Intraspecific differences in physiological response of 20
wheat cultivars to enhanced ultraviolet-B radiation under
field conditions
Li Yuan
a,b,*, Zu Yanqun
a, Chen Jianjun
a, Chen Haiyan
a, Yang Jilong
a,
Hu Zhide
baDepartment of En6ironmental Science,Yunnan Agricultural Uni6ersity,Kunming650201,People’s Republic of China bDepartment of Chemistry,Lanzhou Uni6ersity,Lanzhou730000,People’s Republic of China
Received 11 November 1999; received in revised form 18 April 2000; accepted 23 April 2000
Abstract
Field studies were conducted to determine the potential for alterations in physiology and the intraspecific variation in sensitivity of 20 wheat (Triticum aesti6um) cultivars to enhanced ultraviolet-B (UV-B, 280 – 315 nm) radiation. The
supplemental UV-B radiation was 5 kJ m−2, simulating a depletion of 20% stratospheric ozone. Out of 20 wheat
cultivars (from South China, North China and Mexico) tested, 13 showed significant changes in total chlorophyll content. In most of these sensitive species, chlorophyll a content was strongly reduced, and chlorophyll b content decreased in a lesser extent, leading to a decrease in chlorophyll a/b ratio. However, some species had an increased chlorophyll a/b ratio under enhanced UV-B. The effect of UV-B on flavonoid content also showed intraspecific differences, a significant increase for one cultivar, decreases in 12 cultivars and no effect on the other seven cultivars. Superoxide dismutase (SOD) activity of five cultivars was significantly increased, and that of six cultivars significantly decreased. Membrane permeability of 12 cultivars significantly increased, while only that of Dali 905 was significantly decreased. Malonaldehyde (MDA) contents of eight cultivars were increased significantly, while that of three cultivars was significantly decreased. Although large intraspecific differences were found for the different parameters measured, there was no clear correlation between them under UV-B radiation. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Chlorophyll; Flavonoid; Malonaldehyde; Membrane permeability; Superoxide dismutase; Triticum aesti6um; UV-B
radiation
www.elsevier.com/locate/envexpbot
1. Introduction
The rapid decline in stratospheric ozone con-centrations has been confirmed by satellite mea-surements. The most pronounced thinning of the ozone layer has been measured over the Antarctic * Corresponding author.
E-mail address:[email protected] (L. Yuan).
L.Yuan et al./En6ironmental and Experimental Botany44 (2000) 95 – 103
96
continent with up to 71% depletion during the Antarctic spring (Kerr, 1993). Recent mathemati-cal models predict a further increase in solar UV-B irradiation in future years (Madronich et al., 1995). UV-B effects on plants have been the subjects of considerable research (Caldwell et al., 1995). An examination of more than 200 plant species reveals that roughly 20% are sensitive, 50% are mildly sensitive or tolerant and 30% are completely insensitive to UV-B radiation (Tera-mura, 1983). Whilst the impact of enhanced UV-B radiation on plant physiology, morphology, growth and biomass have been investigated exten-sively, little is known about intraspecific differ-ences in physiological response to enhanced UV-B. Recently, intraspecific differences in flavonoid in cucumber (Murali and Teramura, 1986), soybean (Teramura and Murali, 1986; D’surney et al., 1993) andArabidopsis thaliana(Li et al., 1993; Fiscus et al., 1999), flavonoid and chlorophyll in rice (Teramura et al., 1991) have been reported. Plant species and even genotypes within species can differ greatly in their responses to UV-B. In the recent experiment, the substantial growth suppression of A. thaliana flavonoid mu-tants suggests that the disruption of secondary metabolism at the chalcone isomerase site is af-fecting critical aspects of secondary metabolism. This may result in an impairment of the normal growth and differentiation process (Fiscus et al., 1999). Due to our lack of understanding of the role of intraspecific response differences to UV-B radiation, further studies on its importance should be undertaken.
On the other hand, most of the UV-B research in the past two decades has been conducted in growth chambers and greenhouses where the un-natural spectral balance of radiation can lead to unrealistic conclusions, which may have substan-tially changed plant sensitivity to UV-B. It is important in experiments to maintain a realistic balance between various spectral regions since both UV-A (315 – 400 nm) and visible (400 – 700 nm) radiation can have ameliorating effects on responses of plants to UV-B (Caldwell et al., 1995). In growth chamber and greenhouse experi-ments, the visible and UV-A radiation is usually much less than in sunlight, thus, even if realistic
levels of UV-B are used in simulating ozone re-duction, the plant response may be exaggerated relative to field conditions. Unfortunately, only 15% of the studies have been conducted under field conditions. While the laboratory and glasshouse studies provide information on mecha-nisms and processes of UV-B action, only field studies can provide realistic assessments of what will happen as the stratospheric ozone layer thins (Caldwell et al., 1995; Yue et al., 1998).
