Water requirements of terrestrial and epiphytic orchid seeds and
seedlings, and evidence for water uptake by means of mycotrophy
Jay A. Yoder
a,*, Lawrence W. Zettler
a, Scott L. Stewart
baDepartment of Biology,The Illinois College,Jackson6ille,IL62650,USA
bUndergraduate Research Program in Biology,Department of Biology,The Illinois College,Jackson6ille,IL62650,USA
Received 28 December 1999; received in revised form 23 February 2000; accepted 25 February 2000
Abstract
The use of endomycorrhizal fungi as an energy source (=mycotrophy) initiates seedling development and supplements or replaces photosynthesis in all orchids in nature. Fungus-infected and non-infected seeds of the monkey face orchid,Platanthera integrilabia, a US Federally-threatened terrestrial species, had a different set of water relations than seeds of the green fly orchid,
Epidendrum conopseum, a subtropical epiphyte. Seeds of the terrestrial species had lower water loss rates, smaller activation energies for water loss and absorbed water from lower relative humidities. Thus, the epiphyte lacks the enhanced water retention capacity associated with the terrestrial species, implying that epiphytic orchids are capable of germinating quickly given an adequately moist substrate. After germination, water content of fungus-infected seeds was higher. These results provide first time fundamental information related to habitat preference by analyzing seed. Germination is considerably enhanced with mycorrhizal fungi that facilitate the absorption of free water by their orchid seed hosts. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Water relations; Mycorrhizal fungi; Protocorms; Orchidaceae; Mycotrophy
www.elsevier.com/locate/plantsci
1. Introduction
Orchids (Orchidaceae) utilize mycorrhizal fungi as an energy source to stimulate seed germination and seedling development [1]. Fungal hyphae make contact with the seed, enter via micropores, infect the embryo from the suspensor region, and penetrate adjacent cortical cells [2]. Upon infecting the cortex, masses of fungal hyphae (pelotons) are digested (=mycotrophy; [1]), supplying carbohy-drate that promotes seedling development [3]. Symbiotic seed germination has been re-created in the laboratory [4 – 8] and is of importance to or-chid cultivation and conservation. It has been assumed that the orchid’s alternative nutritional capability is partly responsible for the difficulties associated with asymbiotic (terrestrial) orchid
propagation. Because water imbibition triggers germination [9], it is conceivable that an additional role of the fungus may be to affect water levels of the seed. Developing seedlings may also utilize fungi as a water source to resist desiccation.
Adjustment of water levels is a function of water gain and water loss, a relationship called water balance [10,11]. To achieve water balance (gain=loss), orchid seeds must gain sufficient amounts of water to counter large losses of net water across the seed coat (testa) due to their small size (0.18 – 3.85 mm in length; [12]) and large surface area to volume ratio [3]. Water relations match moisture requirements for life in a particu-lar environment [11]. Thus, we would anticipate that the water balance strategies of orchids occu-pying different habitats may differ. This could be of considerable value for conservation programs, as it would help determine habitat preference and ecological suitability for cultivation and establish-ment of orchids into suitable areas.
* Corresponding author.
E-mail address:[email protected] (J.A. Yoder).
Accordingly, we compared water balance char-acteristics of orchid seeds and seedlings from two different habitats: a terrestrial, bog-inhabiting threatened species, the monkey face orchid, Pla
-tanthera integrilabia, native to the Cumberland Plateau (Tennessee) (north temperate) and an epi-phytic species, the green fly orchid, Epidendrum conopseum, native to Florida (subtropical). The impact of the presence of mycorrhizal fungi on the water balance profile of these seeds was also examined.
