www.elsevier.comrlocateranireprosci
The causes of reduced fertility with cryopreserved
semen
P.F. Watson
Department of Veterinary Basic Sciences, Royal Veterinary College, Royal College Street, London NW1 0TU, UK
Abstract
Cryopreserved mammalian semen is generally acknowledged to have an impaired fertility by comparison with fresh semen. The reduction arises from both a lower viability post-thaw and sublethal dysfunction in a proportion of the surviving subpopulation. The reasons for the loss of
Ž
fertility are various. In this paper, factors affecting the proportion of survivors e.g., cold shock
.
susceptibility, cooling rate, diluent composition and osmotic stress and factors influencing
Ž
functional status of survivors e.g., membrane stability, oxidative damage, membrane receptor
.
integrity, nuclear structure are briefly reviewed. The possible effects of cryopreservation on the role of spermatozoa in the early stages of embryogenesis are considered. In the light of this review, indications for new approaches for improving the performance of cryopreserved semen are offered.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Spermatozoa; Cryopreservation; Fertilizing capacity; Sublethal damage; Capacitation;
Cryoprotec-tant
1. Introduction
Cryopreservation of semen has long been seen as a means of benefiting the breeding of animals of agricultural importance, and has been recognised as contributing to the conservation of endangered species and to overcoming aspects of male infertility in humans. Nevertheless, with the possible exception of bull semen, a lower fertility is
Ž .
E-mail address: [email protected] P.F. Watson .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
generally accepted as a consequence of cryopreservation. In this paper I want to offer a perspective on the causes of lower fertility.
Cryopreserved bull semen has been used commercially in dairy cattle for decades and conception results are now comparable or better than with natural mating. However, this is not so for the majority of mammalian species where fertility is clearly reduced by the cryopreservation protocol. Indeed, the losses from cryopreservation are compensated by the insemination of larger numbers of spermatozoa, and this is true for cattle semen as well as other species. The difference between species is explained by the extent of the compensation that can be achieved in cattle, where relatively few spermatozoa are required for good fertility, complete compensation can be achieved by inseminating of the order of 20 million total spermatozoa, still allowing for sufficient dilution of semen to make the process commercially viable. With other species many more spermatozoa are necessary to achieve reasonable fertility, and the losses associated with cryopreserva-tion are such as to preclude the process being commercially viable. As a generalisacryopreserva-tion, some 40–50% of the population do not survive cryopreservation even with optimised protocols. When comparisons are made on the basis of similar numbers of motile Žassumed viable spermatozoa, results are still generally poorer than with fresh semen,. indicating that even the viable subpopulation after cryopreservation is compromised. An approach to overcome the problem in some species has been to utilise surgical insemination permitting the inseminate to be introduced high in the reproductive tract to achieve a more satisfactory fertility, but at the higher cost of surgery.
This phenomenon can be explained by the need to have a sufficient number of fully competent spermatozoa capable of achieving fertilisation over the period when ovulation is likely to occur. The isthmus of the oviduct acts as a functional sperm reservoir providing a source of potentially fertilising spermatozoa over the ovulatory period ŽHunter, 1984; Hunter and Nichol, 1983 . Only a minute proportion of spermatozoa. introduced into the lower reaches of the female tract enter the oviducal reservoir, the majority being expelled through the vulva or phagocytosed in the tract. In the ampulla at
Ž .
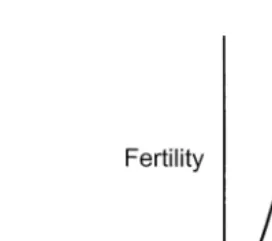
the time of fertilisation, the sperm:oocyte ratio approaches unity Hunter, 1996 . The numerical and temporal competency of this sperm cohort depends on both the number and quality of spermatozoa introduced into the lower reaches of the tract. Apart from maternally-related aspects, conception at artificial insemination can be seen as a probability event determined by considerations of the inseminate. When the sperm number or quality in the inseminate is reduced fertility declines on an exponential curve ŽFig. 1 ; normally, insemination is performed with sufficient competent spermatozoa to. achieve results on the asymptote of this curve. Both the slope of the curve and the asymptote vary with individual sire and can be influenced by cryopreservation
proce-Ž .
dures Amann and Hammerstedt, 1993 . If the total number of fully functional spermato-zoa in a cryopreserved inseminate falls below the number needed to achieve a high probability of fertilisation, then fertility is reduced.