Wheat is one of the major world food crops (Teramura, 1983), the effects of enhanced UV-B radiation on photosynthetic characteristics, growth, development, leaf quality, morphology, tiller number, crop structure, plant nutrients, de-composition, competition interaction between wheat and wild oat, intraspecific response differ-ences, total biomass and yield have been studied (Li et al., 1998, 1999; Yue et al., 1998). Unfortu-nately, only few studies have been conducted un-der field conditions. In this study, we grew 20 wheat cultivars in field under ambient and supple-mental levels of UV-B radiation with the objective to (1) determine, if UV-B radiation affects wheat physiology under field conditions; and (2) evalu-ate intraspecific differences in physiological re-sponse of 20 wheat cultivars to UV-B radiation in the field. We hypothesized that enhanced UV-B radiation will decrease chlorophyll content, and affect other physiological processes, i.e. insuffi-cient protection by flavonoid will result in the production of oxygen radicals, and their insuffi-cient removal by SOD will cause membrane dam-age which can be measured by membrane permeability and MDA content. These changes will result in intraspecific differences in physiolog-ical response under field conditions.
2. Materials and methods
2.1. Plant materials and growth conditions
The field experiment was conducted on a up-land red soil at Yunnan Agricultural University, Kunming, China. No fertilization was necessary during the season. Seeds of 20 wheat (Triticum
cultivars in China (19 cultivars) and Mexico (MY 94-9), were obtained from Lanzhou Agri-cultural Science Research Institute, Yunnan Academic of Agricultural Sciences, Gansu demic of Agricultural Sciences and Henan Aca-demic of Agricultural Sciences. They were sown in rows spaced 0.2 m apart at a density of 80 seeds m−1 in 120 plots of 2×1 m each on July
26, 1998. Five border rows were sown round each plot in order to minimize heterogeneity in microclimate. The overall experimental design was a randomized complete block with two UV-B treatments and three replications. At the three-leaf stage, plants were thinned to 60 m−1
for uniformity in growth. This planting density is within common sowing practice for the Kun-ming region.
2.2. UV-B radiation
Supplemental UV-B radiation was provided by filtered Gucun brand (Gucun Instrument Factory, Shanghai, China) 30 W sunlamps fol-lowing the procedure outlined in Lydon et al. (1986). Lamps were suspended above and per-pendicular to the planted rows (rows oriented in an east – west direction to minimize shading) and filtered with either 0.13 mm thick cellulose diac-etate (transmission down to 290 nm) for supple-mental UV-B radiation or 0.13 mm polyester plastic films (absorbs all radiation below 320 nm) as a control (Sullivan and Teramura, 1990). Cellulose diacetate filters were presolarized for 8 h and changed weekly to ensure uniformity of UV-B transmission. The spectral irradiance from the lamps was determined with an Optronic Model 742 (Optronic Laboratories Inc., Orlando, FL, USA) spectroradiometer. The spectral irradiance was weighted with the gener-alized plant response action spectrum (Caldwell, 1971) and normalized at 300 nm to obtain UV-BBE. Six lamps were installed above each plot.
Plants were irradiated for 7 h daily from three-leaf stage to ripening stage, centered around so-lar noon. Plants under polyester-filtered lamps received only ambient levels of UV-B radiation (10 kJ m−2 UV-B
BE during clear sky conditions
on the summer solstice). Plants beneath the cel-lulose diacetate filters received ambient plus sup-plemental levels of UV-B. The lamp height above the plants was adjusted weekly to main-tain a distance of 0.45 m between the lamps and the top of the plants, and provided supplemen-tal irradiances of five effective kJ m−2 UV-B
BE.
This supplemental level was similar to that which would be experienced at Kunming (25°N, 1950 m) with a 20% stratospheric ozone reduc-tion during a clear day on the summer solstice (10 kJ m−2
UV-BBE) according to a
mathemati-cal model of Madronich et al. (1995). Total daily photosynthetic photon fluence (PPF be-tween 400 and 700 nm) under lamp fixtures was 90% of that above the lamps.
2.3. Measurements and statistical analyses
Physiological indicators of the first three fully expanded leaves of plants were tested in the stages of tillering and elongation of wheat. Two samples each of ten plants were taken from each plot. chlorophyll content was determined ac-cording to the method of Arnon (1949). Leaf discs of 100 mm2
were taken from leaves and extracted in 10-ml of acidified methanol (79:20:1 v/v, methanol:water:HCl) for flavonoid measure-ment, according to the procedure of Mirecki and Teramura (1984). Extract absorbance at 305 nm measured on a spectrophotometer was used as a measure of flavonoid. Superoxide dismutase (SOD) activity was determined according to the method of Giannopolitis and Ries (1977). Mem-brane permeability was determined according to the method of Fan and Blake (1994), and the conductivity of the bathing solutions was tested using a conductivity meter (DDS-IIC, Shanghai, China). Malonaldehyde (MDA) contents were determined according to the method of Heath and Packer (1968).