2. Methods
Seeds of P. integrilabia (Correll) Luer were col-lected from McMinn County, TN from mature yellowing capsules on ten to 15 inflorescences, and seeds of E. conopseum R. Brown were collected from a mature capsule from a specimen growing on the limb of a live oak (Quercus 6irginiana
Miller) in Alachua County, FL. Capsules were desiccated (0% RH, CaSO4, Drierite, 22 – 24°C),
and up to 16 days later, the seeds were removed and were stored for over 2 years at 692°C (LD 0:24 h). Mycorrhizal fungi, Epulorhiza inquilina
Currah, Zettler and McInnis and Epulorhiza sp. were isolated from each, respectively, maintained in culture on agar, and deposited into the Univer-sity of Alberta Microfungus Collection and Herbarium (UAMH 7809, 9203, respectively). Symbiotic seed germination was conducted using standard techniques [13,14]. Briefly, seeds of P.
integrilabiaand E. conopseum(50 – 300) were sown on modified oats medium in a 9-cm i.d. Petri plate [15]. A 1-cm3block of fungal mycelium was added
and plates were stored at 2192°C. Trypan blue (0.05%) staining [16] was used to confirm fungal infection. Seeds incubated in the absence of fungi (asymbiotic seed germination) served as controls. Once about 1 mm in length (developmental ‘Stage 2’; [17]), seedlings were transferred with an aspira-tor to a steel mesh grid held on a porcelain plate [18].
Mass measurements were made with an elec-trobalance (Model AD-4; Perkin-Elmer, Atlanta, GA); precision was 90.2 mg S.D. and accuracy
was 96 mg at 1 mg. Specimens were weighed and
monitored singly to conform with standard prac-tice; weighing took B1 min. Basic observations were conducted at 22 – 24°C, LD 14:10 h, and
other experiments used environmental cabinets (90.5°C). Relative humidities (92% RH) were generated as described [19,20] and CaSO4
pro-vided 0% RH [21].
To ensure that mass changes equal changes in water levels, specimens were held at 33% RH, 30°C, until 4 – 6% of weight had been lost [10]. Water content was calculated from the difference between initial and dry mass (obtained by placing specimens at 0% RH, 90°C, until mass remained constant, 3 – 5 days, plus 1 full day of drying). The amount of water lost is the water mass (m), and was expressed as a percentage [11]. Water loss rates were derived at 0% RH from the slope of a regression on a semi-logarithmic plot of mt/m0
(mt=water mass at time t and m0=initial water
mass) with 1-h weighing intervals for 12 h, and was expressed as %/h [10]. The critical transition temperature (CTT), the temperature threshold of a particularly rapid water loss, was determined simi-larly, except temperature was varied. Activation energies for water loss (Ea), from which the CTT is
derived, were determined from the slope of a regression on an Arrhenius plot (rate vs. reciprocal absolute temperature) [10]. The point of intersec-tion on the plot of two different Eas, where the
correlation coefficient, R, of each is ]0.95 [22], was defined as the CTT. Changes in mass were calculated for specimens after a day’s exposure to various % RH and were expressed as %/h. A 0% change in mass denotes the % RH where water balance (gain=loss) is estimated to be achieved under these test conditions and is the equilibrium humidity (EH). Results (mean9S.E.;n=45) were compared with an analysis of variance (ANOVA), using the arcsin transformation for percentage data and a test for the equality of several slopes [23].
3. Results and discussion
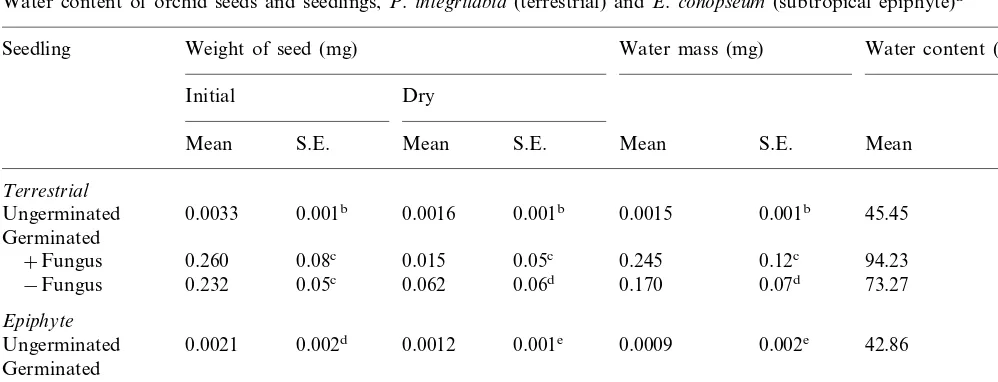
striking increase in initial mass and an approxi-mate doubling in percentage water content for seeds after germination (Table 1). Germinated seeds in contact with moisture, supplied by the water-based agar medium, showed a remarkable increase (30 – 50%) in water content (Table 1). Interestingly, seedlings infected with fungi had higher water contents than seedlings lacking fungi
(Table 1), and this effect was more pronounced in the terrestrial species.