When the inseminate is placed higher in the female tract than is normally the case with artificial insemination, fewer spermatozoa are required to achieve the same probability of fertility since a greater proportion will survive to colonise the oviduct. Thus, with sheep, intrauterine insemination is far more effective than posterior cervical
Ž .
Fig. 1. The theoretical relationship between sperm number in the inseminate and fertility. Normally, for artificial insemination, the sperm dose should be sufficient to reach the asymptote. Both the slope and the maximum response are determined by semen characteristics.
spermatozoa were required to achieve greater than 50% fertility, whereas it is recognised that cervical insemination requires 10 times that dose. If the insemination was made into
Ž .
the oviduct itself, less than 1 million spermatozoa were needed Maxwell et al., 1993 . Another observation relating to cryopreserved semen is manifested with boar semen. In the pig, ovulation can occur over an extended period of oestrus such that spermatozoa
Ž .
may be required to survive up to 40 h in the oviduct. Waberski et al 1994 have noted that fertility with cryopreserved semen may be high providing the insemination is made in the period 4 h before ovulation. Outside this period, fertility with cryopreserved spermatozoa declined dramatically, but fresh semen maintained its fertility for a much longer period. Cryopreserved spermatozoa do not survive in the female tract compared with fresh spermatozoa.
In summary, the cryopreservation process results in reduced fertility compared with fresh semen. It has been shown that this arises from a combination of both loss of sperm viability and an impairment of function in the population of survivors. This situation needs to be borne in mind when strategies to improve the results are contemplated. We need to consider not only the cryopreservation protocol to optimise the number of survivors, but also the functional ability of the surviving population.
2. Increasing the proportion live
The cryopreservation protocol has a number of potentially damaging stresses: firstly, the change in temperature; secondly, the osmotic and toxic stresses presented by exposure to molar concentrations of cryoprotectants; and thirdly, the formation and dissolution of ice in the extracellular environment. We shall discuss these in turn.
2.1. Change in temperature
It has long been known that cooling ungulate semen too rapidly between 308C and 08C induces a lethal stress in some of the cells proportional to the rate of cooling, the
Ž .
known as cold shock, also variably affects a number of other species and, as a result, cooling in this range prior to cryopreservation is usually conducted carefully.
In boar spermatozoa, the phenomenon is mostly manifested immediately after ejaculation but the cells become progressively less sensitive over the next few hours ŽPursel et al., 1972 . We have investigated this phenomenon, and found that boar sperm. incubated at room temperature in their own seminal plasma become quite resistant to
Ž .
cold shock over 16 h after ejaculation Tamuli and Watson, 1994 . The membranes are altered such that they do not respond to the cold stress. This observation suggests that the ideal time to cryopreserve boar semen may not be as soon after ejaculation as possible, i.e. within 6 h, as is commonly practised, but after 18–24 h when the resistance is at a maximum. Modern diluents now permit the maintenance of a high proportion of spermatozoa in a viable state for at least 24 h.
Even with slow cooling, however, temperature change induces stresses on mem-branes. It is probable that these are related to phase change in lipids and altered functional state of membranes. Cold shock is then seen merely as the extreme state of a continuum of stress, influenced by the rate of onset of the phenomenon. Such stresses on the membranes may be continued below 08C since phase changes are not complete at
Ž .
08C, but our preliminary studies unpublished were unable to show cold shock injury in bull spermatozoa below 08C. However, it is well known that a major phase change
Ž .
occurs in the vicinity of 5–158C Drobnis et al., 1993 , and this may well be the prime temperature range for temperature dependent injury.
The suggestion that membrane injury results from phase events in the lipid bilayer is well attested. Freeze fracture studies of membranes before, during and after cooling show clear evidence of phase separation events, which are only partially reversed after
Ž . Ž .
rewarming Holt and North, 1984; de Leeuw et al., 1990 . Pettitt and Buhr 1998 have shown the importance of modulation of the lipid environment of the plasma membranes during cooling, implicating the lipid component in mechanisms of injury.
Nevertheless, one should not overlook the other membrane elements that may be altered by temperature stress. Integral membrane proteins are clustered by lipid phase separation, and this may be expected to alter function, especially of proteins which undergo a structural modulation to carry out their function, such as ion channel proteins.