Statistical differences between mean of control and UV-B radiation treatment of any measured parameter were determined by T-test at the PB
L.Yuan et al./En6ironmental and Experimental Botany44 (2000) 95 – 103
98
3. Results
3.1. Chlorophyll contents
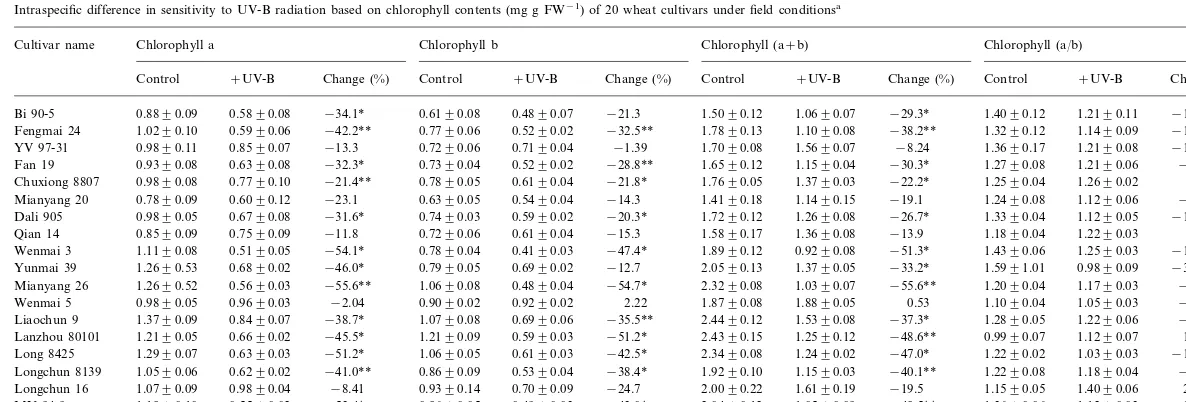
UV-B radiation had obvious effects on chloro-phyll contents of the most of 20 wheat cultivars under field conditions (Table 1). Total chlorophyll contents of 13 cultivars were significantly reduced under UV-B radiation, mostly due to a strong reduction in chlorophyll a content and to a lesser extent reduction in chlorophyll b content. How-ever, there were large intraspecific differences in chlorophyll a/b ratio with significant increases (PB0.05) in cultivars Longchun 16 and Huining 18 and significant decreases (PB0.05) for four cultivars, namely, Dali 905, Yunmai 39, Long 8425 and MY 94-9.
3.2. Fla6onoid contents
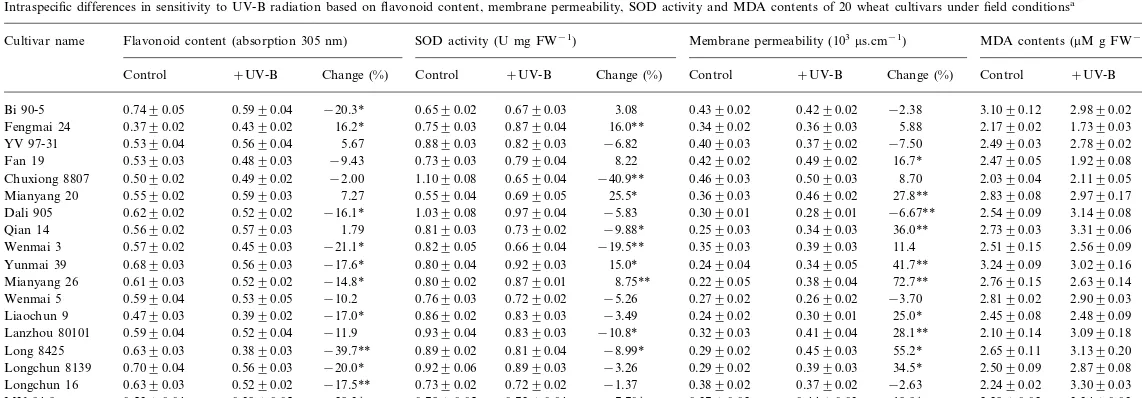
The effect of UV-B on flavonoid content also showed intraspecific differences, a significant in-crease for Fengmai 24 (PB0.01); decreases in 12 cultivars (PB0.01 or 0.05); and no effect on the other seven cultivars (P\0.05) (Table 2).
3.3. SOD acti6ity
UV-B radiation had an obvious effect on SOD activity of most of the 20 wheat cultivars under field conditions (Table 2). SOD activity of five cultivars was significantly increased (PB0.01 or 0.05), while that of six cultivars was significantly decreased (PB0.05).
3.4. Membrane permeability
Table 2 showed that UV-B radiation had obvi-ous effects on membrane permeability of most of the 20 wheat cultivars under field conditions. Membrane permeability of 12 cultivars was sig-nificantly increased (PB0.01 or 0.05), while mem-brane permeability of Dali 905 (PB0.01) only significantly decreased.
3.5. MDA contents
Effects of enhanced UV-B radiation on MDA contents were shown in Table 2. MDA contents of eight cultivars increased significantly (PB0.01 or 0.05) by UV-B radiation, that of Huining 18 (PB0.0l), Fengmai 24 (PB0.05), and Fan 19 (PB0.05) significantly decreased.