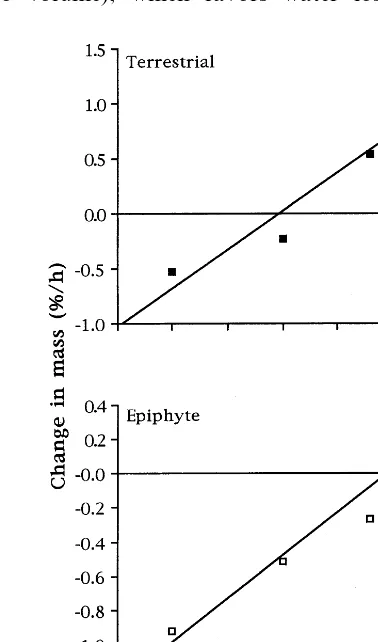
Water loss rates were nearly 2× higher for seeds of the epiphyte than the terrestrial (Table 2; Fig. 1). Over a broad temperature range (0 – 60°C), water retention varied inversely with temperature (RB0.95; Fig. 2), exhibiting a Boltzmann temper-ature function with no abrupt increase in water
Table 1
Water content of orchid seeds and seedlings,P.integrilabia(terrestrial) andE.conopseum(subtropical epiphyte)a
Weight of seed (mg)
Seedling Water mass (mg) Water content (mg)
Dry
Ungerminated 0.0033 0.001b 0.0016 Germinated
aValues followed by the same letter within a column are not significantly different (ANOVA;P\0.05;n=3 replicates each of
15 seedlings or until 45 individuals had been tested). Seeds germinated on potato dextrose agar (PDA, Difco, St. Louis, MO) yielded identical results.
Table 2
Ability to retain (water loss rate: activation energy for water loss,Ea) and to absorb (equilibrium humidity, EH) water by orchid
seeds and seedlings,P.integrilabia(terrestrial) and E.conopseum(subtropical epiphyte)a
Seedling Water loss Water gain from the air (EH) % RH
Ea, kJ/mol
Ungerminated 1.035 0.018b
Germinated
Ungerminated 0.78 0.07d 93–95d
Germinated
aThe slope of the regression corresponds to the activation energy. Seedlings were dehydrated at 33% (22–24°C) before use.
Fig. 1. Water loss from seeds of P. integrilabia (terrestrial) and E. conopseum (subtropical epiphyte) at 0% RH and 22 – 24°C;mtis the water mass at timet,m0is the initial water
mass (15 seeds per replicate;n=3; mean9S.E.50.004).
nated (Table 2). In all cases, the amount of water retained correlated positively with the amount of water in the air (Fig. 3; R]0.97; ANOVA; PB
0.001); the higher the % RH, the more water adsorbed. The terrestrial species took 1 month to germinate in the presence of fungi and 3 months to germinate in the absence of fungi. Seeds of the epiphyte took considerably less time to germinate: 2 – 3 weeks with fungi and 1 month without fungi. Seeds of both species held at 93% RH, 22 – 24°C, failed to germinate during the course of this investigation.
The only difference observed in this study be-tween seeds of the epiphyte,E.conopseum, and the terrestrial, P. integrilabia, was the faster rates of water loss by the epiphyte. This is likely due to smaller seed size of the epiphyte (large surface area to volume), which favors water loss, a poorly
Fig. 2. Arrhenius analysis for seeds ofP.integrilabia (terres-trial) and E.conopseum (subtropical epiphyte). No tempera-ture threshold (CTT, critical transition temperatempera-ture) of a particularly rapid water loss is apparent; the regression slope, corresponding to activation energy, is continuous (15 seeds per replicate;n=3; mean9S.E.50.021).