Ž
Indeed, it is known that membrane permeability is increased after cooling Robertson .
and Watson, 1986; Robertson et al., 1988 and this may be due to a generally increased membrane leakiness, but could be due to effects on specific protein channels. Calcium regulation is clearly affected by cooling and this undoubtedly has serious consequences
Ž .
in terms of cell function Bailey and Buhr, 1994 ; in severe cases, the change may be incompatible with continuing cell viability. The uptake of calcium during cooling
Ž .
contributes both to capacitative changes see below and fusion events between the plasma membrane and underlying outer acrosomal membrane. There are strong similari-ties between membrane damage during cooling and the acrosome reaction, the one being a disorganised version of the other.
In addition, cytoskeletal elements are temperature-sensitive. The cooling of other Ž
cells results in a premature depolymerisation of actin filaments Hall et al., 1993;
. Ž .
membrane and the underlying outer acrosomal membrane promoting acrosomal exocyto-sis. Perhaps, this also could contribute to a disorganised fusion of membranes following cooling or cryopreservation.
2.2. CryopreserÕatiÕe stresses
The addition and removal of cryoprotectant in molar proportions applies a substantial but transient osmotic stress to the plasma membrane of spermatozoa, depending upon
Ž .
the relative permeability of the cryoprotectant Gao et al., 1993 . Generally, the Ž
cryoprotectant of choice for spermatozoa is glycerol or occasionally, dimethyl
sulphox-. Ž .
ide , which induces osmotic stresses. Gao et al 1995 have shown that when human spermatozoa were exposed to 1 M glycerol in a single step, the volume excursion exceeded tolerable limits both on addition and removal. They showed that the stress could be reduced to tolerable limits by stepwise addition and removal and this substantially improved the proportion of spermatozoa surviving.
Furthermore, spermatozoa are also sensitive to toxic effects of cryoprotectants, resulting in the unsuitability of some compounds commonly used for other cells being
Ž .
less useful for spermatozoa Storey et al., 1998 . Even with glycerol, care should be
Ž .
exercised in its use with spermatozoa Katkov et al., 1998 .
2.3. Ice crystal formation and dissolution
The stresses induced by ice crystal formation are mainly associated with the Ž
accompanying osmotic pressure changes in the unfrozen fraction Watson and Duncan, .
1988 . When a solution is cooled below the freezing point, ice crystals are nucleated and pure water crystallises out as ice. The solutes are dissolved in the remaining liquid water fraction and the osmotic strength of the solution rises. The proportion of the water crystallising out as ice, and hence the osmotic strength of the remaining solution, depends on the temperature — the lower the temperature, the smaller the unfrozen fraction and hence the higher the osmotic strength of the solution. It is generally recognised that the duration of exposure to such events should be minimised for optimal cell survival implying that the cooling rate should be rapid. However, the cooling rate must be slow enough to allow water to leave the cells by osmosis preventing intra-cellular ice formation which is lethal. Sperm cells are generally frozen at quite rapid rates in the range 15–608Crmin, which have been empirically determined as giving the best survival rates. Obviously, if the cell water permeability and its activation energy were known it should be possible to predict the maximal cooling rate compatible with osmotic equilibrium and so determine optimal cryopreservation protocols. Such
consid-Ž .
erations have been shown to be important for other cell types Mazur, 1984 .
We and others have measured the water permeability of the membranes of
spermato-Ž .
zoa from a number of species. With the exception of the rabbit Curry et al., 1995a , most species seem to have a rather high water permeability in comparison to the
Ž
permeability of other cell types Watson et al., 1992; Noiles et al., 1993, 1997; Gilmore .
et al., 1996 . We have also shown that modulating the glucose channels of the sperm
Ž .
However, when these figures for water permeability are used in equations to calculate the maximum cooling rate compatible with approximate osmotic equilbrium during
Ž cooling, the rate works out much higher than is known empirically to be optimal Curry
.
et al., 1994 . We have, therefore, examined the assumptions on which the calculations are based. The first assumption is that the water permeability of the membrane remains unchanged in the presence of cryoprotectant, an assumption recently shown to be
Ž .
questionable Gilmore et al., 1998 although the magnitude of the change by no means accounted for all the discrepancy. Another possibility is that the permeability of the sperm plasma membrane is regionally variable. The assumption that a single value applies to the whole cell is unlikely to be true given the known regionalization of the sperm plasma membrane. Thus, a measured value may not represent, say, the sperm
Ž head when the majority of the water transport may occur across the tail membrane Holt
.
and North, 1994 . Alternatively, the techniques for estimating water permeability simply overestimate the true value. More recent methodology showed this to be the case but
Ž
again, the magnitude did not rectify the discrepancy Gilmore et al., 1996; Curry and .