4. Discussion
This is the first report to suggest that intraspe-cific differences existed in physiological response of 20 wheat cultivars to enhanced ultraviolet-B radiation under field conditions. This is supported by the earlier findings of intraspecific differences in flavonoid metabolism in Cucumis sati6us
(Mu-rali and Teramura, 1986), soybean (D’surney et al., 1993) andA.thaliana(Li et al., 1993; Fiscus et al., 1999), and in flavonoid content and chloro-phyll content in rice (Teramura et al., 1991).
Reductions in chlorophyll contents have often been used to assess the degrees of UV-B radiation sensitivity. UV-B radiation decreased chlorophyll contents in rice (Teramura et al., 1991) and soy-bean (Mirecki and Teramura, 1984) in a green-house experiment have been observed. In this study, chlorophyll contents were sensitive to en-hanced UV-B radiation. So, they may be used as response indicator for assessing the degrees of UV-B radiation sensitivity. Correlation analyses showed that percentage change of chlorophyll a/b ratio was positively correlated with percentage change of contents of chlorophyll a (r=0.577,
PB0.0l), and negatively correlated with percent-age change of contents of chlorophyll b (r= − 0.076, P\0.05). UV-B radiation significantly decreased chlorophyll contents, primarily because it destroyed the structure of chloroplasts, inhib-ited synthesis of new chlorophyll, and increased the degradation of chlorophyll (Sakaki et al., 1983). The mechanism of UV-B radiation decreas-ing chlorophyll contents is still not clear.
Yuan
et
al
.
/
En
6
ironmental
and
Experimental
Botany
44
(2000)
95
–
103
99
Table 1
Intraspecific difference in sensitivity to UV-B radiation based on chlorophyll contents (mg g FW−1) of 20 wheat cultivars under field conditionsa
Chlorophyll (a/b) Cultivar name Chlorophyll a Chlorophyll b Chlorophyll (a+b)
Change (%) Control +UV-B Change (%) Control +UV-B Change (%) Control +UV-B Change (%) Control +UV-B
−21.3 1.5090.12 1.0690.07 −29.3* 1.4090.12
0.6190.08 1.2190.11
Bi 90-5 0.8890.09 0.5890.08 −34.1* 0.4890.07 −13.6
1.0290.10 0.5990.06 −42.2** 0.5290.02 −32.5** 1.7890.13 1.1090.08 −38.2** 1.3290.12 1.1490.09 −13.6
Fengmai 24 0.7790.06
−1.39 1.7090.08 1.5690.07 −8.24 1.3690.17
YV 97-31 0.9890.11 0.8590.07 −13.3 0.7290.06 0.7190.04 1.2190.08 −11.0
−28.8** 1.6590.12 1.1590.04 −30.3* 1.2790.08 1.2190.06
0.5290.02 −4.72
−32.3* 0.7390.04 Fan 19 0.9390.08 0.6390.08
0.6190.04
0.9890.08 0.7790.10 −21.4** 0.7890.05 −21.8* 1.7690.05 1.3790.03 −22.2* 1.2590.04 1.2690.02 0.80 Chuxiong 8807
−14.3 1.4190.18 1.1490.15 −19.1 1.2490.08 1.1290.06
Mianyang 20 0.7890.09 0.6090.12 −23.1 0.6390.05 0.5490.04 −9.68
−20.3* 1.7290.12 1.2690.08 −26.7* 1.3390.04 1.1290.05
0.5990.02 −15.8*
0.9890.05
Dali 905 0.6790.08 −31.6* 0.7490.03
0.6190.04
0.8590.09 0.7590.09 −11.8 0.7290.06 −15.3 1.5890.17 1.3690.08 −13.9 1.1890.04 1.2290.03 3.39 Qian 14
−47.4* 1.8990.12 0.9290.08 −51.3* 1.4390.06 1.2590.03
Wenmai 3 1.1190.08 0.5190.05 −54.1* 0.7890.04 0.4190.03 −12.6
−12.7 2.0590.13 1.3790.05 −33.2* 1.5991.01 0.9890.09
0.6990.02 −38.4*
1.2690.53
Yunmai 39 0.6890.02 −46.0* 0.7990.05
0.4890.04
1.2690.52 0.5690.03 −55.6** 1.0690.08 −54.7* 2.3290.08 1.0390.07 −55.6** 1.2090.04 1.1790.03 −2.50 Mianyang 26
2.22 1.8790.08 1.8890.05 0.53 1.1090.04 1.0590.03
Wenmai 5 0.9890.05 0.9690.03 −2.04 0.9090.02 0.9290.02 −4.55
−35.5** 2.4490.12 1.5390.08 −37.3* 1.2890.05 1.2290.06
0.6990.06 −4.69
1.3790.09
Liaochun 9 0.8490.07 −38.7* 1.0790.08
0.5990.03
1.2190.05 0.6690.02 −45.5* 1.2190.09 −51.2* 2.4390.15 1.2590.12 −48.6** 0.9990.07 1.1290.07 13.1 Lanzhou 80101
−42.5* 2.3490.08 1.2490.02 −47.0* 1.2290.02 1.0390.03 −15.6* Long 8425 1.2990.07 0.6390.03 −51.2* 1.0690.05 0.6190.03
−38.4* 1.9290.10 1.1590.03 −40.1** 1.2290.08 1.1890.04
0.5390.04 −3.28
−41.0** 0.8690.09 Longchun 8139 1.0590.06 0.6290.02
0.7090.09
1.0790.09 0.9890.04 −8.41 0.9390.14 −24.7 2.0090.22 1.6190.19 −19.5 1.1590.05 1.4090.06 21.7* Longchun 16
−43.0* 2.0490.12 1.0590.09 −48.5** 1.3690.06 1.1290.03 −17.6* MY 94-9 1.1890.10 0.5590.02 −53.4* 0.8690.05 0.4990.03
−23.6 2.1590.22 1.7090.21 −20.9 1.0290.03 1.0990.06
0.8190.10 6.86
1.0690.14 Longchun 15 1.0890.14 0.8890.10 −18.5
Huining 18 1.2690.05 1.3090.02 3.17 1.0590.15 0.8090.11 −23.8 2.3190.18 2.0990.13 −9.52 1.2090.08 1.6290.09 35.0*
L
.
