J. Appl. Ecol. 3, 127–137.
Douillet, P., 1998. Disinfection of rotifer cysts leading to bacteria-free populations. J. Exp. Mar. Biol. Ecol. 224, 183–192.
Douillet, P., 1999. Bacterial additives that consistently enhance rotifer growth under synxenic culture conditions: 1. Evaluation of commercial products and pure isolates. Aquaculture.
Douillet, P., Langdon, C.J., 1993. Effects of marine bacteria on the culture of axenic oyster Crassostrea gigas
ŽThunberg larvae. Biol. Bull. 184, 36–51..
Ž
Douillet, P., Langdon, C.J., 1994. Use of a probiotic for the culture of larvae of the Pacific oyster Crassostrea
.
gigas Thunberg . Aquaculture 119, 25–40.
Gatesoupe, F.J., 1991. The effect of three strains of lactic bacteria on the production rate of rotifers,
Brachionus plicatilis, and their dietary value for larval turbot, Scophthalmus maximus. Aquaculture 96,
335–342.
Hagiwara, A., Hamada, K., Hori, S., Hirayama, K., 1994. Increased sexual reproduction in Brachionus
Ž .
plicatilis Rotifera with the addition of bacteria and rotifer extracts. J. Exp. Mar. Biol. Ecol. 181, 1–8.
Leeman, M., Den Ouden, F.M., Van Pelt, J.A., Cornelissen, C., Bakker, P.A.H.M., Schippers, B., 1996. Suppression of Fusarium wilt of radish by co-inoculation of fluorescent Pseudomonas spp. and of root colonizing fungi. Eur. J. Plant Pathol. 102, 21–31.
Lemanceau, P., Alabouvette, C., 1993. Biological control of Fusarium diseases by fluorescent Pseudomonas and nonpathogenic Fusarium. Crop Protect. 10, 279–286.
Maeda, M., Hino, A.,1991. Environmental management for mass culture of rotifer, Brachionus plicatilis. In:
Ž .
Fulks, W., Main, K.L. Eds. , Rotifer and Microalgae Culture Systems, Proceedings US–Asia Workshop, Honolulu, USA, 28–31 January 1991. The Oceanic Institute, pp. 125–133.
Pearl, H.W., Pinckney, J.L., 1996. A mini-review of microbial consortia: their roles in aquatic production and biogeochemical cycling. Microb. Ecol. 31, 225–247.
Serra, R., Villani, M., 1997. Modeling bacterial degradation of organic compounds with genetic networks. J. Theor. Biol. 189, 107–119.
Skjermo, J., Vadstein, O., 1993. Characterization of the bacterial flora of mass cultivated Brachionus
plicatilis. Hydrobiologia 255r256, 185–191.
Sokal, R.R., Rohlf, F.J., 1981. Biometry. Freeman, San Francisco, CA, 859 pp.
Stuart, C.A., McPherson, M., Cooper, H.J., 1931. Studies on bacteriologically sterile Moina macrocopa and their food requirements. Physiol. Zool. 4, 87–100.
Walz, N., Hintze, T., Rusche, R., 1997. Algae and rotifer turbidostats: studies on stability of live feed cultures. Hydrobiologia 358, 127–132.
Yasuda, K., Taga, N., 1980. Culture of Brachionus plicatilis Muller using bacteria as food. Bull. Jpn. Soc.¨ Sci. Fish. 46, 933–939.
Yoshimura, K., Usuki, K., Yoshimatsu, T., Kitajima, C., Hagiwara, A., 1997. Recent development of a high density mass culture system for the rotifer Brachionus rotundiformis Tschugunoff. Hydrobiologia 358, 139–144.
Yu, J.-P., Hino, A., Hirano, R., Hirayama, K., 1988. Vitamin B -producing bacteria as a nutritive12