Watson, unpublished . Fourthly, the activation energy measured above 08C may not reflect the activation energy below 08C, an hypothesis shown to be true for human spermatozoa but still inadequate to account for the discrepancy between calculated and
Ž .
empirical optimal cooling rates Noiles et al., 1993 . Perhaps, the discrepancy is explained by the sum of all these errors in the assumptions on which the theoretical calculations are based. A recent study with mouse spermatozoa has suggested that water permeability is markedly less at subzero temperatures in the presence of ice crystals and
Ž cryopreservatives, and in this instance, the discrepancy was largely eliminated
De-. vireddy et al., 1999 .
Nevertheless, it is possible that there are other stresses that are additional to those
Ž .
included in these calculations. Holt and North 1994 have shown that the signs of distress were manifested during rewarming and that these were related to osmotic stress, although not necessarily implying that the damage occurred during rewarming phase.
These considerations led us to hypothesise that there were indeed other factors determining the optimal cooling rate independent of the risk of intracellular ice formation. We and others have shown the extreme sensitivity of sperm membranes to osmotic stresses. We believe these may determine the optimum cooling rate, not by permeability to water but by the rate of displacement required of the plasma membrane to accommodate the volume change, and we suggested that this may stress the
Ž .
attachments of the cytoskeleton Watson, 1995 . In support of this hypothesis, when Ž
mouse or Koala spermatozoa were exposed to cytochalasin D which disrupts f-actin .
filaments they were more able to withstand extremes of osmotic stress without
Ž .
membrane rupture Noiles et al., 1997; Holt and Johnston, 1999 . It appears that we need a more elaborate theory of plasma membrane disruption during cryopreservation than is currently in vogue.
however, are not determined stochastically. The fact that indiÕiduals can often be
classified as ‘‘good freezers’’ or ‘‘bad freezers’’ implies that certain characteristics of membrane structure, which may be genetically determined, predispose towards survival under cryopreservation stress. Attempts to modify lipid composition have not demon-strated any dramatic benefits, perhaps implying that other membrane elements may be more important, e.g. cytoskeleton. This is an area for further investigation.
Nevertheless, even if we optimise the process and minimise the cell death, there will still be a proportion of cells which fail to survive. We need, therefore, to concentrate on the function of the surviving population.
3. Increasing the quality of survivors
3.1. Capacitation-like changes
A significant breakthrough in understanding has come with the recognition that
Ž .
cooled and rewarmed spermatozoa behave as if they were capacitated Watson, 1995 . However, we are unsure at present whether spermatozoa manifest the changes of capacitation or simply are enabled to by-pass capacitation and proceed directly to acrosomal exocytosis. We have begun to study this phenomenon and have shown that cooled spermatozoa display chlortetracycline staining and an increase in intracellular free Ca2q, typical of capacitated spermatozoa, but that the tyrosine phophorylation
Ž
patterns are somewhat different in cooled and rewarmed spermatozoa Watson and
. 2q
Green, in press . Fig. 2 shows the free Ca in cooled spermatozoa compared with that seen in capacitated spermatozoa. We are continuing to investigate these events to determine the nature of the membrane changes resembling capacitation.
Fig. 2. The intracellular free Caq response of boar spermatozoa either to incubation in a capacitating medium or to cooling. The illustrations are of ‘‘dot plots’’ from the flow cytometer of spermatozoa labelled with the
Ž .
viability probe, propidium iodide vertical axis, FL3 LOG and a calcium-sensitive indicator, Fluo-3 AM ester
Žhorizontal axis, FL1 LOG . A Control at time zero, B after 2-h incubation at 39. Ž . Ž . 8C in modified Tyrode’s
Ž . Ž .
medium incubation at ambient temperature does not produce this change , and C after slow cooling at 0.188Crmin to 58C and rapid rewarming to 398C. Area P represents dead cells, area Q represents cells with a low calcium concentration and area R represents those with high calcium concentration. A strong similarity
Ž . Ž .