Fig. 3. Equilibrium humidity, gain=loss, determination of seeds of P. integrilabia (terrestrial) and E. conopseum (sub-tropical epiphyte). The equilibrium humidity (EH) is found by dropping a perpendicular line to thex-axis (% RH) from the point of intersection of the regression through the points and 0% mass change (15 seeds per replicate;n=3; mean9S.E.5 0.09).
loss (no CTT).Eawas higher for the epiphyte than
the terrestrial (Table 2), implying greater escape of water molecules through the seed coat of the epiphyte. After germination water loss rates were two to three times higher, with correspondingly higher Eas. No distinction with regards to water
loss could be made between germinated terrestrial and epiphytic seeds (Table 2), because they had been standardized for size (1 mm) and were devel-opmentally equivalent (‘Stage 2’). Equilibrium hu-midities were 84 – 86% RH for the terrestrial, close to saturation, 92 – 94% RH, for the epiphyte (Table 2; Fig. 3) and at or above saturation,
germi-water-proofed seed coat (as indicated by the larger
Ea), and probably various phylogenetic factors.
Consequently, seeds of the terrestrial show water gain from lower relative humidities (EH=84 – 86% RH) because they lose water less, while the faster water loss rate of the epiphyte masks the gains until relative humidities are close to saturation (EH=93 – 95%). Greater influx of water into the epiphytic seed as a result of being leaky is likely responsible for their more rapid germination time than the terrestrial, which reflects the high perme-ability to water of the epiphyte.
Evidence that symbiotic germination resulted in seeds with higher water contents than seeds germi-nated asymbiotically was a highlight of this study. That this effect was more pronounced in the ter-restrial species compared to the epiphyte is likely due to greater water retention capacity (lower water loss rate) than the epiphyte. By digesting masses of water-rich fungal hyphae (pelotons) in-ternally following infection, it is conceivable that the seedlings have immediate access to water; thus, the fungus itself may be the source of the water. The most likely scenario is that invading fungal hyphae promote water uptake by breaching the testa, or perhaps opening the micropores to water during the infection process, resulting in seedlings with higher water contents. Accelerated rates of germination are observed in orchid seeds with fungi [24] than without because the presence of fungi facilitates seed germination by enhancing water uptake of the seedling. Germination is de-layed for seeds in the absence of fungi because they lack the rapid increase of water acquisition associated with symbiotic seed germination.
Taken together, seeds of the epiphyte,E. conop
-seum, are hydrophilic with regards to water bal-ance. As such, their ability to gain water is more important than their ability to retain water. The emphasis is on water retention instead of gain for the terrestrial, P. integrilabia. For terrestrial or-chids, the water-repellent nature of the testa may facilitate seed dispersal on ground surfaces via water runoff, and slow water imbibition until the lipid layer is stripped by natural weathering [1]. By retaining water prior to germination, the embryo is protected on the surface of a dry terrestrial substrate, until the seed is carried to soil depths where moisture is more prevalent. For epiphytic orchids, the hydrophilic nature of the testa may enable such rain-assisted seeds to infiltrate a moist
porous substrate (e.g. roots/rhizomes of the resur-rection fern, Polypodium polypodioides (L.) Watt) and remain in the canopy. Once anchored in a substrate harboring suitable mycorrhizal fungi, germination is accelerated due to the seed’s ability to absorb water rapidly, with rainfall serving as a cue to coordinate germination. Developing seedlings are prone to desiccation when the sub-strate loses water during dry weather. The utiliza-tion of fungi via mycotrophy as a means to conserve water, therefore, may be especially useful for survival in the canopy. For terrestrial orchids, fungal hyphae in contact with soil could serve as a conduit of water movement from soil to seedling.
References
[1] H.N. Rasmussen, Terrestrial Orchid from Seed to My-cotrophic Plant, Cambridge University Press, Cam-bridge, 1995.
[2] M.A. Clements, Orchid mycorrhizal associations, Lind-leyana 3 (1988) 73 – 86.