Yuan
et
al
.
/
En
6
ironmental
and
Experimental
Botany
44
(2000)
95
–
103
100
Table 2
Intraspecific differences in sensitivity to UV-B radiation based on flavonoid content, membrane permeability, SOD activity and MDA contents of 20 wheat cultivars under field conditionsa
MDA contents (mM g FW−1)
SOD activity (U mg FW−1)
Cultivar name Membrane permeability (103
ms.cm−1)
Flavonoid content (absorption 305 nm)
Change (%) Control +UV-B Change (%) Control +UV-B Change (%) Control +UV-B Change (%) Control +UV-B
3.08 0.4390.02 0.4290.02 −2.38 3.1090.12
0.6590.02 2.9890.02
Bi 90-5 0.7490.05 0.5990.04 −20.3* 0.6790.03 −3.87
0.3790.02 0.4390.02 16.2* 0.8790.04 16.0** 0.3490.02 0.3690.03 5.88 2.1790.02 1.7390.03 −20.3*
Fengmai 24 0.7590.03
−6.82 0.4090.03 0.3790.02 −7.50 2.4990.03
YV 97-31 0.5390.04 0.5690.04 5.67 0.8890.03 0.8290.03 2.7890.02 11.6*
8.22 0.4290.02 0.4990.02 16.7* 2.4790.05 1.9290.08
0.7990.04 −22.3*
−9.43 0.7390.03 Fan 19 0.5390.03 0.4890.03
0.6590.04
0.5090.02 0.4990.02 −2.00 1.1090.08 −40.9** 0.4690.03 0.5090.03 8.70 2.0390.04 2.1190.05 3.94 Chuxiong 8807
25.5* 0.3690.03 0.4690.02 27.8** 2.8390.08 2.9790.17
Mianyang 20 0.5590.02 0.5990.03 7.27 0.5590.04 0.6990.05 4.95
−5.83 0.3090.01 0.2890.01 −6.67** 2.5490.09 3.1490.08
0.9790.04 23.6*
0.6290.02
Dali 905 0.5290.02 −16.1* 1.0390.08
0.7390.02
0.5690.02 0.5790.03 1.79 0.8190.03 −9.88* 0.2590.03 0.3490.03 36.0** 2.7390.03 3.3190.06 21.2* Qian 14
−19.5** 0.3590.03 0.3990.03 11.4 2.5190.15 2.5690.09 1.99 Wenmai 3 0.5790.02 0.4590.03 −21.1* 0.8290.05 0.6690.04
15.0* 0.2490.04 0.3490.05 41.7** 3.2490.09 3.0290.16
0.9290.03 −6.79
0.6890.03
Yunmai 39 0.5690.03 −17.6* 0.8090.04
0.8790.01
0.6190.03 0.5290.02 −14.8* 0.8090.02 8.75** 0.2290.05 0.3890.04 72.7** 2.7690.15 2.6390.14 −4.71 Mianyang 26
−5.26 0.2790.02 0.2690.02 −3.70 2.8190.02 2.9090.03
Wenmai 5 0.5990.04 0.5390.05 −10.2 0.7690.03 0.7290.02 3.20*
−3.49 0.2490.02 0.3090.01 25.0* 2.4590.08 2.4890.09
0.8390.03 1.22
0.4790.03
Liaochun 9 0.3990.02 −17.0* 0.8690.02
0.8390.03
0.5990.04 0.5290.04 −11.9 0.9390.04 −10.8* 0.3290.03 0.4190.04 28.1** 2.1090.14 3.0990.18 47.1* Lanzhou 80101
−8.99* 0.2990.02 0.4590.03 55.2* 2.6590.11 3.1390.20 18.1* Long 8425 0.6390.03 0.3890.03 −39.7** 0.8990.02 0.8190.04
−3.26 0.2990.02 0.3990.03 34.5* 2.5090.09 2.8790.08
0.8990.03 14.8*
−20.0* 0.9290.06 Longchun 8139 0.7090.04 0.5690.03
0.7290.02
0.6390.03 0.5290.02 −17.5** 0.7390.02 −1.37 0.3890.02 0.3790.02 −2.63 2.2490.02 3.3090.03 47.3** Longchun 16
−7.70* 0.3790.02 0.4490.03 18.9* 2.2890.02 3.3490.03 46.5** MY 94-9 0.5390.04 0.3890.05 −28.3* 0.7890.05 0.7290.04
−3.70 0.4290.02 0.5690.04 33.3* 3.0190.14 3.2690.13
0.7890.02 8.31
0.8190.03 Longchun 15 0.5790.02 0.4790.03 −17.5*
Huining 18 0.6890.05 0.5190.02 −25.0* 0.6990.05 0.8790.06 26.1** 0.3990.04 0.5790.05 46.2* 4.9990.32 2.5190.29 −49.7**
results from studies with cucumber suggest that intraspecific responses could be at least partly due to inherent intraspecific differences in the accumu-lation of leaf flavonoids (Teramura and Murali, 1986). The study by Reuber et al. (1996) suggested that a flavonoid mutant of barley (Hordeum