3.2. Motility impairment
One obvious characteristic of cryopreserved spermatozoa is the decline in motility of the cells. While a minority seem to exhibit vigorous forward progression, the majority show a variable degree of impairment. This would seem to be an important contribution to their relatively poor fertilising potential when introduced into the reproductive tract at artificial insemination. A study of cryopreserved human spermatozoa under IVF
condi-Ž tions found that both motility and forward progression were important factors Kelly et
.
al., 1997 . However, one recent observation reached a different conclusion; in a study of in vitro fertility of asthenoteratozoospermic dogs, the quality of motility though poor did
Ž .
not impair fertility Hewitt and England, unpublished . These studies relate only to events associated with the final contact with the oocyte; transport to the site of fertilisation may still require a minimum capability of forward motility, which may be compromised in the majority of cryopreserved spermatozoa.
3.3. OxidatiÕe damage
More important is the observation that oxidative damage could impair sperm func-tion. This subject is difficult to tackle because of the recognition that a degree of
Ž superoxide radical formation is necessary for the events preceding fertilisation de
.
Lamirande et al., 1997 . However, it is now generally accepted that cryopreservation induces the formation of reactive oxygen species that are detrimental to subsequent
Ž .
performance Alvarez and Storey, 1992; Bell et al., 1993; O’Flaherty et al., 1997 . Much of the published work on lipid peroxidation and cryopreservation relates to work on human spermatozoa.
The use of antioxidants in cryopreservation diluents is not commonplace, partly because there are a number of possible agents from which to select, and partly because
Ž .
different pathways and hence, different antioxidants are utilised in different species ŽAskari et al., 1994; O’Flaherty et al., 1997 . Moreover, it is clear that oxidative damage.
Ž is only one of a number of stresses the cryopreserved sperm cell experiences Alvarez
.
and Storey, 1993 . This is clearly an area for further investigation.
3.4. Surface changes affecting recognition of receptors
An issue that has been recently examined is the extent to which the surviving Ž
population is impaired in its ability to interact with the oviductal epithelium Ellington et .
al., 1999 . These interactions are now recognised as receptor–ligand interactions, often involving intracellular signalling mechanisms, which are actively being explored. A
Ž
similar interaction involves spermatozoa and the oocyte and its vestments Bwanga et .
al., 1991; Oehninger et al., 1993 . The freeze-fracture observations, suggesting that the clustering of membrane proteins during lipid phase separations induced by cooling are
Ž .
not entirely reversible see above , may well have implications for receptor–ligand interactions. Although the evidence from IVF studies generally indicates that
cryopre-Ž .
reality may be quite different when only a few spermatozoa reach the site of fertilisation Žsee above ..
3.5. Ability of sperm to sustain embryonic deÕelopment
Finally, another area that has not been adequately studied is the ability of cryopre-served spermatozoa to sustain embryonic development. Technically, this is difficult to investigate since the evidence of early embryonic death often passes without overt signs. Nevertheless, a persistent suggestion is that frozen–thawed spermatozoa are associated
Ž .
with an increased incidence of early embryonic mortality Salamon and Maxwell, 1995 . The potential mechanisms can now be studied more effectively. DNA damage can be
Ž .
investigated with the COMET assay Hughes et al., 1996 or with flow cytometry ŽKarabinus et al., 1990; Royere et al., 1991 and may indicate functional damage to the.
Ž .
nuclear structures Ellington et al., 1998 . Moreover, the suggestion that the spermato-Ž
zoon’s contribution to the zygote is more than the haploid male genome Navara et al., .
1995 implies that there are more subtle ways in which the spermatozoon’s function could be disrupted during cooling and cryopreservation. The post-syngamy fate of other
Ž
sperm structures is now known better than ever before Sutovsky et al., 1996;
Sathanan-. Ž
than et al., 1997 , and the possible importance of sperm RNA Rohwedder et al., 1996; .
Miller, 1997 to the events before the embryonic genome is activated cannot be disregarded. Any of these structures may suffer alteration during cryopreservation and can all now be investigated.
4. Conclusion
The effects that cryopreservation can induce in spermatozoa, ranging from lethal injury to those which merely impair subsequent function, are numerous. In the last few years, the considerable increase in our understanding both of the cell physiology of spermatozoa and of the stresses of cryopreservation has contributed to a renewed interest in improving the performance of cryopreserved semen.