[3] C.C. Baskin, J.M. Baskin, Seeds: Ecology, Biogeogra-phy, and Evolution of Dormancy and Germination, Academic Press, San Diego, 1997.
[4] J.H. Warcup, Symbiotic germination of some Australian terrestrial orchids, New Phytol. 72 (1973) 387 – 392. [5] M.A. Clements, R.K. Ellyard, The symbiotic
germina-tion of Australian terrestrial orchids, Am. Orchid Soc. Bull. 48 (1979) 810 – 816.
[6] E.A. Smreciu, R.S. Currah, Symbiotic germination of seeds of terrestrial orchid of North America and Europe, Lindleyana 1 (1989) 6 – 15.
[7] M.A. Clements, H. Muir, P.J. Cribb, A preliminary report on the symbiotic germination of European terres-trial orchids, Kew Bull. 41 (1986) 437 – 445.
[8] L.W. Zettler, Symbiotic seed germination of terrestrial orchids in North America during the last decade — a progress report, in: C. Allen (Ed.), North American Native Terrestrial Orchid Propagation and Production, National Arboretum, Washington, DC, 1996, pp. 43 – 53.
[9] M. Fenner, Seed Ecology, Chapman and Hall, London, 1985.
[10] G.W. Wharton, Water balance of insects, in: G.A. Kerkut, L.I. Gilbert (Eds.), Comprehensive Insect Physi-ology, Biochemistry and PharmacPhysi-ology, vol. 4, Perga-mon, Oxford, 1985, pp. 565 – 603.
[11] N.F. Hadley, Water Relations of Terrestrial Arthropods, Academic Press, New York, 1994.
[12] H.T. Clifford, W.K. Smith, Seed morphology and clas-sification of Orchidaceae, Phytomorphology 19 (1969) 133 – 139.
[14] L.W. Zettler, T. Delaney, J.A. Sunley, Seed propagation of the epiphytic orchid, Epidendrum conopseum R. Brown, using its endophytic fungus, Selbyana 19 (1998) 249 – 253.
[15] K. Dixon, Raising terrestrial orchids from seed, in: W.K. Harris (Ed.), Modern Orchid Growing for Pleasure and Profit, Orchid Club of South Australia, Adelaide, Aus-tralia, 1987, pp. 47 – 54.
[16] J.M. Phillips, D.S. Hayman, Improved procedures for clearing roots and staining parasitic and vesicular-arbus-cular mycorrhizal fungi for rapid assessment of infec-tion, Trans. Br. Mycol. Soc. 55 (1970) 158 – 161. [17] L.W. Zettler, C.J. Hofer, Propagation of the little
club-spur orchid (Platanthera cla6ellata) by symbiotic seed
germination, and its ecological implications, Environ. Exp. Botany 39 (1998) 189 – 195.
[18] J.A. Yoder, D.L. Denlinger, Water balance in flesh fly pupae and water vapor absorption associated with dia-pause, J. Exp. Biol. 157 (1991) 273 – 286.
[19] P.W. Winston, D.S. Bates, Saturated solutions for the control of humidity in biological research, Ecology 41 (1960) 232 – 237.
[20] C.G. Johnson, The maintenance of high atmospheric humidities for entomological work with glycerol-water mixtures, Ann. Appl. Biol. 27 (1940) 295 – 299.
[21] E.C. Toolson, Diffusion of water through the arthropod cuticle: thermodynamic consideration of the transition phenomenon, J. Thermal Biol. 3 (1978) 69 – 73.
[22] J.A. Yoder, A. Spielman, Differential capacity of larval deer ticks (Ixodes dammini) to imbibe water from sub-saturated air, J. Insect Physiol. 38 (1992) 863 – 869. [23] R.R. Sokal, F.J. Rohlf, Biometry, W.H. Freeman, New
York, 1981.
[24] M.A. Clements, Developments in the symbiotic germina-tion of Australian terrestrial orchids, in: J. Stewart, C.N. van der Merne (Eds.), Proceedings of the 10th World Orchid Conference, South African Orchid Council, Jo-hannesburg, South Africa, 1982, 1982, pp. 269 – 273.