6ulgare) exhibited increased sensitivity to UV-B
radiation especially in the primary leaf. The con-tent of flavonoid in barley mutant was only 7% as compared with the mother variety in the primary leaf. Differences in UV-absorption characteristics between the mutant and the mother line were clear from the spectrophotometric analysis (Reuber et al. 1996).
For barley cv. Atlas,L-phenylalanine ammonia-lyase (PAL) was assumed to play a key role in the UV-B acclimatization by a synthesis of flavonoids (Liu and McClure, 1995). In primary leaves of barley cv. Gerbel, the UV-mediated increased ac-cumulation of flavonoid was closely correlated with increased activity and amount of immuno-logically detectable chalcone synthase (CHS). In leaves of several plant species, including rye
(Secale cereale), CHS was found to play a major
role in UV regulation of the flavonoid pathway (Reuber et al., 1996). In another experiment, the substantial suppression of growth of A. thaliana
flavonoid mutants by UV-B radiation suggests that the disruption of secondary metabolism at the chalcone isomerase site caused an impairment of the normal growth and differentiation process (Fiscus et al., 1999).
A considerable amount of data demonstrated how UV radiation altered membrane structure or function: inhibited K+-ATPase and peroxidized
lipids in T. aesti6um (Wright et al., 1981), and
decreased membrane resistance inChara corallina
(Doughty and Hope, 1973). The damage to non-photosynthetic membranes that was detected by electron microscopy generally required high fluence or occurred only after a long lag time following irradiation. In the latter case, the effect of UV-B can be regarded as an acceleration of normal senescence processes (Skokut et al., 1977). Damage to components of isolated plant mem-branes can also be determined chemically, lipid damage required high fluence; inactivation of AT-Pases occurred at lower fluence. The physiological
effects of UV-B stimulated membrane changes are uncertain. There is little evidence that the UV damage to membranes is responsible for cell death. UV-stimulated membrane changes may play a role in the UV-induced synthesis of an-thocyanins (Murphy, 1983).
Intraspecific differences in physiological re-sponses of crop cultivars to enhanced UV-B radi-ation under field conditions were complex as observed in most of the 20 wheat cultivars in this paper. However, out of 20 wheat cultivars, the ranking of every indicator was different (Table 3). Correlations between the indicators were not ob-served (P\0.05). In a flavonoid mutant of barley
(H.6ulgare), the low flavonoid content primary in
leaves was correlated with a decline in apparent quantum yield and the less robust appearance of the mutant plants as compared with the mother variety (Reuber et al., 1996). The responses of SOD activity and MDA contents to enhanced UV-B radiation were not well known. UV-B radi-ation may induce the production of peroxy free radical, such as O2
−, H
2O2, resulting in membrane
lipid peroxidation, which lead to changes in mem-brane structure, and alter memmem-brane permeability finally (Murphy, 1990).