Today, the biotechnological applications of cryopreservation enjoy an interest which is unprecedented. Sperm freezing for human infertility, for addressing adverse fertility consequences of other life-threatening diseases, for conservation and for animal agricul-ture all clamour for successful cryopreservation techniques. We can look forward to a very productive period in our studies.
References
Alvarez, J.G., Storey, B.T., 1992. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J. Androl. 13, 232–241.
Amann, R.P., Hammerstedt, R.H., 1993. In vitro evaluation of sperm quality: an opinion. J. Androl. 14, 397–406.
Askari, H.A., Check, J.H., Peymer, N., Bollendorf, A., 1994. Effect of antioxidants tocopherol and ascorbic acids in maintenance of sperm activity during freeze–thaw process. Arch. Androl. 33, 11–15.
Bailey, J.L., Buhr, M.M., 1994. Cryopreservation alters the Caqqflux of bovine spermatozoa. Can. J. Anim. Sci. 74, 45–51.
Bell, M., Wang, R., Hellstrom, W.J., Sikka, S.K., 1993. Effect of cryoprotective additives and cryopreserva-tion protocol on sperm membrane lipid peroxidacryopreserva-tion and recovery of motile human sperm. J. Androl. 14, 472–478.
Bwanga, C.O., Hofmo, P.O., Grevle, I.S., Einarsson, S., Rodriguez-Martinez, H., 1991. In vivo fertilizing capacity of deep-frozen boar semen packaged in plastic bags and maxi-straws. Zentralbl. Veterinarmed., Reihe A 38, 281–286.
Curry, M.R., Millar, J.D., Watson, P.F., 1994. Calculated optimal cooling rates for ram and human sperm cryopreservation fail to conform with empirical results. Biol. Reprod. 51, 1014–1021.
Curry, M.R., Redding, B.J., Watson, P.F., 1995a. Determination of water permeability coefficient and its activation energy for rabbit spermatozoa. Cryobiology 32, 175–181.
Curry, M.R., Millar, J.D., Watson, P.F., 1995b. The presence of water channel proteins in ram and human spermatozoa. J. Reprod. Fertil. 104, 297–303.
de Lamirande, E., Jiang, H., Zini, A., Kodama, H., Gagnon, C., 1997. Reactive oxygen species and sperm physiology. Rev. Reprod. 2, 48–54.
de Leeuw, F.E., Chen, H.-C., Colenbrander, B., Verkleij, A.J., 1990. Cold-induced ultrastructural changes in bull and boar sperm plasma membranes. Cryobiology 27, 171–183.
Devireddy, R.V., Swanlund, D.J., Roberts, K.P., Bischof, J.C., 1999. Subzero water permeability parameters of mouse spermatozoa in the presence of extracellular ice and cryoprotective agents. Biol. Reprod. 61, 764–775.
Donaghue, A.M., Johnson, L.A., Seal, U.S., Armstrong, D.L., Simmons, L.G., Gross, T., Tilson, R.L., Wildt,
Ž .
D.E., 1992. Ability of thawed tiger Panthera tigris spermatozoa to fertilize conspecific eggs and bind and penetrate domestic cat eggs in vitro. J. Reprod. Fertil. 96, 555–564.
Drobnis, E.Z., Crowe, L.M., Berger, T., Anchordoguy, T., Overstreet, J.W., Crowe, J.H., 1993. Cold shock damage is due to lipid phase transitions in cell membranes: a demonstration using sperm as a model. J. Exp. Zool. 265, 432–437.
Ellington, J.E., Evenson, D.P., Fleming, J.E., Brisbois, R.S., Hiss, G.A., Broder, S.J., Wright, R.W., 1998. Coculture of human sperm with bovine oviduct epithelial cells decreases sperm chromatin structural changes seen during culture in media alone. Fertil. Steril. 69, 643–649.
Ellington, J.E., Samper, J.C., Jones, A.E., Oliver, S.A., Burnett, K.M., Wright, R.W., 1999. In vitro
Ž .
interactions of cryopreserved stallion spermatozoa and oviduct uterine tube epithelial cells or their secretory products. Anim. Reprod. Sci. 56, 51–65.
Gao, G.Y., Ashworth, E., Watson, P.F., Kleinhans, F.W., Mazur, P., Critser, J.K., 1993. Hyperosmotic tolerance of human spermatozoa: separate effects of glycerol, sodium chloride and sucrose on spermolysis. Biol. Reprod. 49, 112–123.
Gao, D.Y., Liu, J., Liu, C., McGann, L.E., Watson, P.F., Kleinhans, F.W., Mazur, P., Critser, E.S., Critser, J.K., 1995. Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum. Reprod. 10, 1109–1122.
Gilmore, J.A., Du, J., Tao, J., Peter, A.T., Critser, J.K., 1996. Osmotic properties of boar spermatozoa and their relevance to cryopreservation. J. Reprod. Fertil. 107, 87–95.
Gilmore, J.A., Liu, J., Peter, A.T., Critser, J.K., 1998. Determination of plasma membrane characteristics of boar spermatozoa and their relevance to cryopreservation. Biol. Reprod. 58, 28–36.
Hall, S.M., Evans, J., Haworth, S.G., 1993. Influence of cold preservation on the cytoskeleton of cultured pulmonary arterial endothelial cells. Am. J. Respir. Cell Mol. Biol. 9, 106–114.
Holt, W.V., Johnston, S.D., 1999. Enhanced osmotic tolerance of Koala spermatozoa following treatment with the cytoskeletal disrupting agent, cytochalasin D. In: Proceedings of the Annual meeting of the Australian Society for Reproductive Biology, September 1999.
Holt, W.V., North, R.D., 1994. Effects of temperature and restoration of osmotic equilibrium during thawing on the induction of plasma membrane damage in cryopreserved ram spermatozoa. Biol. Reprod. 51, 414–424.
Hughes, C.M., Lewis, S.E.M., McElvey-Martin, V.J., Thompson, W., 1996. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified Comet Assay. Mol. Hum. Reprod. 2, 613–619.
Hunter, R.H.F., Nichol, R., 1983. Transport of spermatozoa in the sheep oviduct: preovulatory sequestering of cells in the caudal isthmus. J. Exp. Zool. 228, 121–128.
Hunter, R.H.F., 1984. Pre-ovulatory arrest and periovulatory redistribution of competent spermatozoa in the isthmus of the pig oviduct. J. Reprod. Fertil. 72, 203–211.
Hunter, R.H.F., 1996. Ovarian control of very low spermregg ratios at the commencement of fertilisation to avoid polyspermy. Mol. Reprod. Dev. 44, 417–422.
Karabinus, D.S., Evenson, D.P., Jost, L.K., Baer, R.K., Kaproth, M.T., 1990. Comparison of semen quality in young and mature Holstein bulls measured by light microscopy and flow cytometry. J. Dairy Sci. 73, 2364–2371.
Katkov, I.I., Katkova, N., Critser, J.K., Mazur, P., 1998. Mouse spermatozoa in high concentrations of glycerol: chemical toxicity vs. osmotic shock at normal and reduced oxygen concentrations. Cryobiology 37, 325–338.
Kelly, M.P., Corson, S.L., Gocial, B., Batzer, F.R., Gutmann, J.N., 1997. Discontinuous Percoll gradient preparation for donor insemination: determinants for success. Hum. Reprod. 12, 2682–2686.
Maxwell, W.M.C., 1986. Artificial insemination of ewes with frozen thawed semen at a synchronised oestrus: II. Effect of dose of spermatozoa and site of intrauterine insemination on fertility. Anim. Reprod. Sci. 10, 309–316.
Maxwell, W.M.C., Evans, G., Rhodes, S.L., Hillard, M.A., Bindon, B.M., 1993. Fertility of superovulated ewes after intrauterine or oviducal insemination with low numbers of fresh or frozen–thawed spermatozoa. Reprod. Fertil. Dev. 5, 57–63.
Mazur, P., 1984. Freezing of living cells: mechanisms and implications. Am. J. Physiol. 247, C125–C142. Miller, D., 1997. RNA in the ejaculate spermatozoon: a window into molecular events in spermatogenesis and
a record of the unusual requirements of haploid gene expression and post-meiotic equilibration. Mol. Hum. Reprod. 3, 669–676.
Navara, C.S., Simerly, C., Zoran, S., Schatten, G., 1995. The sperm centrosome during fertilisation in mammals: implications for fertility and reproduction. Reprod. Fertil. Dev. 7, 745–754.
Noiles, E.E., Mazur, P., Watson, P.F., Kleinhans, F.W., Critser, J.K., 1993. Determination of water permeability coefficient for human spermatozoa and its activation energy. Biol. Reprod. 48, 99–109. Noiles, E.E., Thompson, K.A., Storey, B.T., 1997. Water permeability, Lp, of the mouse sperm plasma
membrane and its activation energy are strongly dependent on interaction of the plasma membrane with the sperm cytoskeleton. Cryobiology 35, 79–92.