L.Yuan et al./En6ironmental and Experimental Botany44 (2000) 95 – 103
102
Table 3
Intraspecific sensitivity to UV-B radiation based on percent change in physiological characteristics of 20 wheat cultivars under field conditionsa
Flavonoid content SOD activity
Rank Chlorophyll (a+b) Membrane permeability MDA content
Fengmai 24 (16.2) Huining 18 (26.1)
1 Wenmai 5 (0.53) Mianyang 26 (72.7) Longchun 16 (47.3)
Mianyang 20 (7.27) Mianyang 20 (25.5)
YV 97-31 (−8.24) Long 8425 (55.2)
2 Lanzhou 80101 (47.1)
YV 97-31 (5.67) Fengmai 24 (16.0)
3 Huining 18 (−9.52) Yunmai 39 (41.7) MY 94-9 (46.5)
Qian 14 (1.79) Yunmai 39 (15.0)
Qian 14 (−13.9) Huining 18 (46.2)
4 Wenmai 5 (3.20)
Longchun 16
5 Chuxing 8807 (−2.00) Mianyang 26 (8.75) Qian 14 (36.0) Dali 905 (23.6) (−15.5)
Mianyang 20 Wenmai 5 (−10.2)
6 Fan 19 (8.22) Longchun 15 (33.3) Qian 14 (21.2)
(−19.1)
7 Longchun 15 Fan 19 (−9.43) Bi 90-5 (3.08) Longchun 8139 (34.5) Long 8425 (18.1) (−20.9)
Liaochun 9 (2.05) Lanzhou 80101
Chuxing 8807
8 Longchun 16 Longchun 8139 (14.8)
(−22.2) (−11.9) (−1.37) Mianyang 26 (−14.8) Longchun 8139
Dali 905 (−26.7) Mianyang 20 (27.8)
9 YV 97-31 (11.6)
(−3.26) Dali 905 (−16.1)
10 Bi 90-5 (−29.3) Liaochun 9 (−3.49) Lanzhou 80101 (28.1) Longchun 15 (8.31) Liaochun 9 (−17.0) Longchun 15
Fan 19 (−30.3)
11 MY 94-9 (18.9) Mianyang 20 (4.95)
(−3.70)
Longchun 16 (−17.5) Wenmai 5 (−5.26)
12 Yunmai 39 (−33.2) Fan 19 (16.7) Chuxing 8807 (3.94)
Longchun 15 Liaochun 9 (−37.3)
13 Dali 905 (−5.83) Chuxing 8807 (8.70) Wenmai 3 (1.99)
(−17.54)
Yunmai 39 (−17.6) YV 97-31 (−6.82)
14 Fengmai 24 (−38.2) Fengmai 24 (5.88) Liaochun 9 (1.22)
Longchun 8139 Bi 90-5 (−20.3) MY 94-9 (−7.70)
15 Wenmai 3 (11.4) Bi 90-5 (−3.87)
(−40.1)
Longchun 8139 Long 8425 (−47.0)
16 Long 8425 (−8.99) Wenmai 5 (−3.70) Mianyang 26 (−4.71)
(−20.0)
Lanzhou 80101 Wenmai 3 (−21.01
17 Qian 14 (−9.88) Longchun 16 (−2.63) Yunmai 39 (−6.79) (−48.6)
Huining 18 (−25.0) Lanzhou 80101 MY 94-9 (−48.5)
18 Bi 90-5 (−2.38) Fengmai 24 (−20.3)
(−10.8)
MY 94-9 (−28.3) Wenmai 3 (−19.5)
Wenmai 3 (−51.3) Dali 905 (−6.67)
19 Fan 19 (−22.3)
Mianyang 26 Long 8425 (−39.7) Chuxing 8807
20 YV 97-31 (−7.50) Huining 18 (−49.7)
(−55.6) (−40.9)
aRanking 1–20 is in the order of increasing sensitivity to UV-B radiation; * and **, significant difference between control and UV-B radiation atPB0.01 orPB0.05, according toT-test. Cultivars with the same ranking were preceded by a line. Values in parentheses represent percent change relative to control.
et al., 1993). In spinach and bean, however, such a relationship was not found, suggesting that in-traspecific differences in UV-B sensitivity may arise through a complex of specific responses rather than a generalized response (Murali and Teramura, 1986).
There are clearly important intraspecific differ-ences in response of the measured physiological parameters to enhanced UV-B radiation. Since no correlation between these effects and the response index was found, other parameters may determine
the sensitivity to UV-B.
Acknowledgements
References
Arnon, D.I., 1949. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta 6ulgaris. Plant Physiol. 24,
1 – 15.
Caldwell, M.M., 1971. Solar UV-B irradiation and the growth and development of higher plant. In: Giese, A.C. (Ed.), Photophysiology, vol. 6. Academic Press, New York, pp. 131 – 171.
Caldwell, M.M., Teramura, A.H., Tevini, M., Bornman, J.F, Bjo¨rn, L.O., Kulandaivelu, G., 1995. Effects of increased solar ultraviolet radiation on terrestrial plants. Ambio 24, 166 – 173.
Doughty, C.J., Hope, A.B., 1973. Effects of ultraviolet radia-tion on the membranes ofChara corallina. J. Membr. Biol. 13, 185 – 198.
D’surney, S.J., Tschaplinski, T.J., Edwards, N.T., Shugart, L.R, 1993. Biological responses of two soybean cultivars exposed to enhanced UV-B radiation. Environ. Exp. Bot. 33, 347 – 356.
Fan, S., Blake, T.J., 1994. Abscisic acid induced electrolyte leakage in woody species with contrasting ecological requirements. Physiol. Plant 90, 414 – 419.
Fiscus, E.L., Philbeck, R., Britt, A.B., Booker, F.L., 1999. Growth ofArabidopsisflavonoid mutants under solar radi-ation and UV filters. Environ. Exp. Bot. 41, 231 – 245. Giannopolitis, C.N., Ries, S.K., 1977. Superoxide dismutases.