Oehninger, S., Morshedi, M., Ertunc, H., Philput, C., Bocca, S.M., Acosta, A.A., Hodgen, G.D., 1993.
Ž .
Validation of the hemizona assay HZA I a monkey model: II. Kinetics of binding and influence of cryopreserved–thawed spermatozoa. J. Assist. Reprod. Genet. 10, 292–301.
O’Flaherty, C., Beconi, M., Beorlegui, N., 1997. Effect of natural antioxidants, superoxide dismutase and hydrogen peroxide on capacitation of frozen–thawed bull spermatozoa. Andrologia 29, 269–275. Pettitt, M.J., Buhr, M.M., 1998. Extender components and surfactants affect boar sperm function and
membrane behaviour during cryopreservation. J. Androl. 19, 736–746.
Pursel, V.G., Johnson, L.A., Rampacek, G.B., 1972. Acrosome morphology of boar spermatozoa incubated before cold shock. J. Anim. Sci. 34, 278–283.
Robertson, L., Watson, P.F., 1986. Calcium transport in diluted or cooled ram semen. J. Reprod. Fertil. 77, 177–185.
Robertson, L., Watson, P.F., Plummer, J.M., 1988. Prior incubation reduces calcium uptake and membrane disruption in boar spermatozoa subjected to cold shock. Cryo-Lett. 9, 286–293.
Royere, D., Hamamah, S., Nicolle, J.C., Lansac, J., 1991. Chromatin alterations induced by freeze-thawing influence the fertilizing ability of human sperm. Int. J. Androl. 14, 328–332.
Sathananthan, A.H., Tatham, B., Dharmawardena, V., Grills, B., Lewis, I., Trounson, A., 1997. Inheritance of centrioles and centrosomes in bovine embryos. Arch. Androl. 38, 37–48.
Salamon, S., Maxwell, W.M.C., 1995. Frozen storage of ram semen: II. Causes of low fertility after cervical insemination and methods of improvement. Anim. Reprod. Sci. 38, 1–36.
Saunders, K.M., Parks, J.E., 1999. Effects of cryopreservation procedures on the cytology and fertilization rate of in vitro-matured bovine oocytes. Biol. Reprod. 61, 178–187.
Spungin, B., Margalit, I., Breitbart, H., 1995. Sperm exocytosis reconstructed in a cell free system. Evidence for the involvement of phospholipase C and actin filaments in membrane fusion. J. Cell Sci. 108, 2525–2535.
Storey, B.T., Noiles, E.E., Thompson, K.A., 1998. Comparison of glycerol, other polyols, trehalose, and raffinose to provide a defined cryoprotectant medium for mouse sperm cryopreservation. Cryobiology 37, 46–58.
Sutovsky, P., Navara, C.S., Schatten, G., 1996. Fate of mitochondria, and the incorporation, conversion and disassembly of the sperm tail structures during bovine fertilization. Biol. Reprod. 55, 1195–1205. Tamuli, M.K., Watson, P.F., 1994. Cold resistance in the live acrosome-intact subpopulation of boar
spermatozoa acquired during incubation after ejaculation. Vet. Rec. 135, 160–162.
Waberski, D., Weitze, K.F., Gleumes, T., Schwarz, M., Willmen, T., Petzoldt, R., 1994. Effect of time of insemination relative to ovulation on fertility with liquid and frozen boar semen. Theriogenology 42, 831–840.
Ž .
Watson, P.F., 1981. The effects of cold shock on sperm cell membranes. In: Morris, G.J., Clarke, A. Eds. , Effects of Low Temperatures on Biological Membranes. Academic Press, London, pp. 189–218. Watson, P.F., 1995. Recent developments and concepts in the cryopreservation of spermatozoa and the
assessment of their post-thawing function. Reprod. Fertil. Dev. 7, 871–891.
Watson, P.F., Duncan, A.E., 1988. Effect of salt concentration and unfrozen water fraction on the viability of slowly frozen ram spermatozoa. Cryobiology 25, 131–142.
Watson, P.F., Green, C.E., 2000. Cooling and capacitation of boar spermatozoa: What do they have in
Ž .
common? Reprod. Dom. Anim., in press .