I. Purification and quantitative relationship with water-soluble protein in seedling. Plant Physiol. 59, 315 – 318. Heath, R.I., Packer, L., 1968. Photoperoxidation in isolated
chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189 – 198. Kerr, R.A., 1993. The ozone hole reaches a new low. Science
26, 501.
Li, J., Ou-Lee, T.-M., Raba, R., Amundson, R.G., Last, R.L., 1993.Arabidopsisflavonoid mutants are hypersensitive to UV-B radiation. Plant Cell 5, 171 – 179.
Li, Y., Yue, M., Wang, X.L., 1998. Effects of enhanced ultraviolet-B radiation on crop structure, growth and yield components of spring wheat under field conditions. Field Crops Res. 57, 253 – 263.
Li, Y., Yue, M., Wang, X.L., Hu, Z.D., 1999. Competition and sensitivity of wheat and wild oat exposed to enhanced UV-B radiation at different densities under field condi-tions. Environ. Exp. Bot. 41, 47 – 55.
Li, Y., Zu, Y.Q., Chen, H.Y., Chen, J.J., Yang, J.L., Hu, Z.D., 2000. Intraspecific responses in crop growth and yield of 20 wheat cultivars to enhanced ultraviolet-B radia-tion under field condiradia-tions. Field Crops Res., in press. Liu, L., McClure, J.W., 1995. Effects of UVB on activities of
enzymes of secondary phenolic metabolism in barley pri-mary leaves. Physiol. Plant 93, 734 – 739.
Lydon, J., Teramura, A.H., Summers, E.G., 1986. Effects of ultraviolet-B radiation on growth and productivity of
field-grown soybean. In: Worrest, R.C. (Ed.), Stratospheric Ozone Reduction. Solar Ultraviolet Radiation and Plant Life. Springer, Berlin, pp. 313 – 325.
Madronich, S., Mckenzie, R.L., Caldwell, M.M., Bjo¨rn, L.O., 1995. Changes in ultraviolet radiation reaching the earth’s surface. Ambio 24, 143 – 153.
Mirecki, R.M., Teramura, A.H., 1984. Effects of ultraviolet-B irradiance on soybe. V. The dependence of plant sensitivity on the photosynthetic photon flux density during and after leaf expansion. Plant Physiol. 74, 475 – 480.
Murali, N.S., Teramura, A.H., 1986. Intraspecific differences in Cucumis sati6us sensitivity to ultraviolet-B radiation.
Physiol. Plant 68, 673 – 677.
Murphy, T.M., 1983. Membranes as targets of ultraviolet radiation. Physiol. Plant 58, 381 – 388.
Murphy, T.M., 1990. Effects of broad-band ultraviolet and visible radiation on hydrogen peroxide formation by cul-tured rose cells. Physiol. Plant 80, 63 – 68.
Reuber, S., Bornman, J.F., Weissenbo¨ck, G., 1996. A flavonoid mutant of barley (Hordeum6ulgareL.) exhibits
increased sensitivity to UV-B radiation in the primary leaf. Plant Cell Environ. 19, 593 – 601.
Sakaki, T., Kondo, N., Sugahara, K., 1983. Breakdown of photosynthetic pigments and lipid in spinach leaves with ozone fumigation: role of active oxygen. Physiol. Plant 59, 28 – 34.
Skokut, T.A., Wu, J.G., Daniel, R.S., 1977. Retardation of ultraviolet light accelerated chlorosis by visible light or by benzyladenine in Nicotiana glutinosa leaves: changes in amino acid contents and chloroplast ultrastructure. Pho-tochem. Photobiol. 25, 109 – 118.
Sullivan, J.H., Teramura, A.H., 1990. Field study of the interaction between solar ultraviolet-B radiation and drought on photosynthesis and growth in soybean. Plant Physiol. 92, 141 – 146.
Teramura, A.H., 1983. Effects of ultraviolet-B radiation on the growth and yield of crop plants. Physiol. Plant 58, 415 – 427.
Teramura, A.H., Murali, N.S., 1986. Intraspecific differences in growth and yield of soybean exposed to ultraviolet-B radiation under greenhouse and field conditions. Environ. Exp. Bot. 26, 89 – 95.
Teramura, A.H., Ziska, L.H., Sztein, A.E., 1991. Changes in growth and photosynthetic capactiy of rice with increased UV-B radiation. Physiol. Plant 83, 373 – 380.
Tevini, M., Braun, J., Fieser, G., 1990. The protective function of the epideral layer of rye seedlings against ultraviolet-B radiation. Photochem. Photobiol. 53, 329 – 333.
Wright, L.A., Murphy, T.M., Travis, R.L., 1981. The effect of ultraviolet radiation on wheat root vesicles enriched in plasma membrane. Photochem. Photobiol. 33, 343 – 348. Yue, M., Li, Y., Wang, X.L., 1998. Effects of enhanced
ultraviolet-B radiation on plant nutrients and decomposi-tion of spring wheat under field condidecomposi-tions. Environ. Exp. Bot. 40, 187 – 196.